Genistein Improves the Cytotoxic, Apoptotic, and Oxidative-Stress-Inducing Properties of Doxorubicin in SK-MEL-28 Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Cell Culture Method
2.4. Cell Viability Assessment–The MTT Test
2.5. Combination Index Calculation
2.6. Bright-Field Evaluation of Cell Morphology
2.7. Label-Free Quantification of Cell Confluence
2.8. Immunofluorescence Imaging of Nuclei and Cytoskeletal Filaments
2.9. Caspase-3/7 and Caspase-9 Activity Measurement
2.10. ROS Quantification
2.11. In Ovo Irritation Assay
2.12. Statistical Analysis
3. Results
3.1. GEN–DOX Treatment Triggers a Selective Cytotoxicity in SK-MEL-28 Cells
3.1.1. Impact of GEN–DOX Treatment on Cell Viability
3.1.2. Combination Index Calculation for GEN–DOX Treatment
3.1.3. Impact of GEN–DOX Treatment on Cell Morphology and Confluence
3.1.4. Impact of GEN–DOX Treatment on Nuclear and Cytoskeletal Reorganization
3.2. GEN–DOX Treatment Activates Intrinsic Apoptosis and Generates Oxidative Stress in SK-MEL-28 Cells
3.2.1. Impact of GEN–DOX Treatment on Caspase-3/7 and -9 Activation
3.2.2. Impact of GEN–DOX Treatment on Intracellular ROS Production
3.3. GEN–DOX Treatment Lacks Irritant Effect in Ovo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Cutaneous melanoma |
| DOX | Doxorubicin |
| GEN | Genistein |
| CAM | Chorioallantoic membrane |
| UV | Ultraviolet radiation |
| CTLA-4 | Cytotoxic T-lymphocyte associated protein-4 |
| PD-1 | Programmed cell death protein-1 |
| BRAF | v-Raf murine sarcoma viral oncogene homolog B1 |
| MEK | Mitogen-activated protein kinase |
| GEN–DOX | Genisten–doxorubicin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| BSA | Bovine serum albumin |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| CI | Combination index |
| IC50 | Half-maximal inhibitory concentration |
References
- Lee, K.A.; Nathan, P. Cutaneous melanoma—A review of systemic therapies. Acta Derm. Venereol. 2020, 100, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, 2021161S. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.; Dehelean, C.; Moaca, E.A.; Pinzaru, I.; Bratu, T.; Navolan, D.; Boruga, O. Cutaneous melanoma—A long road from experimental models to clinical outcome: A review. Int. J. Mol. Sci. 2018, 19, 1566. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the Management of Advanced Cutaneous Malignant Melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef]
- Elliott, A.M.; Al-Hajj, M.A. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol. Cancer Res. 2009, 7, 79–87. [Google Scholar] [CrossRef]
- Ebbert, L.; von Montfort, C.; Wenzel, C.K.; Reichert, A.S.; Stahl, W.; Brenneisen, P. A Combination of Cardamonin and Doxorubicin Selectively Affect Cell Viability of Melanoma Cells: An In Vitro Study. Antioxidants 2024, 13, 864. [Google Scholar] [CrossRef]
- Pham, J.P.; Joshua, A.M.; da Silva, I.P.; Dummer, R.; Goldinger, S.M. Chemotherapy in Cutaneous Melanoma: Is There Still a Role? Curr. Oncol. Rep. 2023, 25, 609–621. [Google Scholar] [CrossRef]
- Lori, G.; Paoli, P.; Caselli, A.; Cirri, P.; Marzocchini, R.; Mangoni, M.; Talamonti, C.; Livi, L.; Raugei, G. Targeting LMW-PTP to sensitize melanoma cancer cells toward chemo- and radiotherapy. Cancer Med. 2018, 7, 1933–1943. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Özdemir, D.; Büssgen, M. Effectiveness and cost-effectiveness of combination therapy versus monotherapy in malignant melanoma. J. Pharm. Policy Pract. 2023, 16, 106. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Postow, M.A. Combinatorial Approaches to the Treatment of Advanced Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.; Bastos, V.; Oliveira, H. Hyperthermia enhances doxorubicin therapeutic efficacy against A375 and MNT-1 melanoma cells. Int. J. Mol. Sci. 2022, 23, 35. [Google Scholar] [CrossRef]
- Licarete, E.; Rauca, V.F.; Luput, L.; Drotar, D.; Stejerean, I.; Patras, L.; Dume, B.; Toma, V.A.; Porfire, A.; Gherman, C.; et al. Overcoming Intrinsic Doxorubicin Resistance in Melanoma by Anti-Angiogenic and Anti-Metastatic Effects of Liposomal Prednisolone Phosphate on Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2968. [Google Scholar] [CrossRef]
- Yerragopu, A.K.; Vellapandian, C. Chemoimmunotherapy with doxorubicin and caffeine combination enhanced ICD induction and T-cell infiltration in B16F10 melanoma tumors. J. Biochem. Mol. Toxicol. 2023, 37, e23327. [Google Scholar] [CrossRef]
- Dorasamy, M.S.; Ab, A.; Nellore, K.; Wong, P.F. Synergistic inhibition of melanoma xenografts by Brequinar sodium and Doxorubicin. Biomed. Pharmacother. 2019, 110, 29–36. [Google Scholar] [CrossRef]
- Rostami, Z.; Alizadeh-Navaei, R.; Golpoor, M.; Yazdani, Z.; Rafiei, A. Synergistic effects of cold atmospheric plasma and doxorubicin on melanoma: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 7870. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Veerasamy, T.; Eugin Simon, S.; Tan, K.O. Emerging strategies for sensitization of therapy resistant tumors toward cancer therapeutics by targeting the Bcl-2 family, TGF-β, Wnt/β-Catenin, RASSF and miRNA regulated signaling pathways. Int. J. Biochem. Cell Biol. 2021, 137, 106016. [Google Scholar] [CrossRef]
- de Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, C.W.; Jeon, S.Y.; Go, R.E.; Hwang, K.A.; Choi, K.C. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Lab. Anim. Res. 2014, 30, 14. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/paxillin and MAPK pathways. Oncotarget 2017, 8, 21674–21691. [Google Scholar] [CrossRef]
- Venza, I.; Visalli, M.; Oteri, R.; Beninati, C.; Teti, D.; Venza, M. Genistein reduces proliferation of EP3-expressing melanoma cells through inhibition of PGE2-induced IL-8 expression. Int. Immunopharmacol. 2018, 62, 86–95. [Google Scholar] [CrossRef]
- Chen, M.; Samuel, V.P.; Wu, Y.; Dang, M.; Lin, Y.; Sriramaneni, R.; Sah, S.K.; Chinnaboina, G.K.; Zhang, G. Nrf2/HO-1 Mediated Protective Activity of Genistein Against Doxorubicin-Induced Cardiac Toxicity. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 143–152. [Google Scholar] [CrossRef]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Coricovac, D.; Pinzaru, I.; Marcovici, I.; Macasoi, I.G.; Semenescu, A.; Lazar, G.; Pinzaru, S.C.; Radulov, I.; Alexa, E.; et al. Rutin bioconjugates as potential nutraceutical prodrugs: An in vitro and in ovo toxicological screening. Front. Pharmacol. 2022, 13, 1000608. [Google Scholar] [CrossRef]
- Hernández, J.L.; Padilla, L.; Dakhel, S.; Coll, T.; Hervas, R.; Adan, J.; Masa, M.; Mitjans, F.; Martinez, J.M.; Coma, S.; et al. Therapeutic Targeting of Tumor Growth and Angiogenesis with a Novel Anti-S100A4 Monoclonal Antibody. PLoS ONE 2013, 8, e72480. [Google Scholar] [CrossRef] [PubMed]
- Yunita, E.; Muflikhasari, H.A.; Ilmawati, G.P.N.; Meiyanto, E.; Hermawan, A. Hesperetin alleviates doxorubicin-induced migration in 4T1 breast cancer cells. Future J. Pharm. Sci. 2020, 6, 23. [Google Scholar] [CrossRef]
- Cozma, G.V.; Apostu, A.; Macasoi, I.; Dehelean, C.A.; Cretu, O.M.; Dinu, S.; Gaiță, D.; Manea, A. In Vitro and In Ovo Evaluation of the Potential Hepatoprotective Effect of Metformin. Medicina 2022, 58, 705. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Soica, C.; Antal, D.; Popescu, A.; Ghiulai, R.; Pavel, I.Z.; Avram, S.; Daliana, M.; Dehelean, C. An Update On Natural Compounds and Their Modern Formulations for the Management of Malignant Melanoma. In Natural Products and Cancer Drug Discovery; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Kassouf, E.; Ali Tehfe, M.; Florescu, M.; Soulieres, D.; Lemieux, B.; Ayoub, J.P.M.; Charpentier, D.; Yelle, L.; Daigneault, L.; Colin, P.; et al. Phase I and II studies of the decitabine–genistein drug combination in advanced solid tumors. J. Clin. Oncol. 2015, 33, e13556. [Google Scholar] [CrossRef]
- Liu, H.; Lee, G.; Lee, J.I.; Ahn, T.G.; Kim, S.A. Effects of genistein on anti-tumor activity of cisplatin in human cervical cancer cell lines. Obstet. Gynecol. Sci. 2019, 62, 322–328. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, J.; Tang, L. Genistein inhibits invasion and migration of colon cancer cells by recovering WIF1 expression. Mol. Med. Rep. 2018, 17, 7265–7273. [Google Scholar] [CrossRef]
- Nazim, U.M.D.; Park, S.Y. Genistein enhances TRAIL-induced cancer cell death via inactivation of autophagic flux. Oncol. Rep. 2015, 34, 2692–2698. [Google Scholar] [CrossRef]
- Orzechowska, E.J.; Girstun, A.; Staron, K.; Trzcinska-Danielewicz, J. Synergy of BID with doxorubicin in the killing of cancer cells. Oncol. Rep. 2015, 33, 2143–2150. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Leikeim, A.; Wußmann, M.; Schmidt, F.F.; Neto, N.G.B.; Benz, F.; Tiltmann, K.; Junger, C.; Monaghan, M.G.; Schilling, B.; Groeber-Becker, F.K. A preclinical model of cutaneous melanoma based on reconstructed human epidermis. Sci. Rep. 2022, 12, 16269. [Google Scholar] [CrossRef]
- Available online: https://www.atcc.org/ (accessed on 3 March 2025).
- Simsone, Z.; Freivalds, T.; Bēma, D.; Miķelsone, I.; Patetko, L.; Bērziņš, J.; Harju, L.; Buiķis, I. Cancer microcell initiation and determination. BMC Cancer 2021, 21, 1087. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Ridd, K.; Dhir, S.; Smith, A.G.; Gant, T.W. Defective TPA signalling compromises HaCat cells as a human in vitro skin carcinogenesis model. Toxicol. Vitr. 2010, 24, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; Petkov, P.S.; Vayssilov, G.N.; Garrido, M.J. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef]
- Ikawati, M.; Jenie, R.I.; Utomo, R.Y.; Amalina, N.D.; Nur Ilmawati, G.P.; Kawaichi, M.; Meiyanto, E. Genistein enhances cytotoxic and antimigratory activities of doxorubicin on 4T1 breast cancer cells through cell cycle arrest and ROS generation. J. Appl. Pharm. Sci. 2020, 10, 95–104. [Google Scholar]
- Sakagami, H.; Okudaira, N.; Masuda, Y.; Amano, O.; Yokose, S.; Kanda, Y.; Suguro, M.; Natori, T.; Oizumi, H.; Oizumi, T. Induction of apoptosis in human oral keratinocyte by doxorubicin. Anticancer Res. 2017, 37, 1023–1029. [Google Scholar]
- Pessina, A.; Raimondi, A.; Cerri, A.; Piccirillo, M.; Neri, M.G.; Croera, C.; Foti, P.; Berti, E. High sensitivity of human epidermal keratinocytes (HaCaT) to topoisomerase inhibitors. Cell Prolif. 2001, 34, 243–252. [Google Scholar] [CrossRef]
- Yokomichi, N.; Nagasawa, T.; Coler-Reilly, A.; Suzuki, H.; Kubota, Y.; Yoshioka, R.; Tozawa, A.; Suzuki, N.; Yamaguchi, Y. Pathogenesis of Hand-Foot Syndrome induced by PEG-modified liposomal Doxorubicin. Hum. Cell 2013, 26, 8–18. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Chanvorachote, P.; Nimmannit, U.; Leonard, S.S.; Stehlik, C.; Wang, L.; Rojanasakul, Y. Mitochondrial superoxide mediates doxorubicin-induced keratinocyte apoptosis through oxidative modification of ERK and Bcl-2 ubiquitination. Biochem. Pharmacol. 2012, 83, 1643–1654. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ. Toxicol. 2019, 34, 645–651. [Google Scholar] [CrossRef]
- Ma, J.; Motsinger-Reif, A. Current Methods for Quantifying Drug Synergism. Proteom. Bioinform. Curr. Res. 2019, 1, 43–48. [Google Scholar]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yogosawa, S.; Izutani, Y.; Watanabe, H.; Otsuji, E.; Sakai, T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol. Cancer 2009, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, S.; Zarepour, A.; Nasri, N.; Cordani, M.; Zarrabi, A. A comparison study between doxorubicin and curcumin co-administration and co-loading in a smart niosomal formulation for MCF-7 breast cancer therapy. Eur. J. Pharm. Sci. 2023, 191, 106600. [Google Scholar] [CrossRef]
- Terzioglu-Usak, S.; Yildiz, M.T.; Goncu, B.; Ozten-Kandas, N. Achieving the balance: Biphasic effects of genistein on PC-3 cells. J. Food Biochem. 2019, 43, e12951. [Google Scholar] [CrossRef]
- Sardão, V.A.; Oliveira, P.J.; Holy, J.; Oliveira, C.R.; Wallace, K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biol. Toxicol. 2009, 25, 227–243. [Google Scholar] [CrossRef]
- Merten, K.E.; Jiang, Y.; Feng, W.; Kang, Y.J. Calcineurin activation is not necessary for doxorubicin-induced hypertrophy in H9c2 embryonic rat cardiac cells: Involvement of the phosphoinositide 3-kinase-akt pathway. J. Pharmacol. Exp. Ther. 2006, 319, 934–940. [Google Scholar] [CrossRef]
- Bottone, M.; Santin, G.; Aredia, F.; Bernocchi, G.; Pellicciari, C.; Scovassi, A. Morphological Features of Organelles during Apoptosis: An Overview. Cells 2013, 2, 294–305. [Google Scholar] [CrossRef]
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis. Exp. Cell Res. 2007, 313, 3635–3644. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; De La Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic reorganization of the cytoskeleton during apoptosis: The two coffins hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Ghorbani, N.; Yaghubi, R.; Davoodi, J.; Pahlavan, S. How does caspases regulation play role in cell decisions? apoptosis and beyond. Mol. Cell. Biochem. 2024, 479, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget 2017, 8, 23996–24008. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 1. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.-C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef]
- Marcovici, I.; Chioibas, R.; Zupko, I.; Pinzaru, I.; Moaca, A.; Ledeti, A.; Barbu-Tudoran, L.; Geamantan, A.; Predescu, I.; Dehelean, C.A. Preclinical pharmaco-toxicological screening of biomimetic melanin-like nanoparticles as a potential therapeutic strategy for cutaneous melanoma. Front. Pharmacol. 2025, 16, 1487854. [Google Scholar] [CrossRef]
- de Cristo Soares Alves, A.; Rosane Dallemole, D.; Medeiro Ciocheta, T.; Weber, A.F.; Gündel, S.d.S.; Visioli, F.; Figueiró, F.; Guterres, S.S.; Pohlmann, A.R. Chicken embryo model for in vivo acute toxicological and antitumor efficacy evaluation of lipid nanocarrier containing doxorubicin. Int. J. Pharm. X 2023, 6, 100193. [Google Scholar] [CrossRef]
- Iftode, C.; Iurciuc, S.; Marcovici, I.; Coricovac, D.; Dehelean, C.; Ursoniu, S.; Rusu, A.; Ardelean, S. Genistein-Aspirin Combination Exerts Cytotoxic and Anti-Migratory Effects in Human Colorectal Cancer Cells. Life 2024, 14, 606. [Google Scholar] [CrossRef]
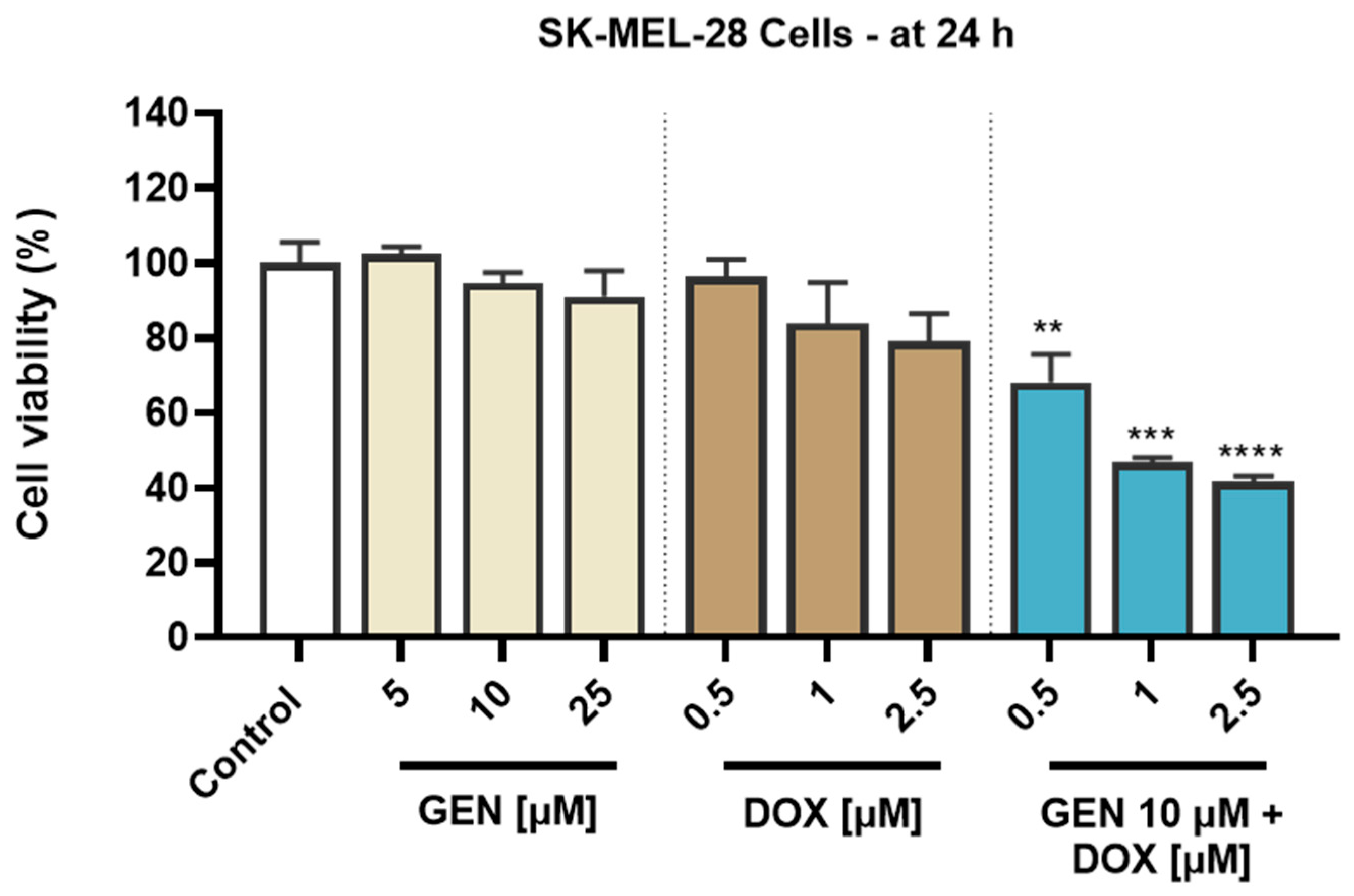
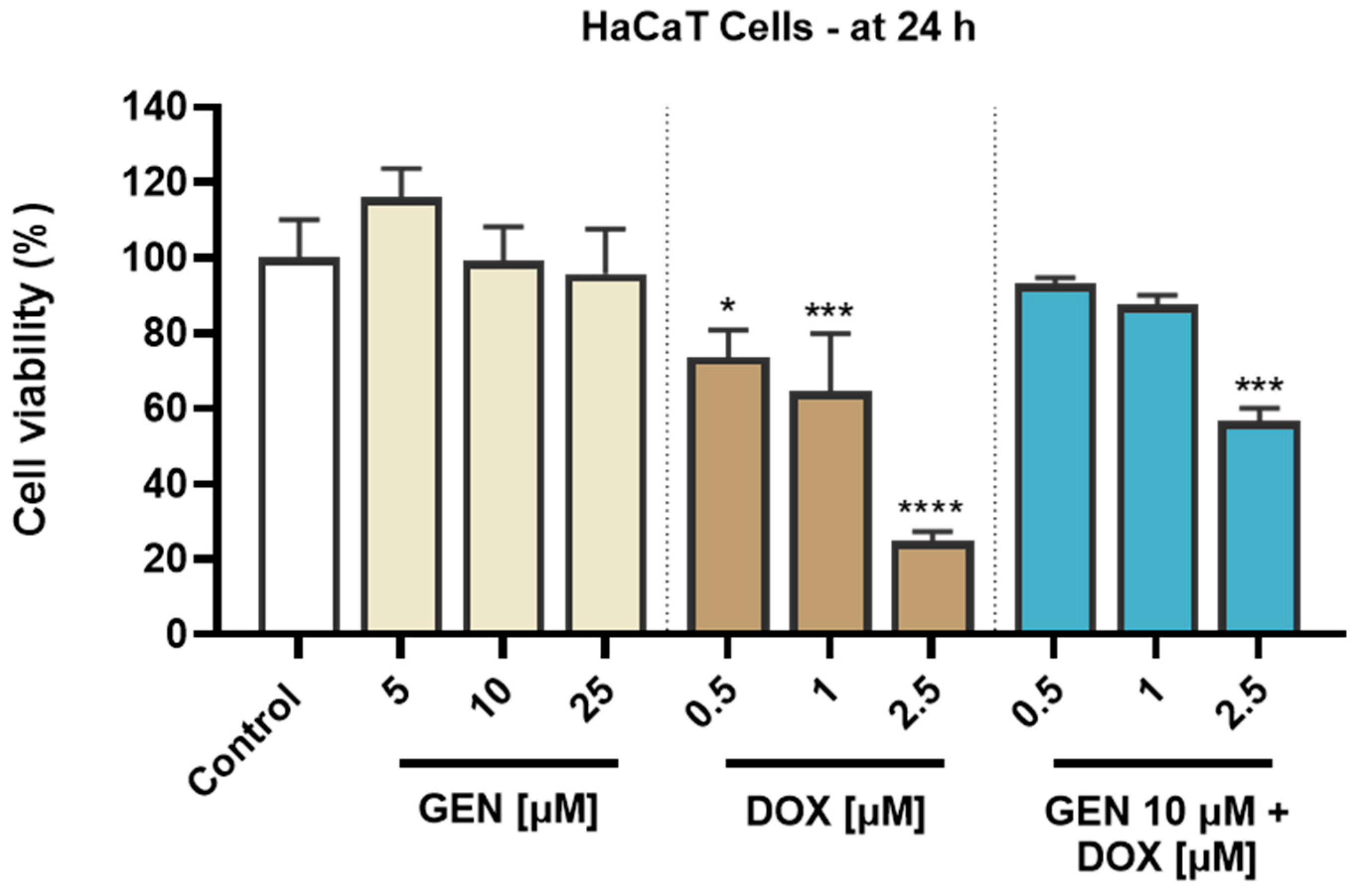

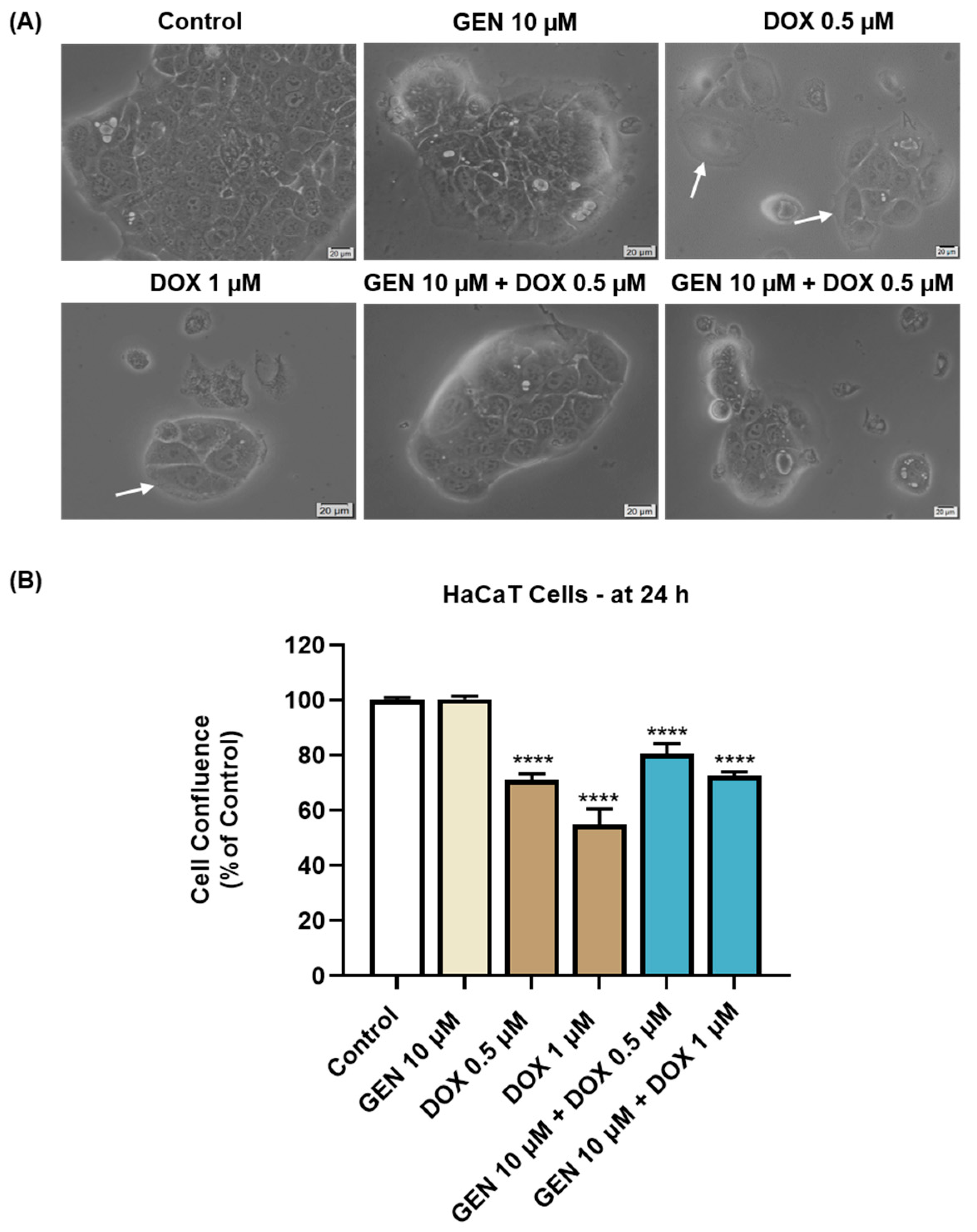
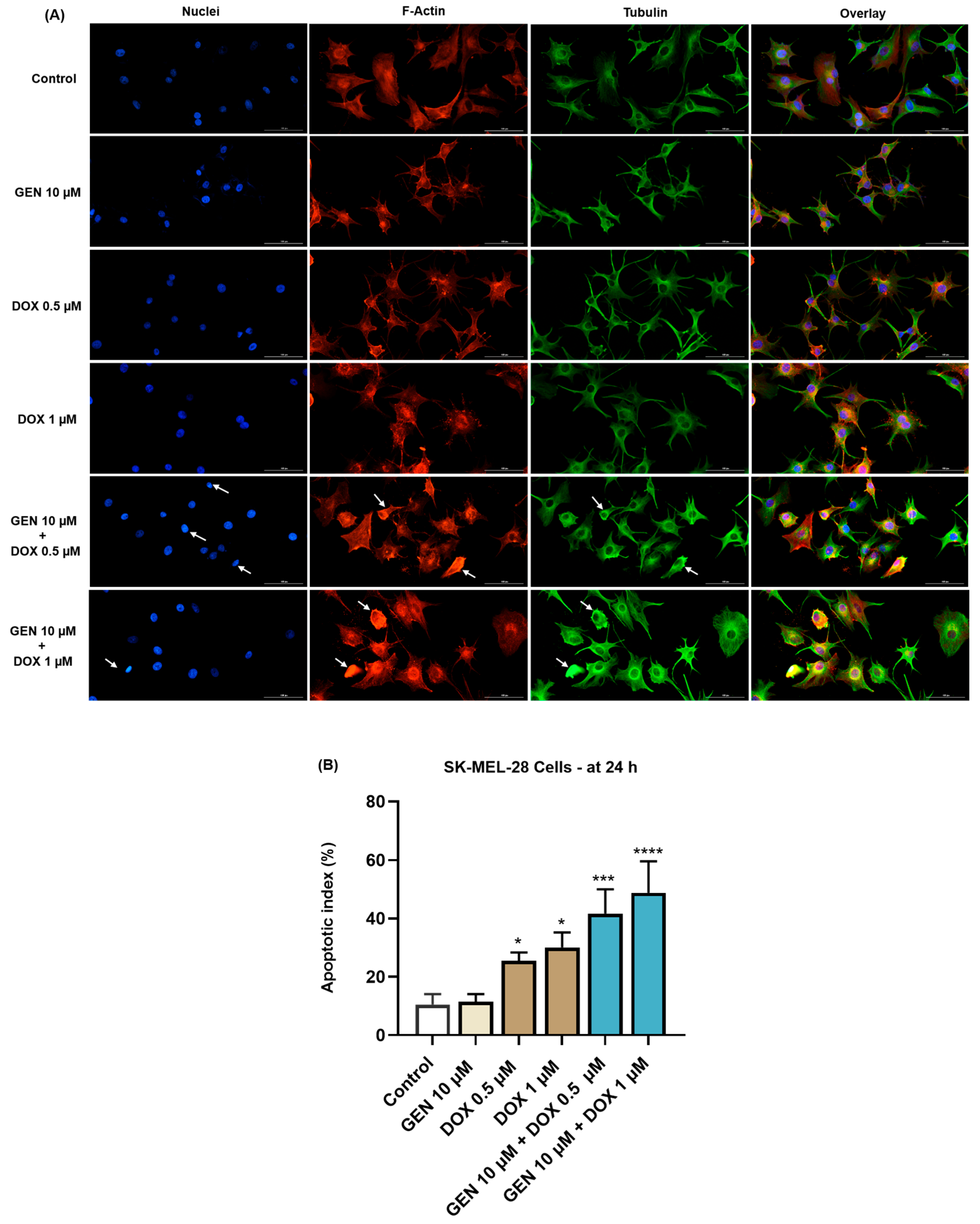
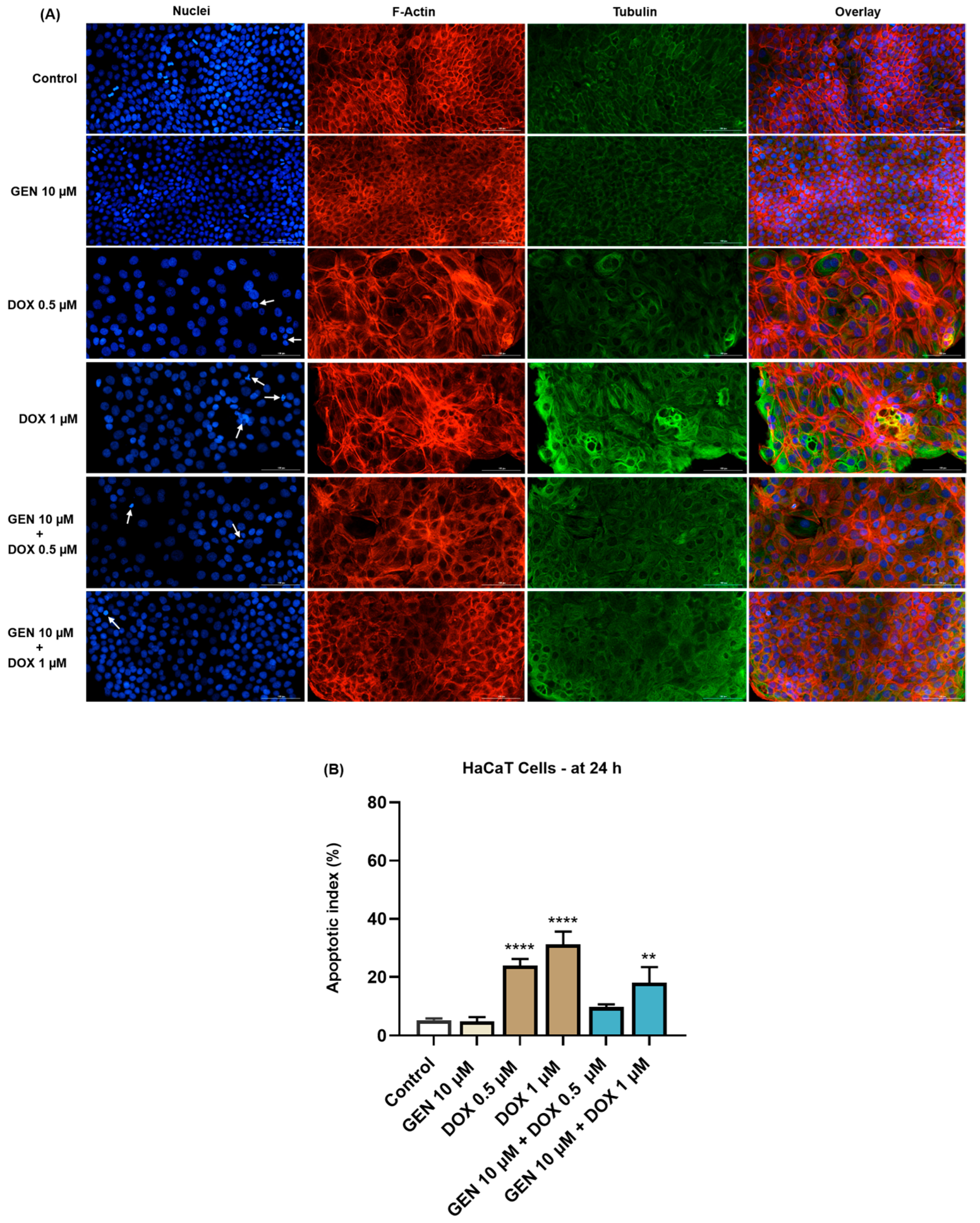
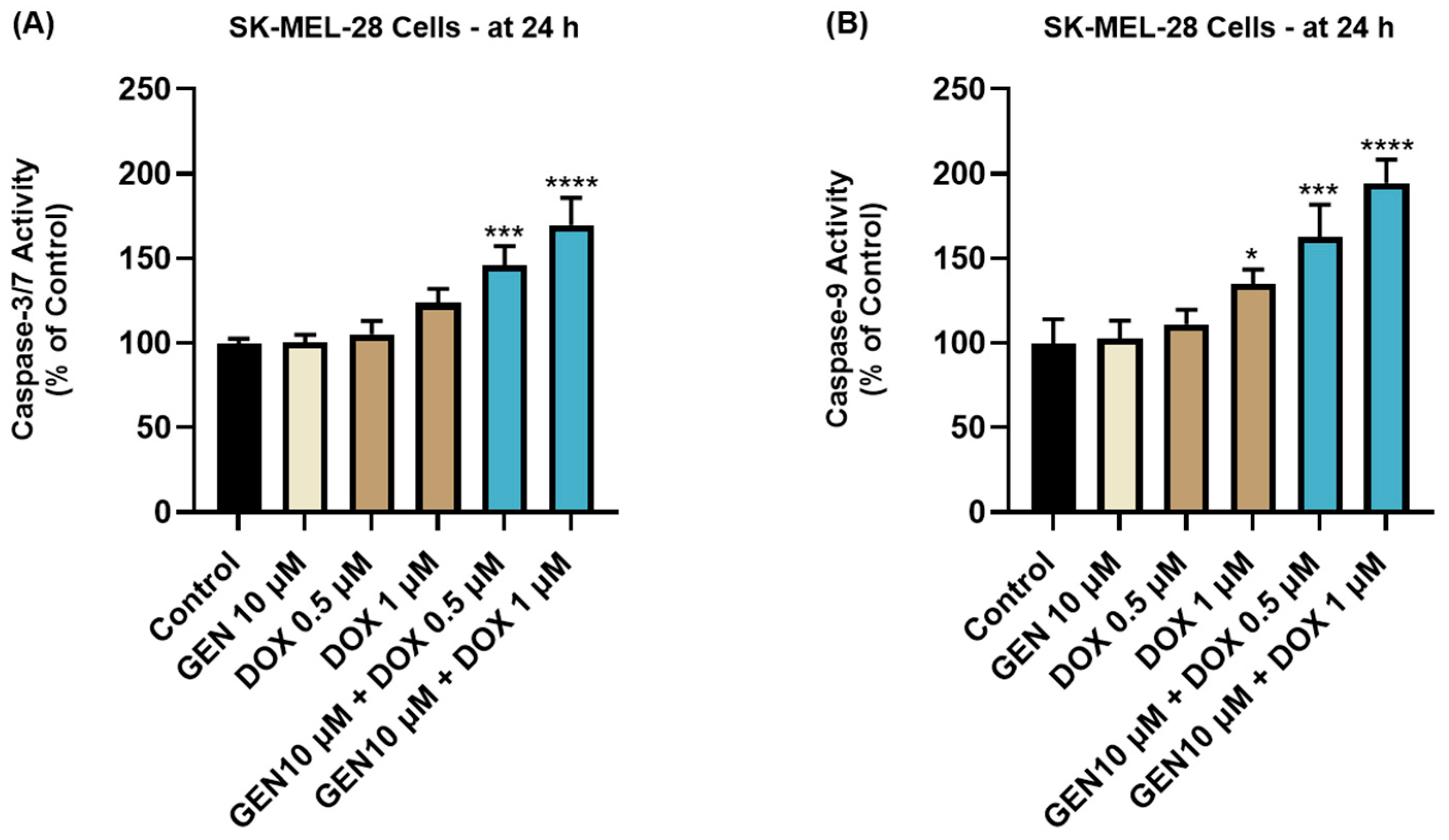
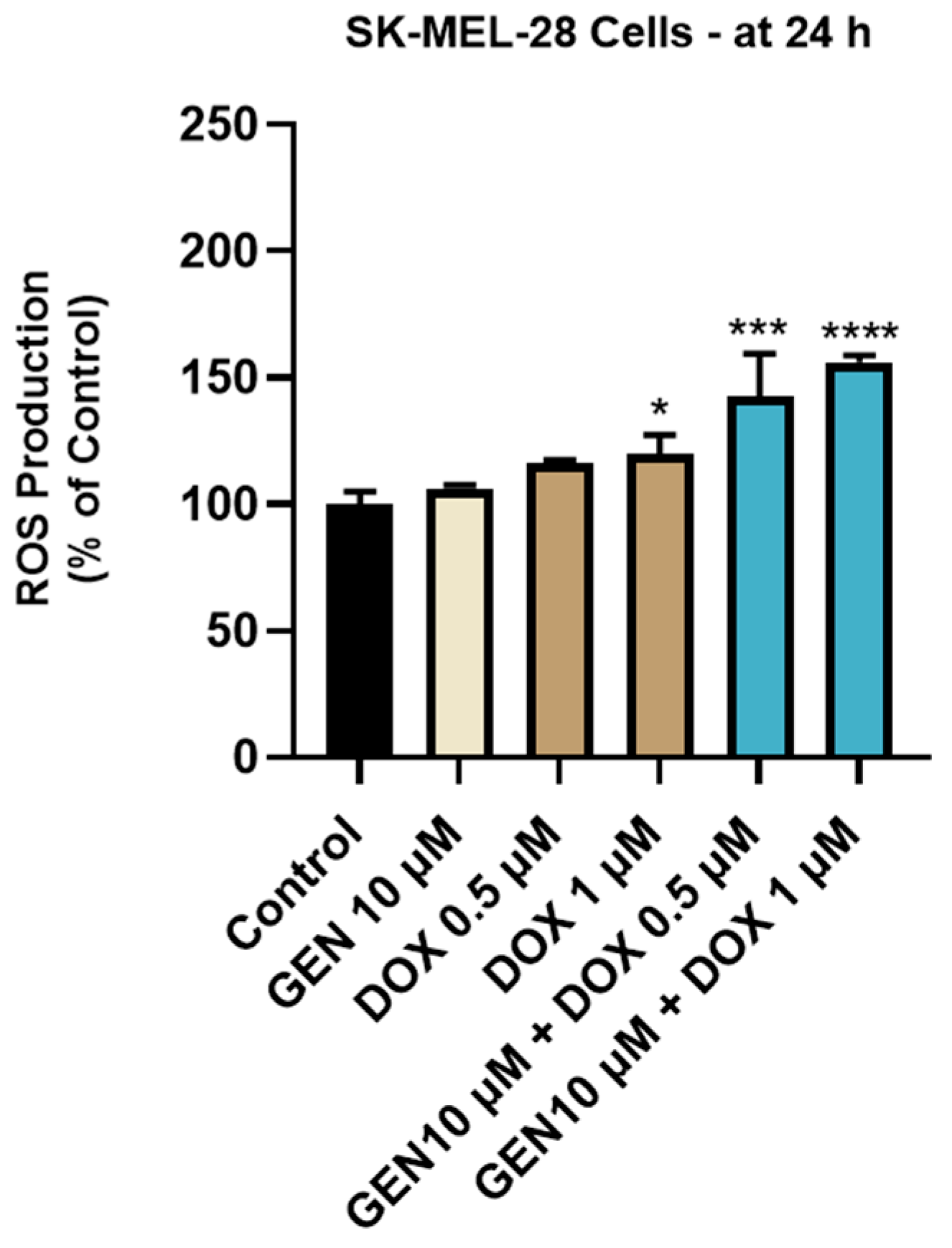
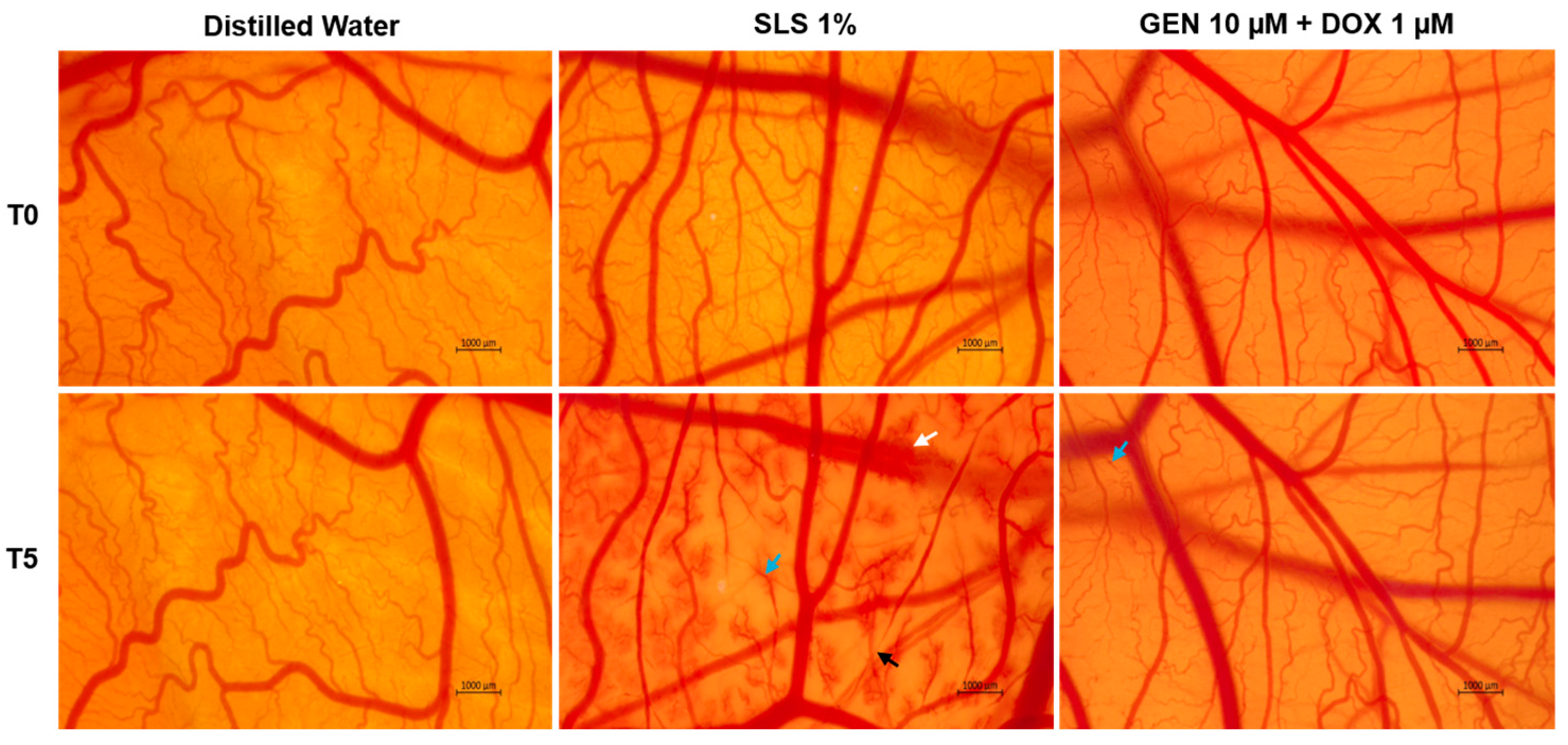
| Cell Line | GEN 10 µM + DOX (µM) | Combination Index (CI) | Type of Interaction |
|---|---|---|---|
| SK-MEL-28 | 0.5 | 0.192 | Strong synergism |
| 1 | 0.170 | Strong synergism | |
| 2.5 | 0.353 | Synergism | |
| HaCaT | 0.5 | 3.635 | Strong antagonism |
| 1 | 4.191 | Strong antagonism | |
| 2.5 | 2.497 | Antagonism |
| Sample | Irritation Score |
|---|---|
| Distilled water | 0.07 |
| SLS 1% | 19.35 |
| GEN 10 µM + DOX 1 µM | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, A.; Motoc, A.; Marcovici, I.; Dehelean, C.; Nicolescu, L.; Boru, C. Genistein Improves the Cytotoxic, Apoptotic, and Oxidative-Stress-Inducing Properties of Doxorubicin in SK-MEL-28 Cancer Cells. Medicina 2025, 61, 798. https://doi.org/10.3390/medicina61050798
Roman A, Motoc A, Marcovici I, Dehelean C, Nicolescu L, Boru C. Genistein Improves the Cytotoxic, Apoptotic, and Oxidative-Stress-Inducing Properties of Doxorubicin in SK-MEL-28 Cancer Cells. Medicina. 2025; 61(5):798. https://doi.org/10.3390/medicina61050798
Chicago/Turabian StyleRoman, Andrea, Andrei Motoc, Iasmina Marcovici, Cristina Dehelean, Laura Nicolescu, and Casiana Boru. 2025. "Genistein Improves the Cytotoxic, Apoptotic, and Oxidative-Stress-Inducing Properties of Doxorubicin in SK-MEL-28 Cancer Cells" Medicina 61, no. 5: 798. https://doi.org/10.3390/medicina61050798
APA StyleRoman, A., Motoc, A., Marcovici, I., Dehelean, C., Nicolescu, L., & Boru, C. (2025). Genistein Improves the Cytotoxic, Apoptotic, and Oxidative-Stress-Inducing Properties of Doxorubicin in SK-MEL-28 Cancer Cells. Medicina, 61(5), 798. https://doi.org/10.3390/medicina61050798








