Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy
Abstract
:1. Introduction
Structure of the Paper
2. Materials and Methods
2.1. Study Workflow Overview
2.2. Imaging and Delineation Protocol
2.3. Treatment Planning and Dose Prescription
2.4. OAR Delineation and Dose Evaluation
2.5. Ethical Considerations
3. Results
3.1. Temporal Lobe Dosimetry
3.2. Hippocampus Dosimetry
3.3. Hippocampal Avoidance Area Dosimetry
3.4. Summary and Interpretation
3.5. Visual Data Summary
3.6. Key Findings
- IMRT and VMAT offer superior target coverage but may increase mean and minimum doses to sensitive neural structures.
- Although median D_max values can be reduced by inverse planning, they may still exceed safe limits if plans are not specifically optimized.
- In several cases, V7.3 values for the hippocampus exceeded the recommended 40% threshold, suggesting elevated risk for neurocognitive decline.
- Temporal lobe D_max values in certain VMAT plans surpassed the suggested 65–70 Gy limit, reinforcing the need for proactive dose constraint application.
4. Discussion
4.1. If We Use Dose-Volume Recommendations from Whole-Brain Radiotherapy (WBRT)
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, W.; Li, Q.; Xi, D.; Tian, Y.; Mo, J.; Pei, H. The Hippocampus Sparing Volume Modulated Arc Therapy does not Influence Plan Quality on Locally Advanced Nasopharyngeal Carcinoma Patients. Sci. Rep. 2017, 7, 3443. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Zhu, X.Z.; Lee, T.F.; Feng, P.B.; Xu, J.H.; Qian, P.D.; Zhang, Y.Q. LASSO-based NTCP model for radiation-induced temporal lobe injury developing after intensity-modulated radiotherapy of nasopharyngeal carcinoma. Sci. Rep. 2016, 6, 26378. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.C.; Chan, A.S.; Law, S.C.; Chan, J.H.; Tse, V.K. Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer 2003, 97, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Nagtegaal, S.H.J.; David, S.; van der Boog, A.T.J.; Philippens, M.E.P.; Snijders, T.J.; Leemans, A.; Verhoeff, J.J.C. Dose-dependent volume loss in subcortical deep grey matter structures after cranial radiotherapy. Radiother. Oncol. 2021, 160, 192–198. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol Phys. 2013, 85, 348–354. [Google Scholar] [CrossRef]
- Monje, M.; Dietrich, J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav. Brain Res. 2012, 227, 376–379. [Google Scholar] [CrossRef]
- Ahles, T.A.; Root, J.C.; Ryan, E.L. Cancer- and cancer treatment–associated cognitive change: An update on the state of the science. J. Clin. Oncol. 2012, 30, 3675–3686. [Google Scholar] [CrossRef]
- Wefel, J.S.; Kesler, S.R.; Noll, K.R.; Schagen, S.B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef]
- Vergeer, M.R.; Doornaert, P.A.; Rietveld, D.H.; Leemans, C.R.; Slotman, B.J.; Langendijk, J.A. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: Results of a nonrandomized prospective study using a standardized follow-up program. Int. J. Radiat. Oncol. 2009, 74, 1–8. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Li, X.A.; El Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. 2010, 76 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Gong, G.; Meng, K.; Du, S.; Yin, Y. Hippocampal sparing in whole-brain radiotherapy for brain metastases: Controversy, technology and the future. Front. Oncol. 2024, 14, 1342669. [Google Scholar] [CrossRef] [PubMed]
- Achanta, P.; Fuss, M.; Martinez, J.L. Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav. Neurosci. 2009, 123, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Morris, S.; Dobbs, J.; Roques, T. Practical Radiotherapy Planning, 4th ed.; Taylor & Francis: Abingdon, UK, 2009. [Google Scholar]
- Konrad, C.; Ukas, T.; Nebe, C. Defining the human hippocampus in cerebral magnetic resonance images—An overview of current segmentation protocols. Neuroimage 2009, 47, 1185–1195. [Google Scholar] [CrossRef]
- Gondi, V.; Tolakanahalli, R.; Mehta, M.P.; Tewatia, D.; Rowley, H.; Kuo, J.S.; Tomé, W.A. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1244–1252. [Google Scholar] [CrossRef]
- Brodin, N.P.; Kabarriti, R.; Garg, M.K.; Guha, C.; Tomé, W.A. Systematic Review of Normal Tissue Complication Models Relevant to Standard Fractionation Radiation Therapy of the Head and Neck Region Published After the QUANTEC Reports. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 391–407. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. Image Guided Radiotherapy (IGRT) and Delta (Δ) Radiomics—An Urgent Alliance for the Front Line of the War against Head and Neck Cancers. Diagnostics 2023, 13, 2045. [Google Scholar] [CrossRef]
- Kazda, T.; Jancalek, R.; Pospisil, P.; Sevela, O.; Prochazka, T.; Vrzal, M.; Burkon, P.; Slavik, M.; Hynkova, L.; Slampa, P.; et al. Why and how to spare the hippocampus during brain radiotherapy: The developing role of hippocampal avoidance in cranial radiotherapy. Radiat. Oncol. 2014, 9, 139. [Google Scholar] [CrossRef]
- Ghosh, G.; Tallari, R.; Malviya, A. Toxicity Profile of IMRT Vs. 3D-CRT in Head and Neck Cancer: A Retrospective Study. J. Clin. Diagn. Res. 2016, 10, XC01. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Holt, A.; Van Gestel, D.; Arends, M.P.; Korevaar, E.W.; Schuring, D.; Kunze-Busch, M.C.; van Vliet-Vroegindeweij, C. Multi-institutional comparison of volumetric modulated arc therapy vs. inten-sity-modulated radiation therapy for head-and-neck cancer: A planning study. Radiat. Oncol. 2013, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Chambers, M.S.; Fuller, C.D.; Rebueno, N.C.; Garcia, J.; Kies, M.S.; Garden, A.S. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Crossen, J.R.; Garwood, D.; Glatstein, E.A.; Neuwelt, E. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994, 12, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.; Welsh, L.; McQuaid, D.; Dean, J.; Gulliford, S.; Hansen, V.; Bhide, S.; Nutting, C.; Harrington, K.; Newbold, K. Brain-sparing methods for IMRT of head and neck cancer. PLoS ONE 2015, 10, e0120141. [Google Scholar] [CrossRef]
- Fowler, J.F. 21 years of biologically effective dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef]
- Khodayari, B.; Michaud, A.L.; Stanic, S.; Wooten, O.H.; Dublin, A.A.; Purdy, J.; Chen, A.M. Evaluation of hippocampus dose for patients undergoing intensity-modulated radiotherapy for nasopharyngeal carcinoma. Br. J. Radiol. 2014, 87, 20130474. [Google Scholar] [CrossRef]
- Tsai PF, Yang CC, Chuang CC Hippocampal dosimetry correlates with the change in neurocognitive function after hip-pocampal sparing during whole brain radiotherapy: A prospective study. Radiat. Oncol. 2015, 10, 253. [CrossRef]
- Santhosh, N.; Sridhar, P.; Pramod, K.P.R.; Loni, R. Hippocampal Sparing Post-Operative Radiation Therapy and Its Impact on Memory Function in Newly Diagnosed High Grade Glioma Patients. Rep. Radiother. Oncol. 2016, 3, e57084. [Google Scholar] [CrossRef]
- Huo, X.; Reyes, T.M.; Heijnen, C.J.; Kavelaars, A. Cisplatin treatment induces attention deficits and impairs synaptic integrity in the prefrontal cortex in mice. Sci. Rep. 2018, 8, 17400. [Google Scholar] [CrossRef]
- Rao, V.; Bhushan, R.; Kumari, P.; Cheruku, S.P.; Ravichandiran, V.; Kumar, N. Chemobrain: A review on mechanistic insight, targets and treatments. Adv. Cancer Res. 2022, 155, 29–76. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Shamsesfandabadi, P.; Patel, A.; Liang, Y.; Shepard, M.J.; Wegner, R. Radiation-Induced Cognitive Decline: Challenges and Solutions. Cancer Manag. Res. 2024, 16, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Simó, M.; Rodríguez-Fornells, A.; Navarro, V.; Navarro-Martín, A.; Nadal, E.; Bruna, J. Mitigating radiation-induced cognitive toxicity in brain metastases: More questions than answers. Neuro-Oncol. Adv. 2024, 6, vdae137. [Google Scholar] [CrossRef]
- Nakkazi, A.; Forster, D.; Whitfield, G.A.; Dyer, D.P.; Dickie, B.R. A systematic review of normal tissue neurovascular unit damage following brain irradiation—Factors affecting damage severity and timing of effects. Neuro-Oncol. Adv. 2024, 6, vdae098. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. New horizons in modulating the radio-sensitivity of head and neck cancer—100 years after Warburg’ effect discovery. Front. Oncol. 2022, 12, 908695. [Google Scholar] [CrossRef]
- Wang, B.; Dong, J.; Xiao, H.; Li, Y.; Jin, Y.; Cui, M.; Zhang, S.-Q.; Fan, S.-J. Metformin fights against radiation-induced early developmental toxicity. Sci. Total. Environ. 2020, 732, 139274. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.-Q.; Zhang, N.; Zhu, X.-D.; Yang, K.-Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.-H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef]
- Iancu, R.I.; Zara, A.; Mirestean, C.; Iancu, D. Radiomics in Head and Neck Cancers Radiotherapy. Promises and Challenges. Maedica 2021, 16, 482–488. [Google Scholar] [CrossRef]
- Haldbo-Classen, L.; Amidi, A.; Lukacova, S.; Wu, L.M.; von Oettingen, G.; Lassen-Ramshad, Y.; Zachariae, R.; Kallehauge, J.F.; Høyer, M. Cognitive impairment following radiation to hippocampus and other brain structures in adults with primary brain tumours. Radiother. Oncol. 2020, 148, 1–7. [Google Scholar] [CrossRef]
- Umfress, A.; Speed, H.E.; Tan, C.; Ramezani, S.; Birnbaum, S.; Brekken, R.A.; Sun, X.; Plattner, F.; Powell, C.M.; Bibb, J.A. Neuropathological Effects of Chemotherapeutic Drugs. ACS Chem. Neurosci. 2021, 12, 3038–3048. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Ismaila, N.; Chua, M.L.K.; Colevas, A.D.; Haddad, R.; Huang, S.H.; Wee, J.T.S.; Whitley, A.C.; Yi, J.-L.; Yom, S.S.; et al. Chemotherapy in Combination with Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Mirestean, C.C.; Iancu, R.I.; Iancu, D.T. Radiation-induced cognitive toxicity in era of precision oncology—From pathophysiology to strategies for limiting toxicities. Ceska Slov. Neurol. Neurochir. 2023, 86, 322–326. [Google Scholar] [CrossRef]
- Bayatfard, P.; Sari, S.Y.; Yazici, G. Chemotherapy, Radiation Therapy, and Nasopharyngeal Carcinoma. JAMA Oncol. 2024, 10, 1292. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.P.T. Simultaneous Integrated Boost (SIB) vs. Sequential Boost in Head and Neck Cancer (HNC) Radiotherapy: A Radiomics-Based Decision Proof of Concept. J. Clin. Med. 2023, 12, 2413. [Google Scholar] [CrossRef]
- Buzea, C.G.; Mirestean, C.; Butuc, I.; Zara, A.; Iancu, D.T. Radiation-induced biological changes of neural structures in the base of the skull tumours. J. Radiother. Pract. 2017, 16, 183–198. [Google Scholar] [CrossRef]
- Huang, L.-W.; Pan, J.-W.; Li, B.; Wu, W.-X.; Guo, L.; Zhou, X.-H.; Zhang, X.; Gao, M.-Y.; Xu, Z.-F. Evaluation of radiation induced brain injury in nasopharyngeal carcinoma patients based on multi-parameter quantitative MRI: A prospective longitudinal study. Radiother. Oncol. 2024, 202, 110621. [Google Scholar] [CrossRef]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. (In English) [Google Scholar] [CrossRef]
- Kossmann, M.R.P.; Ehret, F.; Roohani, S.; Winter, S.F.; Ghadjar, P.; Acker, G.; Senger, C.; Schmid, S.; Zips, D.; Kaul, D. Histopathologically confirmed radiation-induced damage of the brain—An in-depth analysis of radiation parameters and spatio-temporal occurrence. Radiat. Oncol. 2023, 18, 198. [Google Scholar] [CrossRef]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef]
- He, Y.-Q.; Wang, T.-M.; Yang, D.-W.; Xue, W.-Q.; Deng, C.-M.; Li, D.-H.; Zhang, W.-L.; Liao, Y.; Xiao, R.-W.; Luo, L.-T.; et al. A comprehensive predictive model for radiation-induced brain injury in risk stratification and personalized radiotherapy of nasopharyngeal carcinoma. Radiother. Oncol. 2023, 190, 109974. [Google Scholar] [CrossRef]
- Wen, D.-W.; Lin, L.; Mao, Y.-P.; Chen, C.-Y.; Chen, F.-P.; Wu, C.-F.; Huang, X.-D.; Li, Z.-X.; Xu, S.-S.; Kou, J.; et al. Normal tissue complication probability (NTCP) models for predicting temporal lobe injury after intensity-modulated radiotherapy in nasopharyngeal carcinoma: A large registry-based retrospective study from China. Radiother. Oncol. 2021, 157, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Papacocea, S.I.; Vrinceanu, D.; Dumitru, M.; Manole, F.; Serboiu, C.; Papacocea, M.T. Molecular Profile as an Outcome Predictor in Glioblastoma along with MRI Features and Surgical Resection: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 9714. [Google Scholar] [CrossRef]

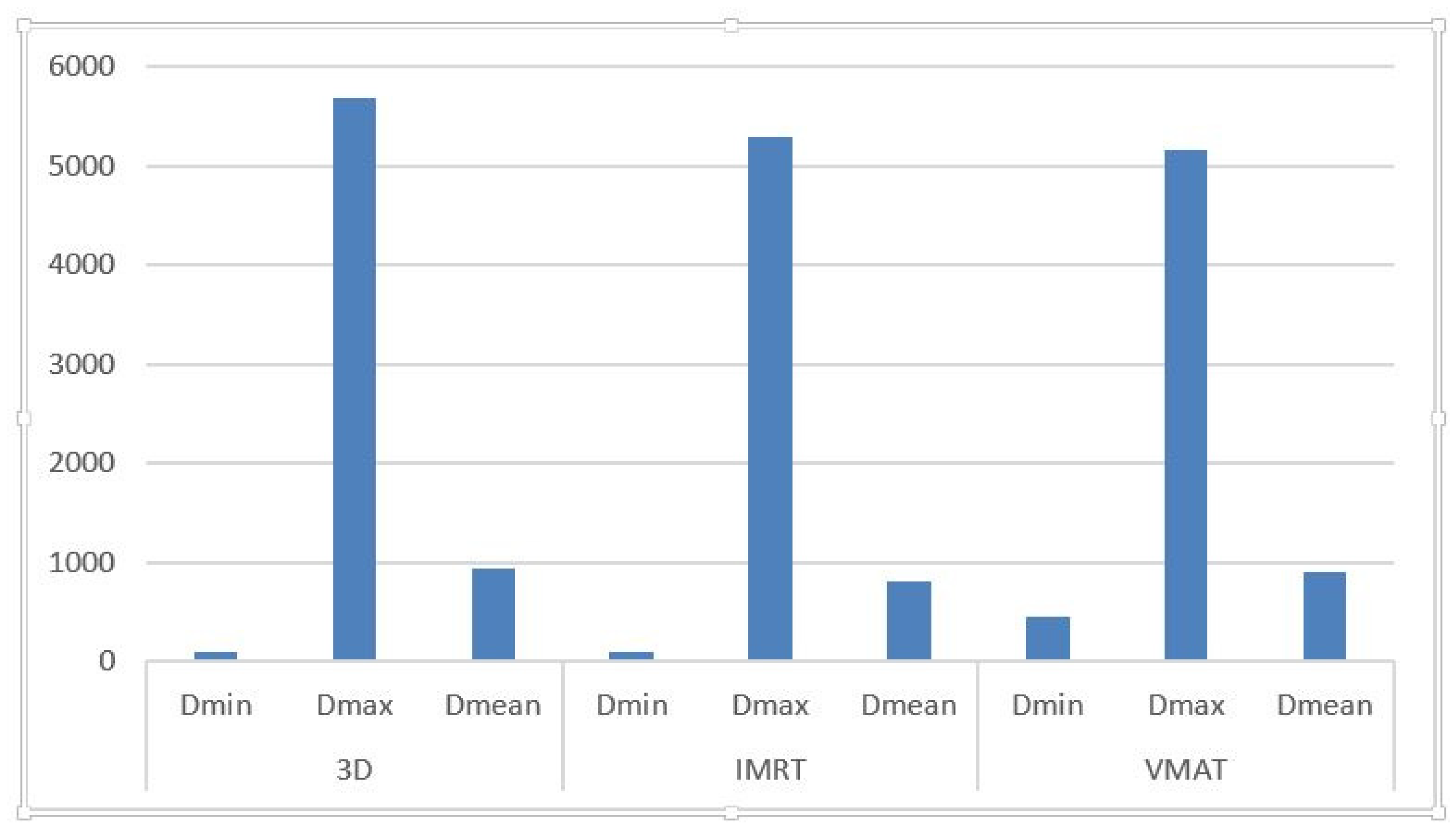
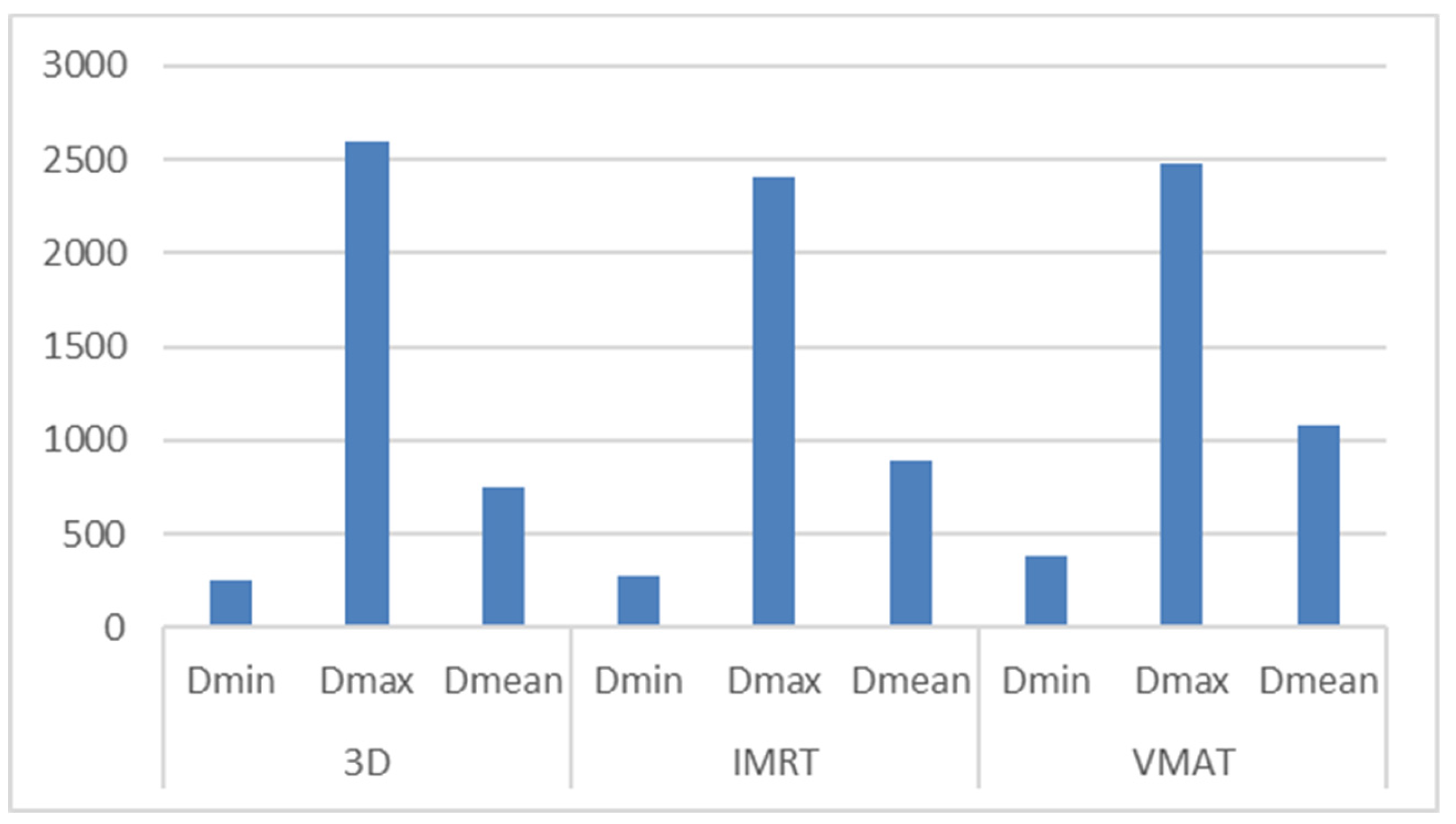
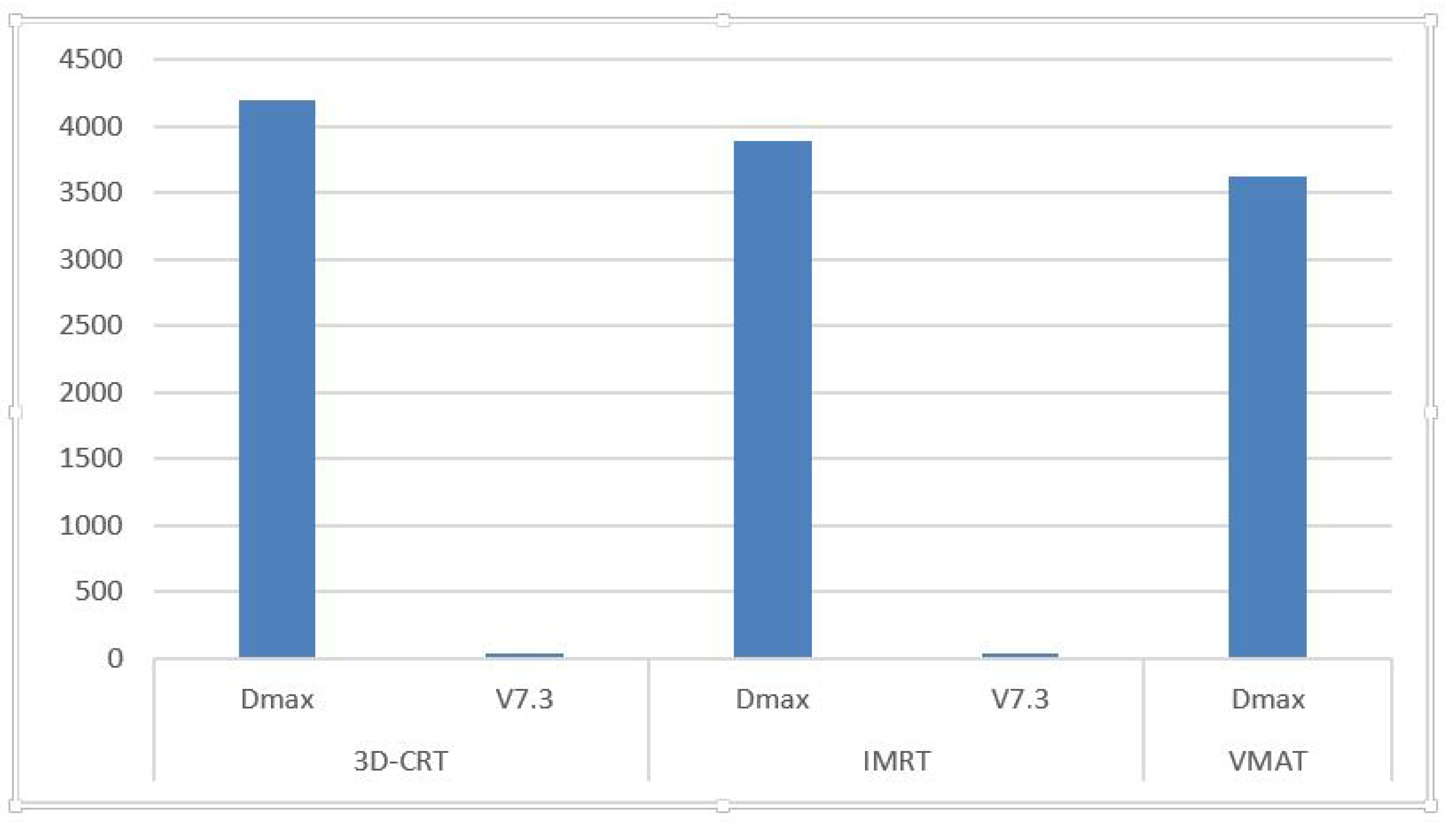
| Technique | D_Min (cGy) | D_Max (cGy) | D_Mean (cGy) |
|---|---|---|---|
| 3D-CRT | 41–146.7 (avg: 95.49) | 1532.2–6640.7 (avg: 5682.09) | 235–2271.8 (avg: 933.5) |
| IMRT | 52.6–163.3 (avg: 101.89) | 1857.6–7232.4 (avg: 5293.93) | 189.6–1928.7 (avg: 813.95) |
| VMAT | 66.9–3540 (avg: 5158.97) | 1481.4–7067.5 (avg: 5158.97) | 277.4–2154.9 (avg: 903.21) |
| Technique | D_Min (cGy) | D_Max (cGy) | D_Mean (cGy) |
|---|---|---|---|
| 3D-CRT | 113–765.8 (avg: 2900.2) | 409.4–6393.9 (avg: 2600.53) | 247.9–1935.2 (avg: 747.51) |
| IMRT | 123.5–550.5 (avg: 271.49) | 376.1–5157.5 (avg: 2409.05) | 217.4–1860.2 (avg: 891.48) |
| VMAT | 179.9–777.9 (avg: 387) | 567.6–5485.7 (avg: 2481.8) | 355.8–2505.2 (avg: 1077.06) |
| Technique | D_Max (cGy) | V7.3 (%) |
|---|---|---|
| 3D-CRT | 6.44–6638.5 (avg: 4201.17) | 0.0–90.81 (mean: 28.67) |
| IMRT | 640.5–7161.8 (avg: 3889.33) | 0.0–88.7 (mean: 37.79) |
| VMAT | 784.7–6606.8 (avg: 3619.91) | 0.0–96.2 (mean: 42.45) |
| Study/Source | Population/Setting | Key Findings | Recommendations/Implications |
|---|---|---|---|
| Current Study (3D-CRT vs. IMRT/VMAT for NPC) | Locally advanced NPC; comparative dosimetric study | D_max exceeded constraints in several plans; IMRT/VMAT reduced D_mean but increased D_min in hippocampus; V7.3 often >40% in VMAT plans | Reinforces the need to routinely delineate hippocampus and temporal lobes as OARs; advocates for individualized planning |
| Gu et al. (VMAT/NPC) [1] | Locally advanced NPC using VMAT | Proposed V7.3 < 40% constraint for hippocampus; higher hippocampal exposure linked to cognitive risk | Advocates for integrating V7.3 as a planning constraint in NPC radiotherapy |
| Gondi et al. (IMRT/WBRT) [16] | Brain metastases, WBRT hippocampal sparing | 87–81% reduction in hippocampal dose with sparing; short-term memory preservation achieved | Supports routine hippocampal sparing to maintain cognitive function |
| Khodayari et al. (IMRT/NPC) [27] | NPC treated with IMRT | In 30% of plans, hippocampus received a higher dose than tumor; lack of OAR constraint resulted in unintended exposure | Highlights need for hippocampus delineation in head and neck radiotherapy planning |
| Tsai et al. (WBRT + Sparing) [28] | WBRT with hippocampal sparing | Verbal memory impairment correlated with EQD2 values of hippocampal subvolumes; dose thresholds proposed for memory loss prevention | Supports subvolume-based dose limits for hippocampus to preserve verbal memory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mireștean, C.C.; Buzea, C.G.; Zară, A.D.; Iancu, R.I.; Iancu, D.P.T. Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy. Medicina 2025, 61, 810. https://doi.org/10.3390/medicina61050810
Mireștean CC, Buzea CG, Zară AD, Iancu RI, Iancu DPT. Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy. Medicina. 2025; 61(5):810. https://doi.org/10.3390/medicina61050810
Chicago/Turabian StyleMireștean, Camil Ciprian, Călin Gheorghe Buzea, Alexandru Dumitru Zară, Roxana Irina Iancu, and Dragoș Petru Teodor Iancu. 2025. "Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy" Medicina 61, no. 5: 810. https://doi.org/10.3390/medicina61050810
APA StyleMireștean, C. C., Buzea, C. G., Zară, A. D., Iancu, R. I., & Iancu, D. P. T. (2025). Potential Risk of Cognitive Impairment Due to Irradiation of Neural Structures in Locally Advanced Nasopharyngeal Cancer Treated by Curative Radiotherapy. Medicina, 61(5), 810. https://doi.org/10.3390/medicina61050810







