Novel Insights into Ethanol-Soluble Oyster Peptide–Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells
Abstract
1. Introduction
2. Results
2.1. Isolation of Zinc-Binding Peptides Using IMAC and Determination of Amino Acidic Profiles and Molecular Weight Distribution
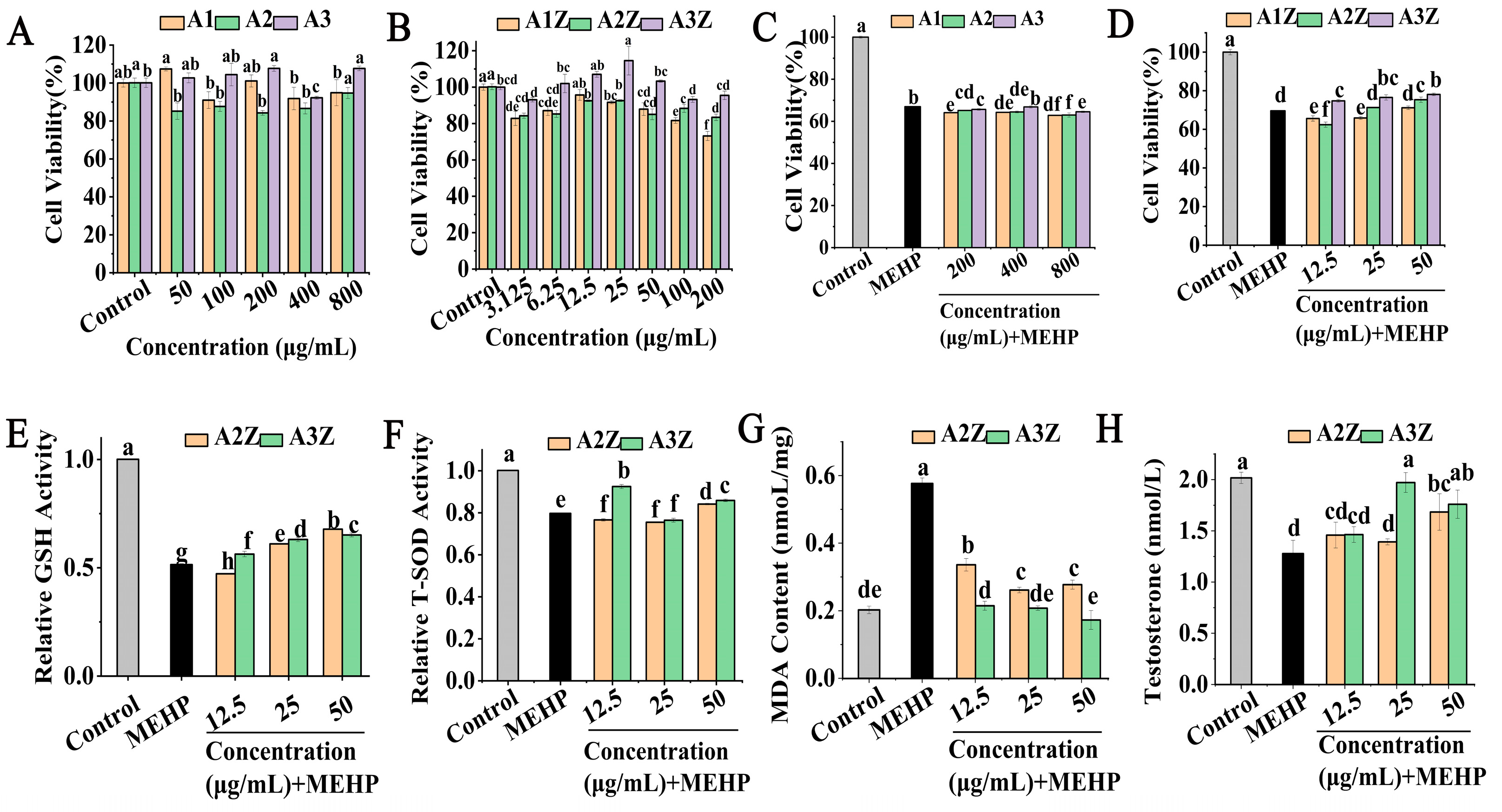
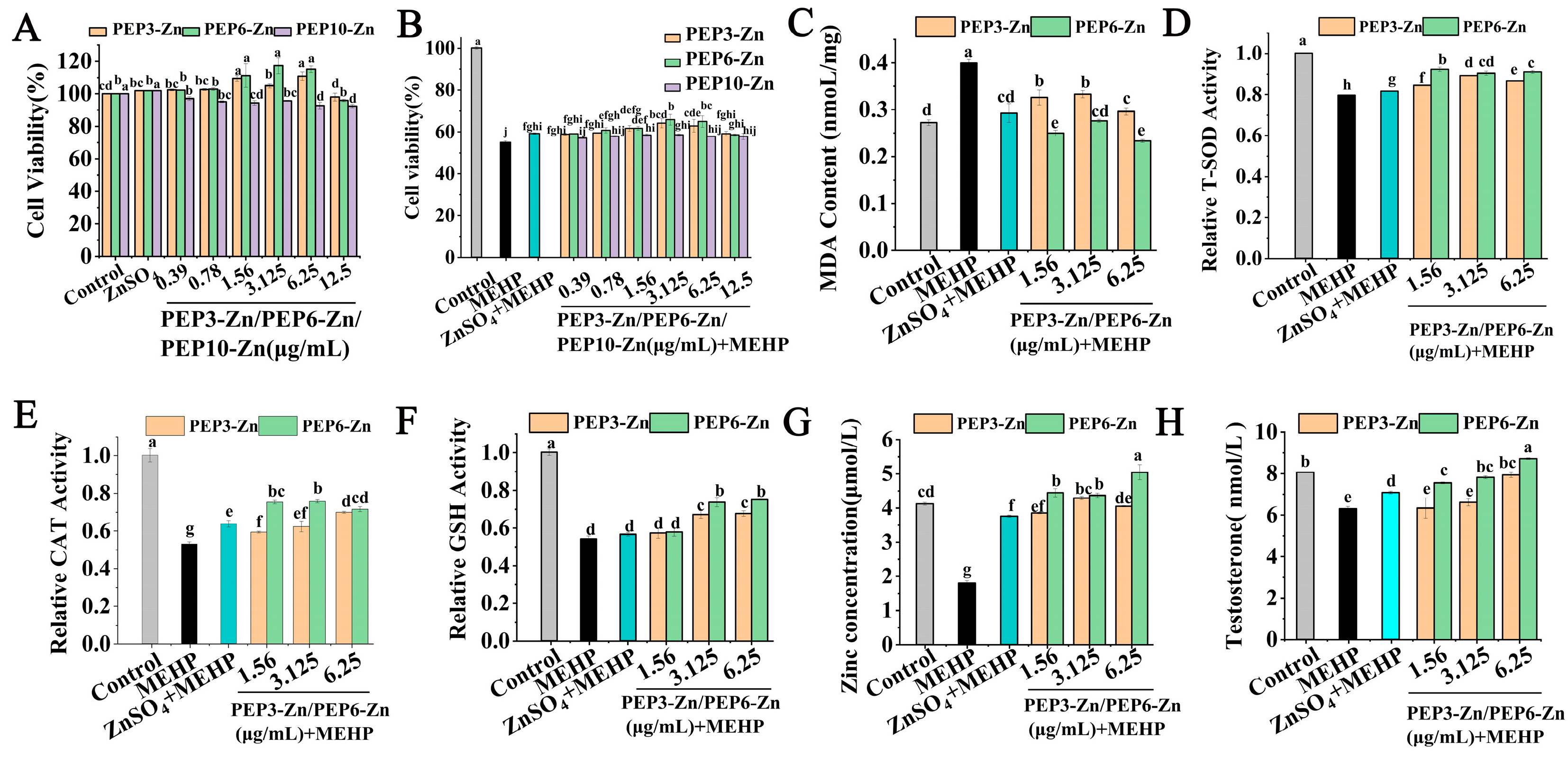
2.2. The Protective Effect of Isolated and Purified Peptides and Their Zinc Chelates in MEHP-TM3 Cells
2.3. Identification of A3 In Silico Prediction and Molecular Docking
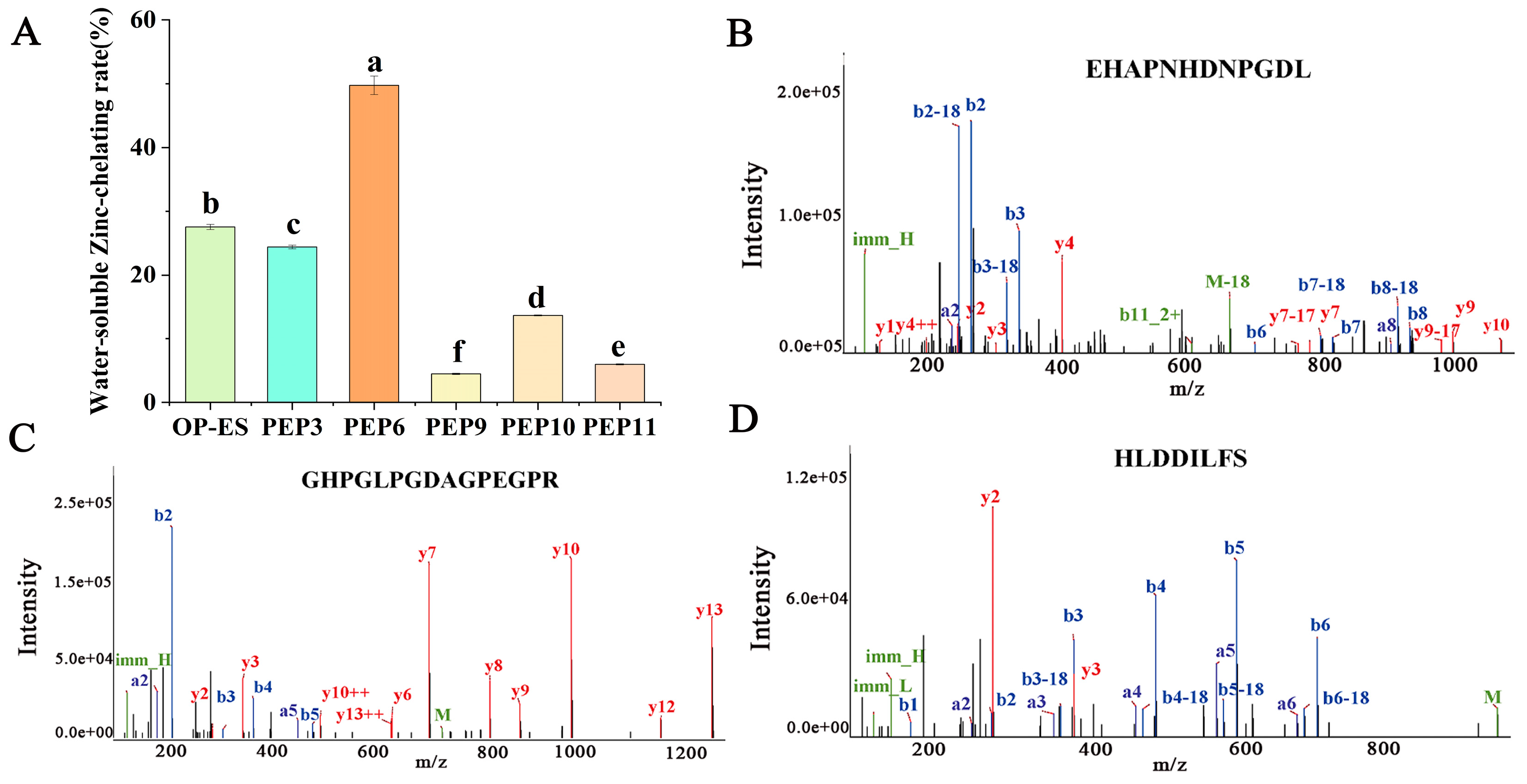
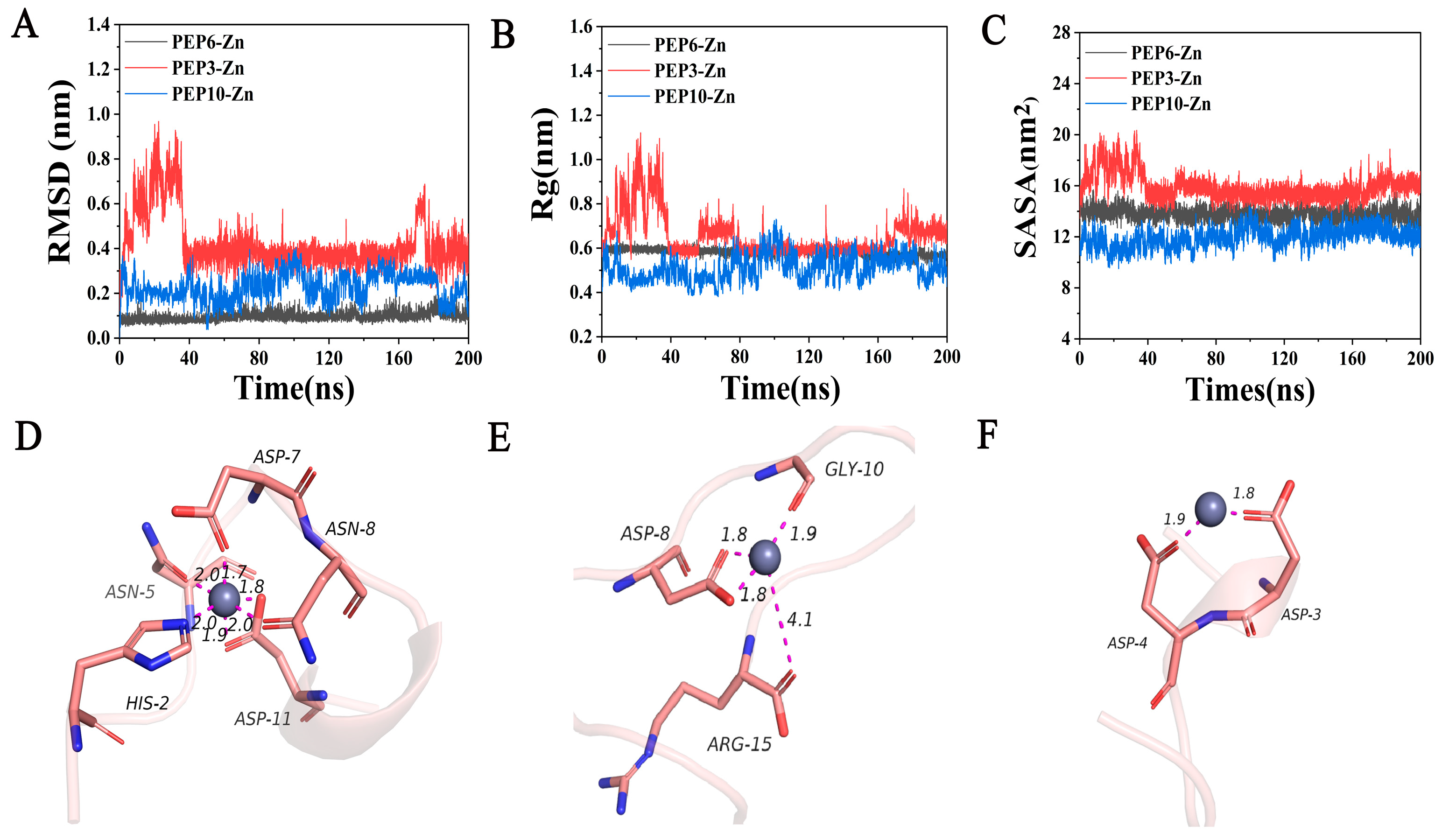
2.4. Determination of Zinc-Chelating Ability and Molecular Dynamics Simulation
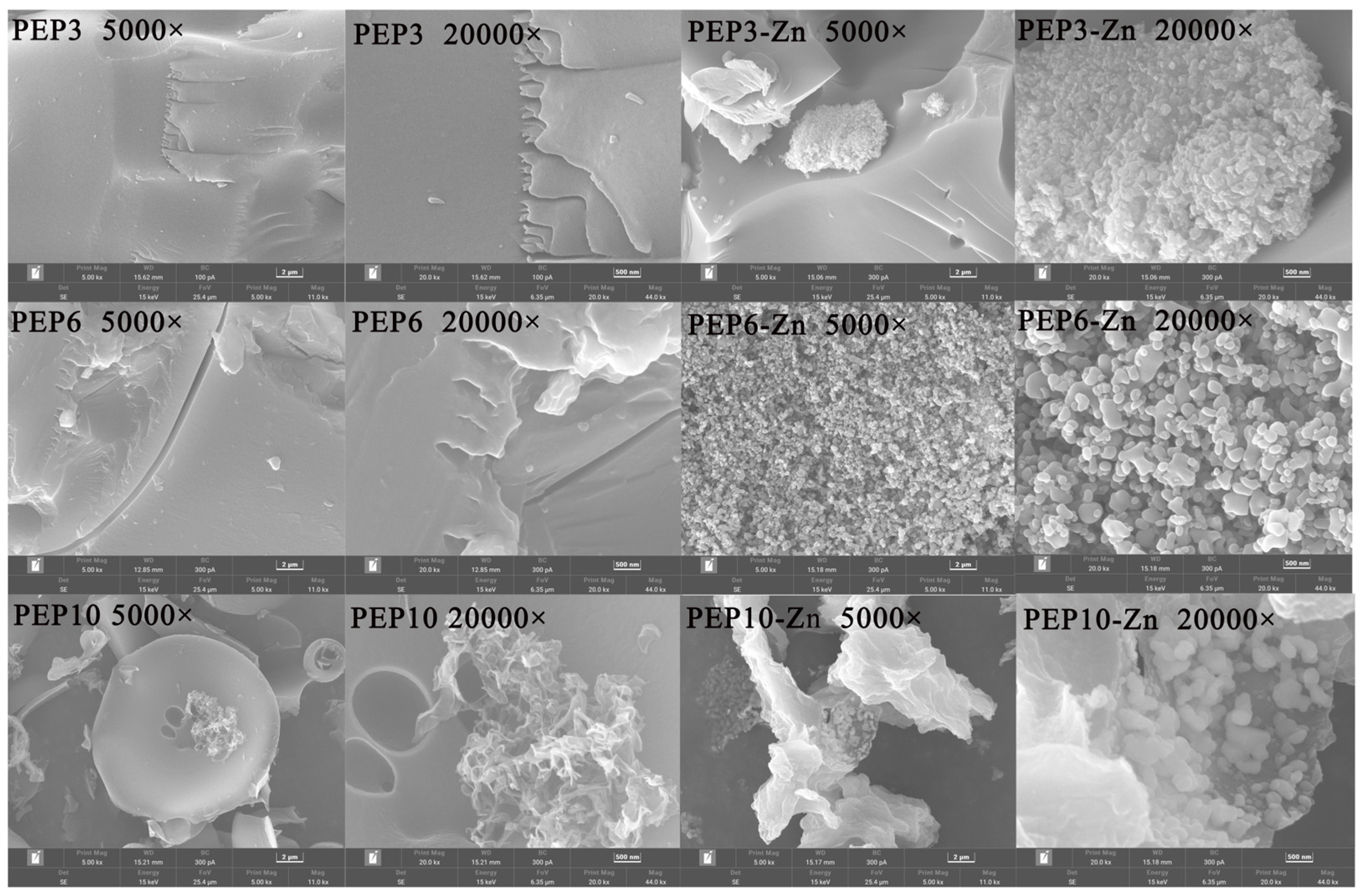
2.5. Morphological Analysis and Structural Characterization of PEP and PEP-Zn
2.6. PEP-Zn Attenuated MEHP-Induced Damage in TM3
2.7. PEP6-Zn Alleviate the MEHP-Mediated TM3 Cell Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Enrichment of Zinc-Chelating Peptides Using Immobilised Metal Affinity Chromatography
4.3. Analysis of Amino Acid Composition
4.4. MALDI-TOF/MS Analysis
4.5. Preparation of Peptide–Zinc Chelates
4.6. Effect of Isolated and Purified Peptides and Their Zinc Chelates in MEHP-TM3 Cells
4.7. Identification of Zinc-Binding Peptides via HPLC-MS/MS
4.8. In Silico Investigation of Identified Peptides and Molecular Docking
4.9. Synthesis of Peptides and Evaluation of Zinc-Chelating Ability
4.10. Molecular Dynamics Simulation
4.11. Physicochemical Characterization of Peptide and Peptide–Zinc Complex
4.12. Effect of Synthetic Peptides and Their Zinc Chelates in MEHP-TM3 Cells
4.13. Western Blotting
4.14. Transmission Electron Microscopy Analysis
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez, M.A.; Rovira, J.; Prasad Sharma, R.; Nadal, M.; Schuhmacher, M.; Kumar, V. Comparing dietary and non-dietary source contribution of BPA and DEHP to prenatal exposure: A Catalonia (Spain) case study. Environ. Res. 2018, 166, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhou, Y.; Shen, L.; Wei, Y.; Long, C.; Fu, Y.; Wu, H.; Wang, J.; Wu, Y.; Wu, S.; et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats. Ecotoxicol. Environ. Saf. 2021, 227, 112889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.; Zhang, Q. Phthalate exposure in Chinese homes and its association with household consumer products. Sci. Total Environ. 2020, 719, 136965. [Google Scholar] [CrossRef]
- Di Bella, G.; Saitta, M.; Lo Curto, S.; Salvo, F.; Licandro, G.; Dugo, G. Production process contamination of citrus essential oils by plastic materials. J. Agric. Food Chem. 2001, 49, 3705–3708. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhang, L.; Zheng, F.; Wang, F.; Leng, J.; Wang, K.; Héroux, P.; Shen, H.M.; Wu, Y.; et al. Mono-2-ethylhexyl phthalate drives progression of PINK1- parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate. Redox Biol. 2021, 38, 101776. [Google Scholar] [CrossRef]
- Beko, G.; Weschler, C.J.; Langer, S. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS ONE 2013, 8, e62442. [Google Scholar] [CrossRef]
- Fong, J.P.; Lee, F.J.; Lu, I.S.; Uang, S.N.; Lee, C.C. Estimating the contribution of inhalation exposure to di-2-ethylhexyl phthalate (DEHP) for PVC production workers, using personal air sampling and urinary metabolite monitoring. Int. J. Hyg. Environ. Health 2014, 217, 102–109. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, Y.; Tang, X.L.; Liu, B.; Shen, L.J.; Long, C.; Lin, T.; He, D.; Wu, S.; Wei, G. Testicular developmental impairment caused by flutamide-induced and DEHPinduced cryptorchid rat models is mediated by excessive apoptosis and deficient autophagy. Toxicol. Mech. Methods 2018, 28, 507–519. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Avellan-Llaguno, R.D.; Zhang, X.; Huang, Q. DEHP-elicited small extracellular vesicles miR-26a-5p promoted metastasis in nearby normal A549 cells. Environ. Pollut. 2021, 272, 116005. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Wu, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Multiple transcriptomic profiling: p53 signaling pathway is involved in DEHP-induced prepubertal testicular injury via promoting cell apoptosis and inhibiting cell proliferation of Leydig cells. J. Hazard. Mater. 2021, 406, 124316. [Google Scholar] [CrossRef]
- Park, J.D.; Habeebu, S.S.; Klaassen, C.D. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology 2020, 171, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.T.; Di, Q.N.; Qian, L.L.; Lu, L.; Li, R.X.; Cao, W.X.; Xu, Q. Zinc supplement ameliorates phthalates-induced reproductive toxicity in male rats. Chemosphere 2020, 246, 125828. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, W.E. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp. Mol. Pathol. 2003, 75, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Joshua Ashaolu, T.; Lee, C.C.; Opeolu Ashaolu, J.; Pourjafar, H.; Jafari, S.M. Metal-binding peptides and their potential to enhance the absorption and bioavailability of minerals. Food Chem. 2023, 428, 136678. [Google Scholar] [CrossRef] [PubMed]
- Syam, A.; Sari, N.P.; Thaha, A.R.; Jafar, N.; Salam, A.; Mallongi, A. The effect of pumpkin seed flour (Cucurbita moschata Durch) on zinc serum levels in malnourished Wistar rats. Enferm. Clin. 2020, 30, 337–340. [Google Scholar] [CrossRef]
- Katimba, H.A.; Wang, R.; Cheng, C. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans. Crit. Rev. Food Sci. Nutr. 2023, 63, 3959–3979. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 2019, 17, 341. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, S.; Wang, Z.; Li, S.; Zeng, M. Oyster hydrolysate-zinc complex ameliorates carrageenan-induced rat prostatitis via an anti-inflammatory mechanism and reduced oxidative stress. J. Funct. Foods 2020, 72, 104066. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, Z.; Chen, M.; Huang, A.; Xie, Y.; Hu, H.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Comparison of Neuroprotection and Regulating Properties on Gut Microbiota between Selenopeptide Val-Pro-Arg-Lys-Leu-SeMet and Its Native Peptide Val-Pro-Arg-Lys-Leu-Met In Vitro and In Vivo. J. Agric. Food Chem. 2023, 71, 12203–12215. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar]
- He, W.; Su, G.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Zhao, M.; Liu, Y. In vivo anti-hyperuricemic and xanthine oxidase inhibitory properties of tuna protein hydrolysates and its isolated fractions. Food Chem. 2019, 272, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, M.; Zhu, D.; Wu, D.; Cheng, S.; Wu, C. Immunomodulatory effect of ethanol-soluble polypeptides from atlantic cod (gadus morhua). Food Sci. Hum. Wellness 2023, 12, 1192–1203. [Google Scholar] [CrossRef]
- Kong, X.Z.; Bao, S.S.; Song, W.G.; Hua, Y.F.; Zhang, C.M.; Chen, Y.M.; Li, X.F. Contributions of ethanol fractionation on the properties of vegetable protein hydrolysates and differences in the characteristics of metal (ca, zn, fe)-chelating peptides. LWT-Food Sci. Technol. 2021, 146, 111482. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhao, Y. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res. Int. 2012, 48, 435–441. [Google Scholar] [CrossRef]
- Xu, Z.L.; Hu, Q.H.; Xie, M.H.; Liu, J.H.; Su, A.X.; Xu, H.; Yang, W.J. Protective effects of peptide KSPLY derived from Hericium erinaceus on H2O2-induced oxidative damage in HepG2 cells. Food Sci. Hum. Wellness 2023, 12, 1893–1904. [Google Scholar] [CrossRef]
- Zhang, T.D.; Ma, Y.B.; Li, H.C.; Chong, T.; Wang, Z.M.; Zhang, L.D. Low Dose of Genistein Alleviates Mono-(2-Ethylhexyl) Phthalate-Induced Fetal Testis Disorder Based on Organ Culture Model. Oxidative Med. Cell. Longev. 2020, 11, 4569268. [Google Scholar] [CrossRef]
- Tu, M.; Liu, H.; Zhang, R.; Chen, H.; Mao, F.; Cheng, S.; Lu, W.; Du, M. Analysis and Evaluation of the Inhibitory Mechanism of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide Derived from Casein Hydrolysate. J. Agric. Food Chem. 2018, 66, 4139–4144. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.; Zhang, Y.; Zhang, C.; Liu, L.; Liu, Y.; Jiang, J.; Xie, P.; Huang, L. Screening and Evaluation of Novel α-Glucosidase Inhibitory Peptides from Ginkgo biloba Seed Cake Based on Molecular Docking Combined with Molecular Dynamics Simulation. J. Agric. Food Chem. 2023, 71, 10326–10337. [Google Scholar] [CrossRef]
- Ali, S.A.; Hassan, M.I.; Islam, A.; Ahmad, F. A review of methods available to estimate solvent-accessible surface areas of soluble proteins in the folded and unfolded states. Curr. Protein Pept. Sci. 2014, 15, 456–476. [Google Scholar] [CrossRef]
- Xia, Q.; Liang, Y.; Cao, A. Preparation and characterization of pH-responsive metal-polyphenol structure coated nanoparticles. Food Sci. Hum. Wellness 2023, 13, 1303–1310. [Google Scholar] [CrossRef]
- Zhou, S.P.; Zhang, J.P.; Yin, X.Y.; Xiong, C.Y.; Zhang, N.; Gao, Z.Y.; Fan, J.F.; Zhang, W.W.; Wang, J.T. Achieving high-performance peptide-based foam via moderate hydrolysis and zinc coordination of oat proteins. Food Hydrocoll. 2024, 50, 109685. [Google Scholar] [CrossRef]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T.; et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Hao, C.; Yang, S.; Wang, J.; Chen, J. ARTS is essential for di-2-ethylhexyl phthalate (DEHP)-induced apoptosis of mouse Leydig cells. Ecotoxicol. Environ. Saf. 2024, 270, 115882. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, Q.; Chen, F.; Li, E.; Lin, H.; Qin, X. Oyster Peptide-Zinc Complex Ameliorates Di-(2-ethylhexyl) Phthalate-Induced Testis Injury in Male Mice and Improving Gut Microbiota. Foods 2023, 13, 93. [Google Scholar] [CrossRef]
- Peng, M.; Lu, D.; Yu, M.; Jiang, B.; Chen, J. Identification of zinc-chelating pumpkin seed (Cucurbita pepo L.) peptides and in vitro transport of peptide-zinc chelates. J. Food Sci. 2022, 87, 2048–2057. [Google Scholar] [CrossRef]
- Meng, K.; Chen, L.; Xia, G.; Shen, X. Effects of zinc sulfate and zinc lactate on the properties of tilapia (Oreochromis niloticus) skin collagen peptide chelate zinc. Food Chem. 2021, 347, 129043. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Ao, J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC-MS/MS. Food Chem. 2012, 134, 1231–1238. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; Wu, Y.; Han, L.; Chen, J.; Wei, Y.; Shen, L.; Long, C.; Wu, S.; Wei, G. Increased m6A modification of RNA methylation related to the inhibition of demethylase FTO contributes to MEHP-induced Leydig cell injury. Environ. Pollut. 2021, 268, 115627. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, J.; He, S. Zinc Protects against Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in TM3 Leydig Cells. Biol. Trace Elem. Res. 2022, 200, 728–739. [Google Scholar] [CrossRef]
- Li, S.T.; Hu, X.; Li, L.L.; Yang, X.Q.; Chen, S.J.; Wu, Y.Y.; Yang, S.L. Preparation, purification and identification of iron-chelating peptides derived from tilapia (Oreochromis niloticus) skin collagen and characterization of the peptide-iron complexes. LWT–Food Sci. Technol. 2021, 149, 111796. [Google Scholar]
- Pei, P.; Chen, L.; Fan, R.; Zhou, X.R.; Feng, S.; Liu, H.; Guo, Q.; Yin, H.; Zhang, Q.; Sun, F.; et al. Computer-Aided Design of Lasso-like Self-Assembling Anticancer Peptides with Multiple Functions for Targeted Self-Delivery and Cancer Treatments. ACS Nano 2022, 16, 13783–13799. [Google Scholar] [CrossRef]
- Penna, M.J.; Mijajlovic, M.; Biggs, M.J. Molecular-level understanding of protein adsorption at the interface between water and a strongly interacting uncharged solid surface. J. Am. Chem. Soc. 2014, 136, 5323–5331. [Google Scholar] [CrossRef]
- Bellucci, L.; Bussi, G.; Di Felice, R.; Corni, S. Fibrillation-prone conformations of the amyloid-beta-42 peptide at the gold/water interface. Nanoscale 2017, 9, 2279–2290. [Google Scholar] [CrossRef]
- Deguchi, S.; Yokoyama, R.; Maki, T.; Tomita, K.; Osugi, R.; Hakamada, M.; Mabuchi, M. A new mechanism for reduced cell adhesion: Adsorption dynamics of collagen on a nanoporous gold surface. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111461. [Google Scholar] [CrossRef]
- Yan, X.; Yue, Y.; Guo, B.; Zhang, S.; Ji, C.; Chen, Y.; Dai, Y.; Dong, L.; Zhu, B.; Lin, X. Novel microbial fermentation for the preparation of iron-chelating scallop skirts peptides-its profile, identification, and possible binding mode. Food Chem. 2024, 451, 139493. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, S.; Sheng, Y.; Feng, Y.; Wang, C. Isolation and characterization of zinc-binding peptides from mung bean protein hydrolysates. Eur. Food Res. Technol. 2020, 246, 113–124. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Wang, Y.; Yang, M.; Guo, M. Zinc Deficiency Promotes Testicular Cell Apoptosis in Mice. Biol. Trace Elem. Res. 2020, 195, 142–149. [Google Scholar] [CrossRef]
- Santos, H.O.; Teixeira, F.J. Use of medicinal doses of zinc as a safe and efficient coadjutant in the treatment of male hypogonadism. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 669–678. [Google Scholar] [CrossRef]
- Pourhassanali, N.; Roshan-Milani, S.; Kheradmand, F.; Motazakker, M.; Bagheri, M.; Saboory, E. Zinc attenuates ethanol-induced Sertoli cell toxicity and apoptosis through caspase-3 mediated pathways. Reprod. Toxicol. 2016, 61, 97–103. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Li, Y.; Sun, G.; Mo, H. Protective effects of tree peony seed protein hydrolysate on Cd-induced oxidative damage, inflammation and apoptosis in zebrafish embryos. Fish Shellfish Immunol. 2022, 126, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, F.; Roshan-Milani, S.; Fard, A.A.; Ahmadi, K.; Saboory, E.; Pourjabali, M.; Chodari, L.; Amini, M. The protective effect of zinc on morphine-induced testicular toxicity via p53 and Akt pathways: An in vitro and in vivo approach. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2021, 67, 126776. [Google Scholar] [CrossRef]
- Yirong, C.; Shengchen, W.; Jiaxin, S.; Shuting, W.; Ziwei, Z. DEHP induces neutrophil extracellular traps formation and apoptosis in carp isolated from carp blood via promotion of ROS burst and autophagy. Environ. Pollut. 2020, 262, 114295. [Google Scholar] [CrossRef]
- Wang, X.; Simpson, E.R.; Brown, K.A. p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Res. 2015, 75, 5001–5007. [Google Scholar] [CrossRef]
- Yu, G.; Luo, H.; Zhang, N.; Wang, Y.; Li, Y.; Huang, H.; Liu, Y.; Hu, Y.; Liu, H.; Zhang, J.; et al. Loss of p53 Sensitizes Cells to Palmitic Acid-Induced Apoptosis by Reactive Oxygen Species Accumulation. Int. J. Mol. Sci. 2019, 20, 6268. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef]
- Matsumoto, I.; Mizuno, Y.; Seno, N. Activation of Sepharose with epichlorohydrin and subsequent immobilization of ligand for affinity adsorbent. J. Biochem. 1979, 85, 1091–1098. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Simabuco, F.M.; Bezerra, R.M.N.; da Silva, D.C.; Barbosa, E.A.; Moreira, D.C.; Brand, G.D.; Leite, J.R.S.A.; Pacheco, M.T.B. Isolation and Sequencing of Cu-, Fe-, and Zn-Binding Whey Peptides for Potential Neuroprotective Applications as Multitargeted Compounds. J. Agric. Food Chem. 2020, 68, 12433–12443. [Google Scholar] [CrossRef]
- Wang, Q.L.; Xiong, Y.L. Zinc-binding behavior of hemp protein hydrolysates: Soluble versus insoluble zinc-peptide complexes. J. Funct. Foods 2018, 49, 105–112. [Google Scholar] [CrossRef]
- Mather, J.P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 1980, 23, 243–252. [Google Scholar]
- Du, R.; Li, W.; Li, J.W.; Zeng, S.; Chen, Z.Q.; Gao, J.L.; Zheng, H.N.; Lin, H.S.; Cao, W.-H. Dynamic changes of zinc chemical speciation and zinc-containing peptides release in oysters (Crassostrea hongkongensis) during enzymatic hydrolysis. Food Biosci. 2024, 58, 103649. [Google Scholar] [CrossRef]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.W.; Renfrew, P.D.; Smith, C.A.; Sheffler, W. ROSETTA3: An object-oriented software suite for the simulation and design of macromolecules. Methods Enzym. 2011, 487, 545–574. [Google Scholar]
- Kang, N.; Heo, S.Y.; Kim, E.A.; Cha, S.H.; Ryu, B.; Heo, S.J. Antiviral effect of fucoxanthin obtained from Sargassum siliquastrum (Fucales, Phaeophyceae) against severe acute respiratory syndrome coronavirus 2. Algae 2023, 38, 295–306. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.A.; Heo, S.Y.; Heo, S.J. Structure-based in silico screening of marine phlorotannins for potential walrus calicivirus inhibitor. Int. J. Mol. Sci. 2023, 24, 15774. [Google Scholar] [CrossRef]
- Li, C.; Bu, G.; Chen, F. Preparation and structural characterization of peanut peptide-zinc chelate. CyTA-J. Food 2020, 18, 409–416. [Google Scholar] [CrossRef]
- Sun, X.; Saleh, A.S.M.; Wang, Z.; Yu, Y.; Li, W.; Zhang, D. Insights into the interactions between etheric compounds and myofibrillar proteins using multi-spectroscopy, molecular docking, and molecular dynamics simulation. Food Res. Int. 2024, 175, 113787. [Google Scholar] [CrossRef]
- Shen, L.; Tang, X.; Wei, Y.; Long, C.; Tan, B.; Wu, S.; Sun, M.; Zhou, Y.; Cao, X.; Wei, G. Vitamin E and vitamin C attenuate di-(2-ethylhexyl) phthalate-induced bloodtestis barrier disruption by p38 MAPK in immature SD rats. Reprod. Toxicol. 2018, 81, 17–27. [Google Scholar] [CrossRef]




| No | Peptide Sequence (a) | Molecular Mass (Da) | Instability Index | Toxicity | Estimated Solubility |
|---|---|---|---|---|---|
| PEP1 | SETGAGKHVPR | 1138.59 | 14.1 | Non-Toxin | good |
| PEP2 | KQVHPDTGVSSKAM | 1500.74 | 10.33 | Non-Toxin | good |
| PEP3 | GHPGLPGDAGPEGPR | 1413.68 | 26.81 | Non-Toxin | good |
| PEP4 | KQVHPDTGVSSK | 1282.67 | 10.38 | Non-Toxin | good |
| PEP5 | YHPTKPGDYT | 1178.55 | 2.43 | Non-Toxin | good |
| PEP6 | EHAPNHDNPGDL | 1315.56 | 16.53 | Non-Toxin | good |
| PEP7 | SRLPGQCEMKH | 1358.63 | 37.25 | Non-Toxin | good |
| PEP8 | AIGDHDGHVGL | 1090.52 | 1.37 | Non-Toxin | good |
| PEP9 | DVHPEHPY | 993.44 | 13.85 | Non-Toxin | good |
| PEP10 | HLDDILFS | 959.49 | 32.83 | Non-Toxin | good |
| PEP11 | YHDHDVPCA | 1113.44 | 35.62 | Non-Toxin | good |
| PEP12 | DYTKHPSKPD | 1187.57 | 1.81 | Non-Toxin | good |
| No | Peptide Sequence | Chelating Sites | Docking Energy (kj/mol) | Types | Distance (Å) |
|---|---|---|---|---|---|
| PEP1 | SETGAGKHVPR | Glu-2 | 18.4682 | C-C | 2.2 |
| PEP2 | KQVHPDTGVSSKAM | His-4 | 25.3241 | M-A; P-C | 3.6; 1.9 |
| Asp-6 | M-A; C-C | 2.3; 2.4 | |||
| PEP3 | GHPGLPGDAGPEGPR | Asp-8 | 29.7068 | M-A | 3.0 |
| His-2 | C-C | 3.9 | |||
| Gly-13 | M-A | 2.9 | |||
| PEP4 | KQVHPDTGVSSK | His-4 | 21.8462 | P-C | 2.1 |
| Asp-6 | C-C | 2.1 | |||
| PEP5 | YHPTKPGDYT | Tyr-1 | 13.7164 | M-A | 1.1 |
| Asp-8 | C-C | 1.3 | |||
| PEP6 | EHAPNHDNPGDL | Asn-5 | 32.2966 | M-A; | 2.2 |
| Asp-7 | M-A; C-C | 2.2; 2.7 | |||
| Asn-8 | M-A | 2.2 | |||
| Asp-11 | C-C | 1.9 | |||
| PEP7 | SRLPGQCEMKH | Gln-6 | 16.8824 | M-A | 2.6 |
| His-11 | C-C | 1.9 | |||
| PEP8 | AIGDHDGHVGL | Asp-4 | 19.9915 | C-C | 1.9 |
| Asp-6 | M-A; C-C | 2.0; 2.8 | |||
| PEP9 | DVHPEHPY | Asp-1 | 28.5144 | C-C; M-A | 2.3; 2.1 |
| His-3 | P-C | 1.9 | |||
| His-6 | P-C | 1.9 | |||
| PEP10 | HLDDILFS | His-1 | 26.6488 | P-C | 3.2 |
| Asp-3 | M-A; C-C | 2.3; 3.2 | |||
| Asp-4 | M-A; C-C | 2.3; 2.8 | |||
| PEP11 | YHDHDVPCA | ASP-3 | 28.1060 | M-A | 1.8 |
| His-4 | P-C | 1.7 | |||
| PEP12 | DYTKHPSKPD | Asp-1 | 14.4421 | C-C | 2.9 |
| Type | PEP6-Zn | PEP3-Zn | PEP10-Zn |
|---|---|---|---|
| EVDW | 123.431 ± 14.199 | 73.546 ± 13.435 | 62.876 ± 10.164 |
| EELE | −2026.575 ± 39.846 | −1085.907 ± 33.538 | −1185.357 ± 83.788 |
| EGB | 1777.041 ± 53.483 | 909.404 ± 40.165 | 1036.67 ± 98.489 |
| ESA | −1.641 ± 0.05 | −1.657 ± 0.052 | −1.602 ± 0.105 |
| Gbinding energy | −127.744 ± 17.512 | −104.614 ± 22.714 | −87.413 ± 21.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Huang, Q.; Qin, X.; Chen, F.; Li, E.; Lin, H. Novel Insights into Ethanol-Soluble Oyster Peptide–Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells. Mar. Drugs 2024, 22, 465. https://doi.org/10.3390/md22100465
Lu Z, Huang Q, Qin X, Chen F, Li E, Lin H. Novel Insights into Ethanol-Soluble Oyster Peptide–Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells. Marine Drugs. 2024; 22(10):465. https://doi.org/10.3390/md22100465
Chicago/Turabian StyleLu, Zhen, Qianqian Huang, Xiaoming Qin, Fujia Chen, Enzhong Li, and Haisheng Lin. 2024. "Novel Insights into Ethanol-Soluble Oyster Peptide–Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells" Marine Drugs 22, no. 10: 465. https://doi.org/10.3390/md22100465
APA StyleLu, Z., Huang, Q., Qin, X., Chen, F., Li, E., & Lin, H. (2024). Novel Insights into Ethanol-Soluble Oyster Peptide–Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells. Marine Drugs, 22(10), 465. https://doi.org/10.3390/md22100465









