Non-Dipping Pattern Is Associated with Periprocedural Myocardial Infarction in Hypertensive Patients Undergoing Elective Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ambulatory Blood Pressure Monitoring (ABPM)
2.3. Definition of Periprocedural Myocardial Infarction (PMI)
2.4. PCI Procedure
2.5. Definition of Complex PCI
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
4. Discussion
4.1. Clinical Implications and Future Directions
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chotruangnapa, C.; Tansakun, T.; Roubsanthisuk, W. Clinical risk factors and predictive score for the non-dipper profile in hypertensive patients: A case-control study. Clin. Hypertens. 2021, 27, 22. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunstrom, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Azizi, M.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2024, 42, 194. [Google Scholar]
- Kario, K.; Hoshide, S.; Chia, Y.C.; Buranakitjaroen, P.; Siddique, S.; Shin, J.; Turana, Y.; Park, S.; Tsoi, K.; Chen, C.; et al. Guidance on ambulatory blood pressure monitoring: A statement from the HOPE Asia Network. J. Clin. Hypertens. 2021, 23, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Matsumoto, T.; Murase, K.; Setoh, K.; Kawaguchi, T.; Wakamura, T.; Hirai, T.; Chin, K.; Matsuda, F. Sleep blood pressure measured using a home blood pressure monitor was independently associated with cardiovascular disease incidence: The Nagahama study. J. Hypertens. 2024, 42, 1695–1702. [Google Scholar] [CrossRef]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S.; Hond, E.D.; McCormack, P.; Staessen, J.A.; O’Brien, E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef]
- Chará, C.J.; Andrade, A.F.; Pinzón, M.V.; López, N.A. Non-dipper hypertension: Its pathophysiology, etiology and association with cardiovascular outcomes. Rev. Colomb. Cardiol. 2024, 31, 57–64. [Google Scholar] [CrossRef]
- Pierdomenico, S.D.; Bucci, A.; Costantini, F.; Lapenna, D.; Cuccurullo, F.; Mezzetti, A. Circadian blood pressure changes and myocardial ischemia in hypertensive patients with coronary artery disease. J. Am. Coll. Cardiol. 1998, 31, 1627–1634. [Google Scholar] [CrossRef]
- Babu, G.G.; Walker, J.M.; Yellon, D.M.; Hausenloy, D.J. Peri-procedural myocardial injury during percutaneous coronary intervention: An important target for cardioprotection. Eur. Heart J. 2011, 32, 23–31. [Google Scholar] [CrossRef]
- Idris, H.; Lo, S.; Shugman, I.M.; Saad, Y.; Hopkins, A.P.; Mussap, C.; Leung, D.; Thomas, L.; Juergens, C.P.; French, J.K. Varying definitions for periprocedural myocardial infarction alter event rates and prognostic implications. J. Am. Heart Assoc. 2014, 3, e001086. [Google Scholar] [CrossRef]
- Hojo, Y.; Noma, S.; Ohki, T.; Nakajima, H.; Satoh, Y. Autonomic nervous system activity in essential hypertension: A comparison between dippers and non-dippers. J. Hum. Hypertens. 1997, 11, 665–671. [Google Scholar] [CrossRef]
- Routledge, F.S.; McFetridge-Durdle, J.A.; Dean, C.R.; Canadian Hypertension Society. Night-time blood pressure patterns and target organ damage: A review. Can. J. Cardiol. 2007, 23, 132–138. [Google Scholar] [CrossRef]
- Palatini, P.; Verdecchia, P.; Beilin, L.J.; Eguchi, K.; Imai, Y.; Kario, K.; Ohkubo, T.; Pierdomenico, S.D.; Saladini, F.; Schwartz, J.E.; et al. Association of Extreme Nocturnal Dipping with Cardiovascular Events Strongly Depends on Age. Hypertension 2020, 75, 324–330. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef]
- Piccolo, R.; Leone, A.; Simonetti, F.; Avvedimento, M.; Angellotti, D.; Manzi, L.; Verde, N.; Spaccarotella, C.A.M.; Di Serafino, L.; Cirillo, P.; et al. Periprocedural myocardial infarction in patients undergoing complex versus noncomplex percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2023, 102, 212–220. [Google Scholar] [CrossRef]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive role of the nighttime blood pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Toblli, J.E.; Cardinali, D.P.; Malateste, J.C.; Milei, J. Nocturnal hypertension defined by fixed cut-off limits is a better predictor of left ventricular hypertrophy than non-dipping. Int. J. Cardiol. 2008, 127, 387–389. [Google Scholar] [CrossRef]
- Kurpesa, M.; Trzos, E.; Drozdz, J.; Bednarkiewicz, Z.; Krzemińska-Pakuła, M. Myocardial ischemia and autonomic activity in dippers and non-dippers with coronary artery disease: Assessment of normotensive and hypertensive patients. Int. J. Cardiol. 2002, 83, 133–142. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Guerrieri, M.; Gatteschi, C.; Benemio, G.; Boldrini, F.; Porcellati, C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990, 81, 528–536. [Google Scholar] [CrossRef]
- Lo, L.; Hung, S.W.S.; Chan, S.S.W.; Mak, C.L.; Chan, P.F.; Chao, D.V.K. Prognostic value of nocturnal blood pressure dipping on cardiovascular outcomes in Chinese patients with hypertension in primary care. J. Clin. Hypertens. 2021, 23, 1291–1299. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Winnicki, M.; Schroeder, K.; Phillips, B.G.; Kato, M.; Cwalina, E.; Somers, V.K. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 2002, 39, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Grillo, A.; Guida, V.; Parati, G. Morning blood pressure surge: Pathophysiology, clinical relevance and therapeutic aspects. Integr. Blood Press. Control 2018, 11, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kaplangoray, M.; Toprak, K.; Caglayan, C.; Deveci, E.; Celik, E.; Uyan, U.; Aydın, C. Could the Systemic Inflammatory Response Index be a Marker for the Non-Dipper Pattern in Newly Diagnosed Hypertensive Patients? Cardiovasc. Toxicol. 2025, 25, 559–569. [Google Scholar] [CrossRef]
- Tatsukawa, Y.; Hsu, W.L.; Yamada, M.; Cologne, J.B.; Suzuki, G.; Yamamoto, H.; Yamane, K.; Akahoshi, M.; Fujiwara, S.; Kohno, N. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens. Res. 2008, 31, 1391–1397. [Google Scholar] [CrossRef]
- Manea, V.; Leucuţa, D.C.; Pop, C.; Popescu, M.I. The predictive risk factors associated with non-dipper profile in patients with type 2 diabetes and hypertension. Med. Pharm. Rep. 2024, 97, 270–279. [Google Scholar] [CrossRef]
- Quinaglia, T.; Martins, L.C.; Figueiredo, V.N.; Santos, R.C.; Yugar-Toledo, J.C.; Martin, J.F.V.; Demacq, C.; Pimenta, E.; A Calhoun, D.; Moreno, H. Non-dipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J. Hum. Hypertens. 2011, 25, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, N.H.; Kim, Y.K.; Yoo, J.H.; Shin, S.N.; Ko, J.S.; Kim, Y.K.; Rhee, S.J.; Yun, K.H.; Lee, E.M.; et al. The Number of Endothelial Progenitor Cells is Decreased in Patients with Non-Dipper Hypertension. Korean Circ. J. 2012, 42, 329–334. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Güntürk, E.E.; Güntürk, İ.; Topuz, A.N.; Akkaya, H.; Topuz, M. Serum interleukin-18 levels are associated with non-dipping pattern in newly diagnosed hypertensive patients. Blood Press. Monit. 2021, 26, 87–92. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Sasaki, S.; Goto, C.; Oshima, T.; Chayama, K.; Yoshizumi, M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: A comparison of dippers and non-dippers. J. Am. Coll. Cardiol. 2002, 40, 2039–2043. [Google Scholar] [CrossRef]
- Aksit, E.; Gursul, E.; Aydin, F.; Samsa, M.; Ozcelik, F. Non-dipper hypertension is associated with slow coronary flow among hypertensives with normal coronary angiogram. Cardiovasc. J. Afr. 2017, 28, 14–18. [Google Scholar] [CrossRef]
- Erdogan, D.; Gullu, H.; Caliskan, M.; Yildirim, I.; Ulus, T.; Bilgi, M.; Muderrisoglu, H. Coronary flow reserve in dipper and non-dipper hypertensive patients. Blood Press. 2005, 14, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cinar, A.; Gedikli, O.; Uyanik, M.; Terzi, O. Evaluation of Coronary Artery Calcium Score (CACS) in Dipper and Non-Dipper Hypertensive Patients with Moderate and High Cardiovascular Disease Risks. Medicina 2024, 60, 1999. [Google Scholar] [CrossRef]
- Nishimura, K.; Okamura, T.; Watanabe, M.; Nakai, M.; Takegami, M.; Higashiyama, A.; Kokubo, Y.; Okayama, A.; Miyamoto, Y. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: The suita study. J. Atheroscler. Thromb. 2014, 21, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Pierdomenico, A.M.; Di Tommaso, R.; Coccina, F.; Di Carlo, S.; Porreca, E.; Cuccurullo, F. Morning Blood Pressure Surge, Dipping, and Risk of Coronary Events in Elderly Treated Hypertensive Patients. Am. J. Hypertens. 2016, 29, 39–45. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Cardoso, C.R.; Dias, V.B.; Nascimento, A.C.; Salles, G.F. Prognostic impact of the ambulatory arterial stiffness index in resistant hypertension. J. Hypertens. 2010, 28, 1547–1553. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Casuccio, A.; Di Liberti, R.; Musiari, G.; Zappulla, V.; D’Angelo, A.; Pinto, A. Ambulatory Arterial Stiffness Index (AASI) is Unable to Estimate Arterial Stiffness of Hypertensive Subjects: Role of Nocturnal Dipping of Blood Pressure. Curr. Hypertens. Rev. 2017, 13, 121–131. [Google Scholar] [CrossRef]
- Demircan, S.; Durna, K.; Yaşar, T.; Şahin, M. The incidence of nondipping state in normotensive patients with coronary slow flow and its relationship with prognosis. Arch. Turk. Soc. Cardiol. 2005, 33, 319–325. [Google Scholar]
- Evola, S.; Cuttitta, F.; Evola, G.; Macaione, F.; Piraino, D.; Meschisi, M.C.; Peritore, A.; Di Lisi, D.; Novo, G.; Novo, S. Early detection of coronary artery flow and myocardial perfusion impairment in hypertensive patients evidenced by myocardial blush grade (MBG) and thrombolysis in myocardial infarction (TIMI) frame count (TFC). Intern. Med. 2012, 51, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, H.; Cin, V.G.; Ciçek, D.; Çamsari, A.; Akkus, N.; Döven, O.; Parmaksiz, H.T. Slow coronary flow may be a sign of diffuse atherosclerosis. Contribution of FFR and IVUS. Acta Cardiol. 2004, 59, 127–133. [Google Scholar] [CrossRef]
- Xia, S.; Deng, S.B.; Wang, Y.; Xiao, J.; Du, J.-L.; Zhang, Y.; Wang, X.-C.; Li, Y.-Q.; Zhao, R.; He, L.; et al. Clinical analysis of the risk factors of slow coronary flow. Heart Vessels. 2011, 26, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Mousa, T.; el-Sayed, M.A.; Motawea, A.K.; Salama, M.A.; Elhendy, A. Association of blunted nighttime blood pressure dipping with coronary artery stenosis in men. Am. J. Hypertens. 2004, 17, 977–980. [Google Scholar] [CrossRef]
- Cai, A.; Zhong, Q.; Liu, C.; Zhou, D.; Li, X.; Zhang, Y.; Feng, Y.; Zhou, Y. Associations of systolic and diastolic blood pressure night-to-day ratios with atherosclerotic cardiovascular diseases. Hypertens. Res. 2016, 39, 874–878. [Google Scholar] [CrossRef]
- Kartaler, F.; Şahin, M.; Turan, O.E.; Kutlu, M. The Relationship Between the Dipping Pattern and Coronary Artery Disease Severity Assessed by the SYNTAX Score in Patients with Hypertension. Cureus 2023, 15, e36057. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.; Özderya, A.; Sahin, S.; Kul, S.; Konuş, A.H.; Kara, F.; Uzun, G.; Akyüz, A.R.; Sayin, M.R. Abnormal Circadian Blood Pressure Variation is Associated with SYNTAX Scores in Hospitalized Patients with Acute Coronary Syndrome. A Variação Anormal da Pressão Arterial Circadiana está Associada aos Escores SYNTAX em Pacientes Hospitalizados com Síndrome Coronariana Aguda. Arq. Bras. Cardiol. 2022, 119, 76–84. [Google Scholar]
- Mohammed, A.A.S.; Lin, X.; Yangyang, Y.; Runmin, S.; Juan, H.; Mingming, W.; Jing, Y. The Association of Morning Surge and Night-Time Dipping Blood Pressure with Significant and Complex Coronary Artery Lesions. High. Blood Press. Cardiovasc. Prev. 2021, 28, 467–474. [Google Scholar] [CrossRef]


| Non-Dipping Patients (n = 243) | Dipping Patients (n = 219) | p | |
|---|---|---|---|
| Age (years) | 61.35 ± 9.81 | 61.72 ± 9.35 | 0.680 |
| Gender (male, %) | 191 (78.6) | 167 (76.3) | 0.547 |
| Diabetes mellitus (n, %) | 120 (49.4) | 80 (36.5) | 0.005 |
| Hyperlipidemia (n, %) | 138 (56.8) | 122 (55.7) | 0.815 |
| Active smoking (n, %) | 103 (42.4) | 92 (42) | 0.935 |
| Family history of CAD (n, %) | 93 (38.3) | 80 (36.5) | 0.699 |
| Stroke (n, %) | 9 (3.7) | 4 (1.8) | 0.223 |
| ACEi or ARB use (n, %) | 131 (53.9) | 113 (51.6) | 0.619 |
| Beta-blockers use (n, %) | 153 (63) | 112 (51.1) | 0.01 |
| Aspirin use (n, %) | 144 (59.3) | 114 (52.1) | 0.119 |
| Statin use (n, %) | 77 (31.7) | 67 (30.6) | 0.8 |
| CCB use (n, %) | 86 (35.4) | 74 (33.8) | 0.718 |
| Left ventricular ejection fraction (%) | 55.86 ± 4.58 | 56.62 ± 4.49 | 0.086 |
| Hemoglobin (g/dL) | 13.21 ± 1.77 | 13.11 ± 2.12 | 0.605 |
| White blood cell count (109/L) | 9.53 ± 3.22 | 8.61 ± 2.28 | 0.001 |
| Platelet count (109/L) | 238 (190–287) | 245 (198–291) | 0.295 |
| Baseline GFR (mL/min/1.73 m2) | 88.30 ± 23.63 | 90.60 ± 19.14 | 0.392 |
| Baseline creatinine (mg/dL) | 0.85 (0.72–1.00) | 0.90 (0.77–1.00) | 0.045 |
| Total cholesterol (mg/dL) | 181.30 ± 51.16 | 175.09 ± 45.43 | 0.398 |
| LDL cholesterol (mg/dL) | 105.80 (82–133.5) | 105.00 (81–137) | 0.901 |
| HDL cholesterol (mg/dL) | 39 (33–45) | 35.15 (32–44) | 0.111 |
| Triglyceride (mg/dL) | 151 (109–239) | 168.25 (123.5–286.5) | 0.141 |
| Glucose (mg/dL) | 163.47 ± 82.79 | 136.15 ± 63.39 | 0.001 |
| Hba1c % | 8.09 ± 2.45 | 6.21 ± 1.59 | 0.002 |
| C-reactive protein (mg/L) | 3.94 (2–10.85) | 5.12 (2.91–14.60) | 0.109 |
| SYNTAX Score | 9 (6–13) | 11.25 (8–20.5) | <0.001 |
| Three-vessel disease (n, %) | 49(20.2) | 10(4.6) | <0.001 |
| Complex PCI (n, %) | 54(25.1) | 7(5.04) | <0.001 |
| Proximal lesion (n, %) | 125(59) | 101(59.4) | 0.984 |
| PMI (n, %) | 79(32.5) | 30(13.7) | <0.001 |
| Patients Without PMI (n: 353) | Patients with PMI (n: 109) | p | |
|---|---|---|---|
| Age (years) | 61.29 ± 9.61 | 62.29 ± 9.51 | 0.341 |
| Gender (male, %) | 274 (77.6) | 84 (77.1) | 0.903 |
| Body mass index (kg/m2) | 28.47 ± 3.36 | 28.31 ± 3.55 | 0.674 |
| Diabetes mellitus (n, %) | 143 (40.5) | 57 (52.3) | 0.03 |
| Hyperlipidemia (n, %) | 202 (57.2) | 58 (53.2) | 0.460 |
| Active smoking (n, %) | 155 (43.9) | 40 (36.7) | 0.183 |
| Family history of CAD (n, %) | 126 (35.7) | 47 (43.1) | 0.161 |
| Stroke (n, %) | 11 (3.1) | 2 (1.8) | 0.480 |
| ACEi or ARB use (n, %) | 190 (53.8) | 54 (49.5) | 0.434 |
| Beta-blockers use (n, %) | 191 (54.1) | 74 (67.9) | 0.11 |
| Aspirin use (n, %) | 208 (58.9) | 50 (45.9) | 0.160 |
| Statin use (n, %) | 112 (31.7) | 32 (29.4) | 0.640 |
| CCB use (n, %) | 114 (32.3) | 46 (42.2) | 0.057 |
| Left ventricular ejection fraction (%) | 56.38 ± 4.51 | 55.65 ± 4.69 | 0.164 |
| Hemoglobin (g/dL) | 13.33 ± 1.77 | 12.99 ± 1.78 | 0.109 |
| White blood cell count (109/L) | 8.93 ± 2.76 | 9.63 ± 3.09 | 0.037 |
| Platelet count (109/L) | 244.5 (194.5–292) | 238.5 (195–273.5) | 0.612 |
| Baseline GFR (mL/min/1.73 m2) | 91.98 ± 21.94 | 82.36 ± 20.13 | 0.001 |
| Baseline creatinine (mg/dL) | 0.88 (0.73–1.00) | 0.91 (0.79–1.01) | 0.017 |
| Total cholesterol (mg/dL) | 174.85 ± 45.97 | 202.6 ± 72.44 | 0.008 |
| LDL cholesterol (mg/dL) | 107 (82–134) | 99.75 (81–130) | 0.769 |
| HDL cholesterol (mg/dL) | 38 (33–44) | 34.5 (31–46) | 0.464 |
| Triglyceride (mg/dL) | 155.5 (112–242) | 167 (135–287) | 0.133 |
| Glucose (mg/dL) | 146.16 ± 73.39 | 166.71 ± 80.79 | 0.044 |
| Hba1c (%) | 6.93 ± 2.12 | 8.93 ± 2.46 | 0.003 |
| C-reactive protein (mg/L) | 4.89 (2.12–11.92) | 4.95 (3.02–10.85) | 0.694 |
| Three-vessel disease (n, %) | 31 (8.8) | 28 (25.7) | <0.001 |
| Complex PCI (n, %) | 23 (7.8) | 38 (40.4) | <0.001 |
| Proximal lesion | 157 (54.5) | 69 (73.4) | 0.001 |
| SYNTAX score | 9 (6–12) | 22.25 (14.5–28) | <0.001 |
| Pressure (atm) | 17.92 ± 1.41 | 18.14 ± 1.19 | 0.467 |
| Number of stents | 1.27 ± 0.5 | 1.72 ± 0.74 | <0.001 |
| Stent diameter (mm) | 3.01 ± 0.38 | 3.13 ± 0.43 | 0.009 |
| Stent length (mm) | 24.93 ± 9.22 | 29.88 ± 10.2 | <0.001 |
| Non-Dipping pattern (n, %) | 164 (46.5) | 79 (72.5) | <0.001 |
| Dipping pattern (n, %) | 189 (53.5) | 30 (27.5) | <0.001 |
| Variable | p | Odds Ratio | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
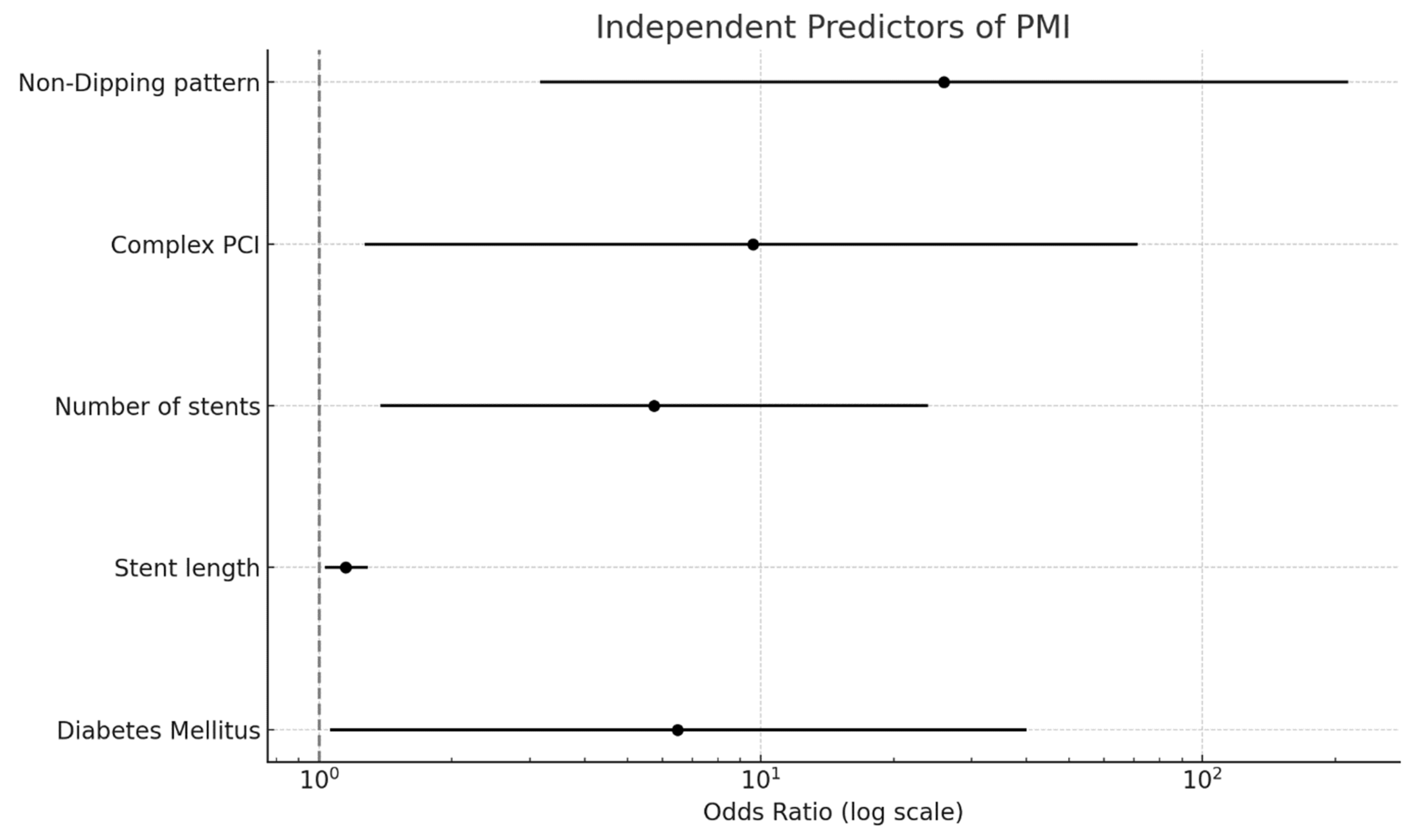
| Non-dipping pattern | 0.002 | 25.986 | 3.157 | 213.923 |
| Stent length | 0.016 | 1.148 | 1.025 | 1.285 |
| Diabetes mellitus | 0.044 | 6.493 | 1.055 | 40 |
| White blood cell | 0.305 | 1.062 | 0.893 | 1.436 |
| Proximal lesion | 0.07 | 5.848 | 0.864 | 40 |
| Stent diameter | 0.185 | 3.237 | 0.569 | 18.407 |
| Number of stents | 0.016 | 5.744 | 1.377 | 23.957 |
| Aspirin | 0.13 | 0.298 | 0.062 | 1.430 |
| Total cholesterol | 0.063 | 1.015 | 0.999 | 1.030 |
| Complex PCI | 0.029 | 9.615 | 1.267 | 71.428 |
| Creatinine | 0.198 | 4.518 | 0.455 | 44.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekler, O.; Kurtul, A. Non-Dipping Pattern Is Associated with Periprocedural Myocardial Infarction in Hypertensive Patients Undergoing Elective Percutaneous Coronary Intervention. Medicina 2025, 61, 794. https://doi.org/10.3390/medicina61050794
Bekler O, Kurtul A. Non-Dipping Pattern Is Associated with Periprocedural Myocardial Infarction in Hypertensive Patients Undergoing Elective Percutaneous Coronary Intervention. Medicina. 2025; 61(5):794. https://doi.org/10.3390/medicina61050794
Chicago/Turabian StyleBekler, Ozkan, and Alparslan Kurtul. 2025. "Non-Dipping Pattern Is Associated with Periprocedural Myocardial Infarction in Hypertensive Patients Undergoing Elective Percutaneous Coronary Intervention" Medicina 61, no. 5: 794. https://doi.org/10.3390/medicina61050794
APA StyleBekler, O., & Kurtul, A. (2025). Non-Dipping Pattern Is Associated with Periprocedural Myocardial Infarction in Hypertensive Patients Undergoing Elective Percutaneous Coronary Intervention. Medicina, 61(5), 794. https://doi.org/10.3390/medicina61050794





