Abstract
Marine bacterial proteases have rarely been used to produce bioactive peptides, although many have been reported. This study aims to evaluate the potential of the marine bacterial metalloprotease A69 from recombinant Bacillus subtilis in the preparation of peanut peptides (PPs) with antioxidant activity and angiotensin-converting enzyme (ACE)-inhibitory activity. Based on the optimization of the hydrolysis parameters of protease A69, a process for PPs preparation was set up in which the peanut protein was hydrolyzed by A69 at 3000 U g−1 and 60 °C, pH 7.0 for 4 h. The prepared PPs exhibited a high content of peptides with molecular weights lower than 1000 Da (>80%) and 3000 Da (>95%) and contained 17 kinds of amino acids. Moreover, the PPs displayed elevated scavenging of hydroxyl radical and 1,1-diphenyl-2-picryl-hydrazyl radical, with IC50 values of 1.50 mg mL−1 and 1.66 mg mL−1, respectively, indicating the good antioxidant activity of the PPs. The PPs also showed remarkable ACE-inhibitory activity, with an IC50 value of 0.71 mg mL−1. By liquid chromatography mass spectrometry analysis, the sequences of 19 ACE inhibitory peptides and 15 antioxidant peptides were identified from the PPs. These results indicate that the prepared PPs have a good nutritional value, as well as good antioxidant and antihypertensive effects, and that the marine bacterial metalloprotease A69 has promising potential in relation to the preparation of bioactive peptides from peanut protein.
1. Introduction
Bioactive peptides, usually containing 2–40 amino acid residues, are peptide compounds that are beneficial to the life activities of organisms or have physiological effects [1,2]. A large number of studies have shown that some oligopeptides have immunoregulatory [3], antibacterial [4], antioxidant [5], antihypertensive [6], and other properties. Bioactive peptides, which are not active in the precursor protein, are usually released from proteins by acid hydrolysis, alkali hydrolysis, or enzymatic hydrolysis, of which enzymatic hydrolysis is the least destructive and the most commonly used. At present, enzymes commonly used for bioactive peptides preparation are pepsin, trypsin, papain, chymotrypsin, Alcalase, and others, all of which are proteases from terrestrial animals or microorganisms. In contrast, few marine proteases have been used in bioactive peptide preparation. Since a lot of marine proteases have been identified, it has important significance to study the potential of marine proteases in the preparation of bioactive peptides.
Protease A69 is a MEROPS M4 family metalloprotease derived from the marine bacterium Anoxybacillus caldiproteolyticus 1A02591 [7]. Protease A69 has been found in Escherichia coli and Bacillus subtilis, and the recombinant protease A69 has been used to prepare bovine bone collagen peptides [7] and soy protein peptides [8]. The prepared collagen peptides have moisturizing and antioxidant properties, and the prepared soybean peptides have angiotensin-converting enzyme (ACE)-inhibitory properties. These studies indicate that protease A69 has good potential in the hydrolyzation of proteins to generate bioactive peptides. Because different proteins have different amino acid sequences, and peptides with different sequences can be released from different proteins by a protease, it is thus necessary to study the potential of protease A69 in the preparation of bioactive peptides from other plant/animal proteins.
In China, peanuts are widely used in the production of cooking oil, and the byproduct of peanut oil extraction is conventionally used in animal feed [9,10]. The byproduct contains 47% to 55% protein and is, therefore, a good source for peanut protein preparation. It has been shown that peanut peptides obtained from peanut protein hydrolysis by terrestrial proteases such as Alcalase and trypsin have antioxidant [11,12], antihypertensive [13,14], and hypoglycemic properties [15], indicating that peanut protein is a good material for bioactive peptide preparation.
This study aimed to evaluate the potential of protease A69 from recombinant Bacillus subtilis in the preparation of peanut peptides (PPs) with antioxidant and ACE inhibitory properties. On the basis of optimizing the hydrolysis parameters, a process to prepare peanut peptides (PPs) from peanut protein hydrolysis with A69 was established. The characteristics of and bioactivities associated with the prepared PPs were further analyzed. The results indicate that the marine bacterial metalloprotease A69 has promising application prospects in the preparation of PPs with good nutritional value and high antioxidant and ACE-inhibitory properties.
2. Results and Discussion
2.1. Optimization of the Hydrolysis Parameters of Protease A69 on Peanut Protein
Protease A69 has been previously shown to be associated with the highest activity at 60 °C and pH 7.0 [7]. To investigate the potential of A69 in the hydrolysis of peanut protein and the preparation of PPs, we determined the optimal conditions for the hydrolysis of peanut protein by A69 to produce PPs through the optimization of three hydrolysis parameters, i.e., hydrolysis temperature, enzyme/substrate (E/S) ratio, and hydrolysis time, by single-factor experiments. According to the hydrolysate yield and the proportion of small peptides (<1000 Da) in the produced PPs, the optimal temperature for the hydrolysis of peanut protein by A69 was determined to be 60 °C (Figure 1A and Table 1). At 60 °C and a pH of 7.0, in the E/S range of 500–6000 U g−1, the hydrolysate yield and the proportion of small peptides (<1000 Da) in the produced PPs increased continuously and they virtually stabilized after 3000 U g−1 (Figure 1B and Table 2). Thus, the optimal E/S ratio was determined to be 3000 U g−1. At 60 °C and a pH of 7.0, with 3000 U g−1 A69, the hydrolysate yield and the proportion of small peptides (<1000 Da) in the produced PPs increased continuously with time, and they virtually stabilized after 4 h of hydrolysis (Figure 1C and Table 3). Thus, 4 h was regarded as the optimal hydrolysis duration.
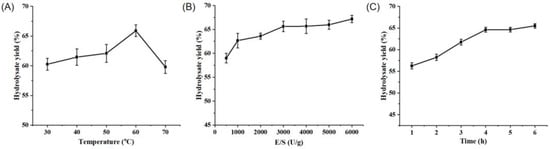
Figure 1.
Optimization of the parameters for protease A69 to hydrolyze peanut protein. (A) The effect of temperature on the hydrolysate yield. Peanut protein was hydrolyzed by A69 (3000 U g−1) for 4 h at pH 7.0 and under different temperatures. (B) The effect of the E/S ratio on the hydrolysate yield. Peanut protein was hydrolyzed at 60 °C and pH 7.0 for 4 h by A69 under different E/S ratios. (C) The effect of hydrolysis time on the hydrolysate yield. Peanut protein was hydrolyzed by A69 (3000 U g−1) at 60 °C and pH 7.0 for different time durations. The graphs show data for triplicate experiments (mean ± SD).

Table 1.
Molecular weight distribution of the PPs from peanut protein hydrolysis by A69 under different hydrolysis temperatures.

Table 2.
Molecular weight distribution of the PPs from peanut protein hydrolysis by A69 under different E/S ratio.

Table 3.
Molecular weight distribution of the PPs from peanut protein hydrolysis by A69 for different hydrolysis time durations.
Protease A69 has been used to prepare collagen peptides from bovine bone collagen and soy protein peptides from soy protein. The optimal hydrolytic parameters for recombinant A69 from E. coli to prepare collagen peptides have been found to be 60 °C, 25 U (collagenolytic activity) g−1, and 2 h [7]. The optimal hydrolytic parameters for recombinant A69 from B. subtilis to prepare soy protein peptides have been shown to be 60 °C, 4000 U (caseinolytic activity) g−1, and 3 h [8]. In this study, the optimal hydrolytic parameters for recombinant A69 from B. subtilis to prepare PPs from peanut protein were determined to be 60 °C, 3000 U (caseinolytic activity) g−1, and 4 h. Thus, some of the hydrolytic parameters for A69 to prepare bioactive peptides from different proteins are different, maybe due to the differences in protein sequences and structures.
2.2. Preparation and Characterization of PPs
Based on the optimized enzymatic hydrolysis conditions, we established a process for the preparation of PPs using protease A69 (Figure 2). In this process, the hydrolysis temperature, E/S ratio, and hydrolysis time were 60 °C, 3000 U g−1, and 4 h, respectively. Using this process, the hydrolysis efficiency of the peanut protein sample was 64.90% based on the weight of the sample before and after hydrolysis. According to the determined protein contents in the sample before and after hydrolysis, the hydrolysis efficiency of the protein in the peanut protein sample was 86.15 ± 0.42%. Compared to the light yellow, coarse peanut protein powder (Figure 3A), the prepared PPs were much finer and exhibited a milky white color (Figure 3B). While the peanut protein powder was poorly soluble in water (Figure 4A), the PPs at 10–30% (w/v) content were completely dissolved in water, resulting in clear solutions (Figure 4B–D). Peptides with a molecular weight lower than 3000 Da, 1000 Da, and 500 Da in the PPs accounted for 96.56%, 82.75%, and 54.19%, respectively (Figure 5 and Table 4), indicating that the majority of the PPs were peptides composed of less than 20 amino acid residues. As bioactive peptides usually contain 2–20 amino acid residues [3,4], this result indicates that the prepared PPs may contain a variety of bioactive peptides and may exhibit good biological activity.
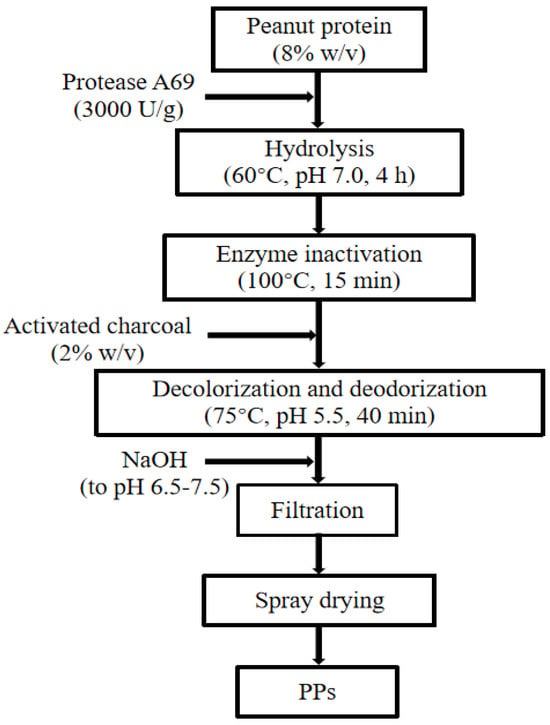
Figure 2.
A flow chart of the preparation of PPs with protease A69.
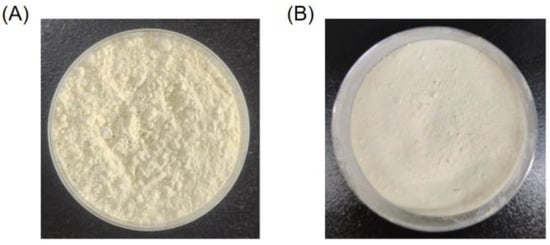
Figure 3.
Peanut protein powder and PPs powder. (A) Peanut protein powder before enzymatic hydrolysis. (B) PPs powder prepared using the process shown in Figure 2.
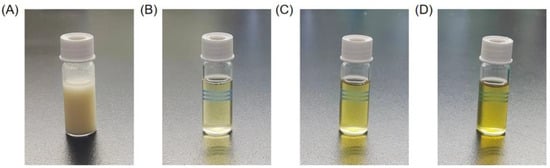
Figure 4.
Solubility of peanut protein powder and PPs powder. (A) Peanut protein solution, 10% (w/v). (B) PPs solution, 10% (w/v). (C) PPs solution, 20% (w/v). (D) PPs solution, 30% (w/v).
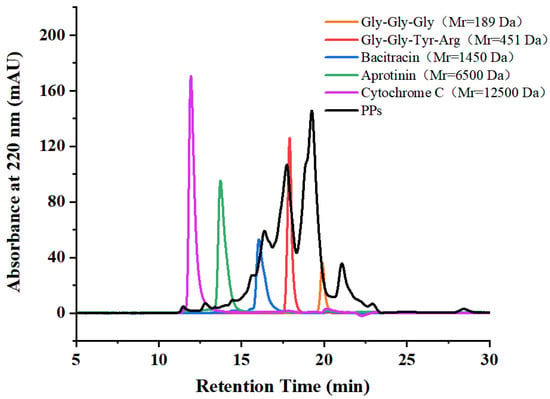
Figure 5.
Molecular weight distribution of the prepared PPs analyzed by HPLC.

Table 4.
Proportion of peptides with different molecular weights in the prepared PPs based on HPLC analysis.
2.3. Amino Acid Composition of the Prepared PPs
The composition of the prepared PPs with respect to free and total amino acids was analyzed by an automatic amino acid analyzer, and the results are shown in Table 5. Due to the use of hydrochloric acid in the hydrolysis process, Trp was destroyed and not detected. The content of free amino acids in the prepared PPs was less than 3%. Among the total amino acids in the PPs, the most abundant amino acid was Glu, which accounted for 14.71 ± 0.62% of the total. The contents of Pro, Asp, and Arg were also high, accounting for 12.37 ± 0.90%, 7.49 ± 0.69%, and 5.11 ± 0.46%, respectively. It is worth noting that there were seven essential amino acids detected in the PPs, accounting for 18.86 ± 1.37% in total. These data show that the prepared PPs have a good nutritional value.

Table 5.
The amino acid composition and content of the PPs prepared using protease A69 a.
It has been reported that aromatic amino acid residues in peptides are very important for their ACE-inhibitory properties [16]. As shown in Table 5, the proportion of aromatic amino acid residues, such as Phe and Tyr, in the PPs was relatively high, implying that the PPs may contain ACE-inhibitory peptides. In addition, it has been reported that some hydrophobic amino acid residues, as well as acidic and basic amino acids, are able to act as antioxidants by acting as proton or electron donors [17,18,19]. The PPs contained large amounts of hydrophobic amino acids such as Pro, Phe, Val, Leu, and Ala, as well as acidic amino acids (Glu and Asp) and basic amino acids (Arg, Lys and His) (Table 5), suggesting that the PPs may contain antioxidant peptides.
2.4. The Antioxidant Activity of the Prepared PPs
Antioxidants play an important role in human health because they protect the body from damage caused by free radicals [20]. Peptides from food sources are potential natural antioxidants that do not cause significant adverse effects. Peptides exhibiting good radical scavenging usually have antioxidant properties and can be widely used in cosmetics and food fields. At present, a variety of peptides with antioxidant properties have been reported, such as carp peptides [21], rice endosperm peptides [22], croceine croaker peptides [23], oyster peptides [24], and salmon peptides [25].
We analyzed the ability of the prepared PPs to scavenge the radicals DPPH•, •OH, and O2−•. Among them, the PPs demonstrated the best scavenging ability with respect to •OH. When the concentration of the PPs was equal to or greater than 10 mg mL−1, the scavenging activity of the PPs relative to •OH was nearly 100%, similar to that of ascorbic acid (Vc) and much higher than that of hyaluronic acid (HA) (Figure 6A). The PPs also demonstrated high DPPH• scavenging activity, which was close to 90% when the concentration of the PPs was 10 mg mL−1 (Figure 6B). The IC50 values of the PPs for •OH and DPPH• were 1.50 ± 0.06 and 1.66 ± 0.06 mg mL−1, respectively. In contrast, the O2−• scavenging activity by the PPs was low, attaining only 30% when the concentration of the PPs was 25 mg mL−1. These results show that the PPs prepared with A69 demonstrate radical scavenging ability, especially high activity against DPPH• and •OH, suggesting that the PPs contain antioxidant peptides.
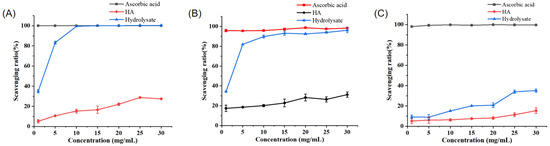
Figure 6.
Antioxidant activity of the prepared PPs. (A) •OH scavenging capacity of PPs, ascorbic acid, and HA. (B) DPPH• scavenging capacity of PPs, ascorbic acid, and HA. (C) O2−• scavenging capacity of PPs, ascorbic acid, and HA.
Several studies have shown that peanut peptides have radical scavenging properties. At the concentration of 10 mg mL−1, the scavenging activity of peanut peptides obtained from papain by Tang et al. was 65.33% with respect to DPPH• and 54.34% with respect to •OH [11]. The peanut protein hydrolysates prepared using pepsin by Ma et al. exhibited high scavenging activity with respect to DPPH• (84.72 ± 0.18%) and •OH (85.62 ± 0.70%) at 50 mg mL−1 [12]. Comparatively, the PPs prepared with the marine bacterial protease A69 demonstrated significantly higher DPPH• and •OH scavenging activity than those prepared using papain or pepsin.
It is common for a protein hydrolysate to demonstrate different degrees of scavenging relative to different radicals. In this study, the PPs demonstrated high DPPH• and •OH scavenging activity, but quite low O2−• scavenging activity. The underlying mechanism for this phenomenon warrants further study.
2.5. The ACE-Inhibitory Activity of the Prepared PPs
Angiotensin I-converting enzyme (ACE, EC 3.4.15.1) plays a role in regulating human blood pressure in the renin–angiotensin system, which catalyzes the conversion of inactive prohormone angiotensin I into active hypertension hormone angiotensin II, and plays a role in the degradation of bradykinin, a vasodilator [26,27,28]. Due to the important role of ACE in regulating blood pressure, synthetic inhibitors of this enzyme have been used to treat high blood pressure [29]. However, these inhibitors usually have some side effects. Therefore, it is important to find safer natural sources of ACE inhibitors. At present, a variety of peptides with ACE-inhibitory properties have been found, such as soybean peptides [30], spinach peptides [31], wheat gliadin peptides [32], bitter melon seed peptides [33], and lizard fish peptides [34].
In order to determine the ACE-inhibitory activity of the PPs prepared with protease A69, the ACE-inhibitory rates of PPs of different concentrations were measured (Figure 7), and the IC50 value was determined to be 0.71 ± 0.03 mg mL−1. Thus, the PPs demonstrated remarkable ACE-inhibitory activity and likely contained ACE-inhibitory peptides.
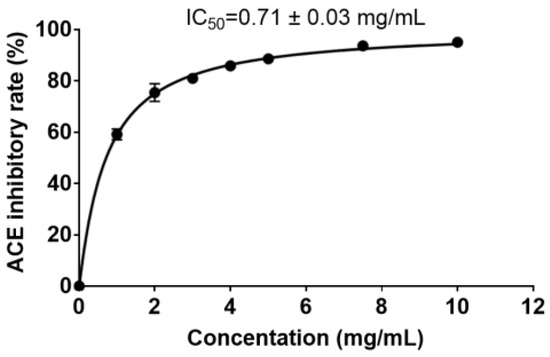
Figure 7.
The ACE-inhibitory rate of PPs of different concentrations.
Several peanut hydrolysates have been reported to have ACE-inhibitory properties. Zhang et al. used a combination of enzymes to hydrolyze peanut protein, and the hydrolysate showed high ACE-inhibitory activity, with an IC50 value of 0.548 mg mL−1 [13]. The peanut hydrolysate prepared with Alcalase by Guang et al. exhibited a high ACE-inhibitory rate, with an IC50 value of 0.134 mg mL−1 [14]. Comparatively, the ACE-inhibitory activity of the PPs prepared with the marine bacterial protease A69 was lower than those reported above.
2.6. Identification of Antioxidant Peptides and ACE-Inhibitory Peptides from the Prepared PPs
The peptide sequences in the prepared PPs were determined by LC-MS/MS, and a total of 1950 peptide sequences from peanut proteins were detected. Based on the determined peptide sequences, the residue frequencies of P1 and P1’ sites of the peptide bonds hydrolyzed by A69 were calculated (Table 6). It has been reported that enzymes in the M4 family preferentially hydrolyze peptide bonds with hydrophobic amino acid residues, and that the P1’ site is most likely to be an aromatic amino acid residue [35,36]. As shown in Table 6, the protease A69 preferentially hydrolyzed peptide bonds in peanut proteins with hydrophobic residues at the P1’ site, such as Leu, Val, Ile, Ala, and Phe, similar to other enzymes in the M4 family. It has been reported that peptides whose P1’ position is hydrophobic (Leu, Val, Ile, Gly, Ala, Phe, and Pro) [37], aromatic (Phe, Tyr, and Trp), Pro or aliphatic (Leu, Ile, Ala, and Met) [38] tend to have ACE-inhibitory properties. Cheung et al. reported that peptides with high ACE-inhibitory activity contained Pro, Phe, or Tyr at the C-terminal and Val or Ile at the N-terminal [39]. Regarding the correlation between the structure and activity of antioxidant peptides, Li et al. reported that polar/charged amino acids such as Arg or His at the P1 position contribute to the antioxidant activity [40]. It has also been reported that one or two residues of His, Pro, Met, Cys, Tyr, Trp, and Phe could enhance the activity of antioxidant peptides [41].

Table 6.
Residue frequency at the P1 and P1’ sites of the peptide bonds in peanut proteins hydrolyzed by A69.
A variety of ACE-inhibitory peptide sequences and antioxidant peptide sequences have been reported, most of which are deposited in the AHTPDB database and the AODB database, respectively. By searching the published ACE-inhibitory peptides in the AHTPDB database, 19 ACE-inhibitory peptides from different sources were identified in our prepared PPs, including four dipeptides, 14 tripeptides, and one tetrapeptides (Table 7). By searching the published antioxidant peptides in the AODB database, 15 antioxidant peptides from different sources were identified in our prepared PPs, including one dipeptides, 12 tripeptides, and two tetrapeptides (Table 8). These data indicate that marine bacterial protease A69 can induce the release of a variety of ACE-inhibitory peptides and antioxidant peptides from peanut proteins; it can be concluded, therefore, that the prepared PPs demonstrate ACE-inhibitory activity and antioxidant activity. As shown in Table 7 and Table 8, these ACE-inhibitory peptides and antioxidant peptides are derived from a variety of plant and animal sources but not peanut. In addition, the IC50 values of these ACE-inhibitory peptides show remarkable differences, and so does the antioxidant activity exhibited by the antioxidant peptides. Notably, the activities of these peptides have mainly been detected in cell-free models; therefore, more in vitro or in vivo experiments would be necessary to confirm the potential effects of these peptides.
So far, five ACE-inhibitory peptides have been identified in peanuts, including KAFR [14], IKP [42], IEY [43], KLYMRP [44], and CVTPALR [45], as well as five antioxidant peptides, including PGCPST [12], TPA [46], SP [46], I/LPS [46], and YGS [47]. However, the sequences of the antioxidant peptides and ACE-inhibitory peptides identified from the PPs in this study (Table 7 and Table 8) are all different from those reported in peanuts, indicating that the 19 ACE-inhibitory peptides (Table 7) and the 15 antioxidant peptides (Table 8) are the first to be identified in peanuts.

Table 7.
The ACE-inhibitory peptides identified in the prepared PPs.
Table 7.
The ACE-inhibitory peptides identified in the prepared PPs.
| Sequence | Molecular Weight (Da) | Source | IC50 (μmol L−1) | References |
|---|---|---|---|---|
| IG | 188.23 | Milk | <20 | [16] |
| IG | 188.23 | Cereals storage protein | - | [48] |
| IG | 188.23 | Pork sarcoplasmic proteins | - | [49] |
| GA | 146.15 | - | <20 | [16] |
| GA | 146.15 | Pork sarcoplasmic proteins | - | [49] |
| GA | 146.15 | Cereals storage protein | - | [48] |
| GA | 146.15 | - | 2000 | [39] |
| KW | 332.4 | Milk | - | [50] |
| KW | 332.4 | - | <20,000 | [16] |
| KW | 332.4 | Wakame (Undaria pinnatifida) | 2.7–43.7 | [51] |
| KW | 332.4 | Pork sarcoplasmic proteins | - | [49] |
| KW | 332.4 | Cereals storage protein | - | [48] |
| KW | 332.4 | Fish (Sardine (Sardina pilchardus muscle)) | 1.63 | [52] |
| KW | 332.4 | Wakame (Undaria pinnatifida) | 10.8 | [53] |
| KW | 332.4 | Synthesized | 7.8 | [54] |
| PG | 172.18 | Pork sarcoplasmic proteins | - | [49] |
| PG | 172.18 | Cereals storage protein | - | [48] |
| PG | 172.18 | - | 17,000 | [39] |
| PG | 172.18 | - | <20 | [16] |
| PLG | 285.34 | Fish (Alaska pollack (Theragra chalcogramma)) | 4.74 | [55] |
| PLG | 285.34 | Cereals storage protein | - | [48] |
| VLP | 327.42 | Milk | - | [50] |
| VLP | 327.42 | Pork sarcoplasmic proteins | - | [49] |
| VLP | 327.42 | Cereals (Finnish) | 0.46 | [48] |
| VLP | 327.42 | - | <20 | [16] |
| VFPS | 448.52 | Synthesized | 0.46 | [54] |
| HIR | 424.5 | Milk derived | 953 | [16] |
| PPL | 325.41 | Insect | >1000 | [56] |
| MKP | 374.5 | Milk-derived | <10 | - |
| VRW | 459.55 | Milk-derived | <10 | - |
| TVY | 381.43 | Milk (digested milk products) | 15 | [16] |
| GAW | 332.36 | Milk-derived | - | [16] |
| QQI | 387.44 | Milk–cheese (goat milk protein and cheeses) | - | [57] |
| IPQ | 356 | Milk (sodium caseinate) | - | [58] |
| YGG | 295.3 | - | <20 | [16] |
| GYK | 366.42 | Bovine beta lactoglobulin | 160 | - |
| YPR | 434.5 | Sake and sake lees | 16.5 | [59] |
| VIF | 377.48 | Fish (sea bream scale) | 7.5 | [60] |

Table 8.
The antioxidant peptides identified in the prepared PPs.
Table 8.
The antioxidant peptides identified in the prepared PPs.
| Sequence | Average Molecular Weight (Da) | Source | Antioxidant Activity | References |
|---|---|---|---|---|
| LLPH | 478.58 | Designed peptide | - | [61] |
| RHI | 424.5 | Synthesis peptide | The trolox equivalent antioxidant capability (TEAC) of the peptide was 0.189 (mMTE) | [62] |
| RWI | 473.57 | Synthesis peptide | The trolox equivalent antioxidant capability (TEAC) of the peptide was 0.702 (mMTE) | [62] |
| YVL | 393.48 | Bovine milk protein | The peptide showed the ACE-inhibitory activity (IC50 > 1000 μmol L−1) and oxygen radical absorption capacity (0.96 ± 0.04 μmol trolox equivalent per μmol of peptide) of chemically synthesized peptides | - |
| TY | 282.29 | Potato protein | - | [63] |
| YGG | 295.29 | Bovine whey protein | The peptide YGG showed very low ABTS+ free radical scavenging activity (75%) at the concentration of 100 μM | [64] |
| FVPH | 498.57 | Chickpea legumin | - | [65] |
| PEQ | 372.37 | bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 0.22 ± 0.02 mmol Fe3+ mol−1 (FARP) | [66] |
| HIR | 424.5 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 1.31 ± 0.12 mmol Fe3+ mol−1 (FARP) | [66] |
| TCG | 279.31 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 4.67 ± 0.17 mmol Fe3+ mol−1 (FARP) | [66] |
| KPT | 344.4 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 0.52 ± 0.06 mmol Fe3+ mol−1 (FARP) | [66] |
| PEG | 301.29 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 0.19 ± 0.04 mmol Fe3+ mol−1 (FARP) | [66] |
| NGE | 318.28 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 0.50 ± 0.09 mmol Fe3+ mol−1 (FARP) | [66] |
| PAV | 285.34 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 1.10 ± 0.02 mmol Fe3+ mol−1 (FARP) | [66] |
| AVF | 335.4 | Bovine whey protein (b-lactoglobulin) | The peptide showed antioxidant activity of 2.28 ± 0.06 mmol Fe3+ mol−1 (FARP) | [66] |
3. Materials and Methods
3.1. Experimental Materials
The peanut protein powder was kindly provided by Dezhou Lanli Biotechnology Limited Company (Dezhou, China). The protease A69 was produced using recombinant Bacillus subtilis, as previously reported [8]. Aprotinin, cytochrome C, salicylic acid, pyrogallol, ACE, hippuric acid, and Hip-His-Leu (HHL) were purchased from Sigma (St Louis, MO, USA). Bacitracin and H2O2 were purchased from Aladdin (Shanghai, China). Tetrapeptide GGYR and tripeptide GGG were synthesized by Qiangyao Co., Ltd. (Shanghai, China). Ascorbic acid was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). HA was purchased from Shandong Freda Bioeng Co., Ltd. (Jinan, China). DPPH• was purchased from Tokyo Chemical Industry (Tokyo, Japan). Other chemicals were of analytical grade and commercially available.
3.2. Optimization of the Hydrolytic Parameters of Protease A69 with Respect to Peanut Protein
The activity of A69 was determined using casein as the substrate by following the method previously reported [67]. One unit (1 U) was defined as the amount of enzyme that released 1 µg of tyrosine from casein per min.
The hydrolysis parameters of peanut protein produced by A69, including hydrolysis temperature, hydrolysis time, and enzyme/substrate (E/S) ratio, were optimized according to the methods previously used in bovine bone collagen hydrolysis by A69 [7] with some modifications. To determine the optimal hydrolysis temperature, 1 g of peanut protein powder in 25 mL ddH2O was hydrolyzed with A69 (3000 U g−1) at different temperatures (30 °C, 40 °C, 50 °C, 60 °C, or 70 °C) for 4 h and the solution was constantly stirred (180 rpm). To determine the optimal hydrolysis time, 1 g of peanut protein powder in 25 mL ddH2O was hydrolyzed with A69 at an E/S ratio of 3000 U g−1 at 60 °C and the solution was constantly stirred (180 rpm) for different time durations (1–6 h). To determine the optimal E/S ratio, 1 g of peanut protein powder in 25 mL ddH2O was hydrolyzed at 60 °C and the solution was constantly stirred (180 rpm) for 4 h with A69 under different E/S ratios (500 U g−1, 1000 U g−1, 2000 U g−1, 3000 U g−1, 4000 U g−1, 5000 U g−1, or 6000 U g−1). Because the optimal pH for A69 activity is 7.0 [7], all the hydrolytic reactions were performed at pH 7.0. After hydrolysis, the mixture was incubated at 100 °C for 15 min to inactivate the enzyme, and then centrifuged at 4 °C and 10,000× g for 30 min. The supernatant containing the PPs and the residual peanut protein were separately freeze-dried. The freeze-dried residual peanut protein was weighed, and the weight was used to calculate the hydrolysate yield using the formula below. The obtained PPs were dissolved in ddH2O to prepare 5 mg mL−1 of sample solution for peptide molecular weight distribution analysis by HPLC (Shimadzu, Kyoto, Japan) on a TSK gel G2000 SWXL column (7.8 × 300 mm; Tosoh, Tokyo, Japan). The column was eluted with 45% acetonitrile containing 0.1% (v/v) trifluoroacetic acid at a flow rate of 0.5 mL min−1. Peptide signals were monitored at 220 nm. The molecular weight standard samples used included cytochrome C (12,500 Da), aprotinin (6500 Da), bacitracin (1450 Da), tetrapeptide GGYR (451 Da), and tripeptide GGG (189 Da). The proportion of peptides with different molecular weights (<500 Da, <1000 Da, 1000–3000 Da, 3000–5000 Da, 5000–10,000 Da, and >10,000 Da) in the PPs was determined as the percentage of the area of the corresponding molecular weight range to the total chromatograph area. The hydrolysate yield of peanut protein was calculated using the formula:
where Wa and Wb are the dry weights of the peanut protein samples before and after being hydrolyzed, respectively.
Hydrolysate yield (%) = (Wa − Wb)/Wa × 100,
3.3. Preparation of PPs Using A69
To prepare PPs, 80 g of peanut protein in 1 L ddH2O was hydrolyzed with A69 (3000 U g−1) at 60 °C, pH 7.0, and the solution was constantly stirred (180 rpm) for 4 h. During hydrolysis, the pH of the mixture was maintained at 7.0 with 10 M NaOH solution. After hydrolysis, the reaction was terminated by incubating the mixture at 100 °C for 15 min to inactivate the protease. Then, the temperature and pH of the mixture were adjusted to 75 °C and 5.5, respectively, and 2% activated carbon (w/v) was added to the mixture. The mixture was incubated at 75 °C and constantly stirred (180 rpm) for 40 min to remove color and odor. After that, the pH of the mixture was adjusted to 6.5–7.0 using 16 M phosphoric acid, and then the mixture was centrifuged and filtered with a 0.22 µm filter membrane. The filtrate was collected and spray dried. The resulting powder constituted the PPs prepared with protease A69.
3.4. Characterization of the Prepared PPs
The PPs powder was dissolved in ddH2O to prepare 5 mg mL−1 of sample solution. The peptide molecular weight distribution of the PPs was analyzed by HPLC as described above.
To analyze the water solubility of the PPs powder, 10%, 20%, and 30% (w/v) of PPs solutions were prepared by dissolving the PPs powder in ddH2O. A total of 10% (w/v) of peanut protein solution was used as the control.
The composition of free amino acids and total amino acids in the PPs was analyzed with the method described previously [7]. Briefly, 5 mg of the prepared PPs powder was dissolved in 1 mL ddH2O containing trifluoroacetic acid (1%, v/v) and the solution was divided into two samples for the analysis of free amino acids and total amino acids. For free amino acid analysis, sulfosalicylic acid (4%, w/v) was added to the sample, and the sample was incubated at 25 °C for 30 min, followed by centrifugation (10,000× g) at 4 °C for 10 min. The supernatant was collected and used for free amino acid analysis. For total amino acid analysis, the sample was hydrolyzed at 110 °C using 6.0 M HCl for 22 h. Subsequently, the HCl was evaporated from the sample by rotation. The sample was redissolved in ddH2O and used for total amino acid analysis. An automatic amino acid analyzer, HITACHI 835 (HITACHI, Tokyo, Japan), was used to analyze the amino acid composition of the two samples.
3.5. Determination of Protein Content
The protein content of the peanut protein samples before and after A69 hydrolysis was determined using the colorimetric method according to the National Standard of the People’s Republic of China for determination of protein in foods (GB 5009.168-2016) [68]. Briefly, the sample (0.1 g) was mixed with 0.1 g of CuSO4, 1 g of K2SO4, and 5 mL (V1) of 18.4 M H2SO4 and the mixture was heated on a light wave furnace. After assuming a clear blue-green coloration, the mixture solution was further heated for 0.5 h. After cooling, the volume of the solution was fixed to 100 mL (V2) with ddH2O. The sample containing only CuSO4, K2SO4, and H2SO4 was used as the blank control. Next, 3 mL (V3) of the sample (containing two drops of 1 g L−1 p-nitrophenol indicator solution) was neutralized by 10 M NaOH until the solution became yellow. The solution was then rendered colorless with 1 M acetic acid and diluted to 5 mL (V4) with ddH2O. A total of 200 µL of the sample solution was heated with 400 µL of sodium-acetate–acetic-acid buffer solution (pH 4.8) and 400 µL of formaldehyde–acetone solution in a 100 °C water bath for 15 min. After cooling to room temperature, the absorbance of the solution was measured at 400 nm. The standard curve was made with ammonia nitrogen standard solutions of different concentrations (0 µg mL−1, 5 µg mL−1, 10 µg mL−1, 20 µg mL−1, 40 µg mL−1, 60 µg mL−1, 80 µg mL−1, 100 µg mL−1). The protein content of the sample was calculated as follows:
where X is the protein content of the sample (g 100 g−1), c is the nitrogen content of the sample solution (μg), c0 is the nitrogen content of the blank solution (μg), V1, V2, V3, and V4 as shown above, m is the sample quantity (g), and F is the conversion coefficient of nitrogen to protein which was taken as 5.46 for peanut and its products.
3.6. Assay of the Antioxidant Activity of the Prepared PPs
The antioxidant activity of the prepared PP samples was analyzed by measuring its free radical scavenging activity toward DPPH•, •OH, and O2−• according to the methods described by Cheng et al. [7]. Vc and HA were used as two positive controls. To determine the DPPH• scavenging activity, 100 µL of PPs (1 mg mL−1, 5 mg mL−1, 10 mg mL−1, 15 mg mL−1, 20 mg mL−1, 25 mg mL−1, or 30 mg mL−1) was reacted with 200 µL of 100 µM DPPH• (dissolved in 50% ethanol solution) at 25 °C in the dark for 40 min. Then, the absorbance of the reaction solution was measured at 525 nm. Under the same reaction conditions, the DPPH• solution replaced by an equal volume of 50% ethanol solution was used as the background group, and the sample replaced by an equal volume of ddH2O was used as the blank control group. To determine the •OH scavenging activity, 200 µL of PPs (1 mg mL−1, 5 mg mL−1, 10 mg mL−1, 15 mg mL−1, 20 mg mL−1, 25 mg mL−1, or 30 mg mL−1) was mixed with 200 µL of 9 mM FeSO4 (dissolved in ddH2O) and 200 µL of 9 mM salicylic acid (dissolved in anhydrous ethanol) at 37 °C. Then, 200 µL of 8.8 mM H2O2 solution was added to the mixture, and the reaction was conducted at 37 °C, in the darkness, for 30 min. The absorbance of the reaction solution was measured at 510 nm. Under the same reaction conditions, 8.8 mM H2O2 replaced by an equal volume of ddH2O was used as the background group, and the sample replaced by an equal volume of ddH2O was used as the blank control group. To determine the O2−• scavenging activity, 200 µL of PPs (1 mg mL−1, 5 mg mL−1, 10 mg mL−1, 15 mg mL−1, 20 mg mL−1, 25 mg mL−1, or 30 mg mL−1) was mixed with 900 µL of 50 mM Tris-HCl buffer (pH 8.2) and 80 µL of 25 mM pyrogallol solution (dissolved in 10 mM HCI). The reaction was conducted at 25 °C, in the darkness, for 5 min. The reaction was terminated by adding 200 µL of 8 mM HCl. The absorbance of the reaction solution was measured at 320 nm. Under the same reaction conditions, 80 µL of 10 mM HCl instead of the pyrocatechol solution was used as the background group, and an equal volume of ddH2O instead of the sample was used as the blank control group. The free radical scavenging activity (D) was calculated by using the following formula:
where Ai is the absorbance of the sample, Aj is the background absorbance of the sample, and A0 is the absorbance of the blank control.
D (%) = [1 − (Ai − Aj)/A0] × 100,
3.7. Assay of the ACE-Inhibitory Activity of the Prepared PPs
The ACE-inhibitory activity of the prepared PPs was analyzed using the method described by Wang et al. [69]. The PPs powder was dissolved in 0.1 M boric acid buffer solution containing 0.3 M NaCl (pH 8.3) to prepare PPs solutions of different concentrations (1 mg mL−1, 2 mg mL−1, 3 mg mL−1, 4 mg mL−1, 5 mg mL−1, 7.5 mg mL−1, and 10 mg mL−1). The PPs solutions (25 μL) of different concentrations were mixed with 25 μL of HHL (5 mM). The mixture was incubated at 37 °C for 5 min. After that, 25 μL of 0.1 U mL−1 ACE was added to the mixture, which was further incubated at 37 °C for 30 min. Then, 25 μL of trifluoroacetic acid solution (4%) was added to the mixture to stop the reaction. Boric acid buffer instead of the PPs solution in the mixture served as the blank control. The amount of hippuric acid released from HHL hydrolysis by ACE was determined by HPLC (Shimadzu, Kyoto, Japan) on a SunFire C18 column (4.6 × 250 mm, 5 μm; Waters, Milford, MA, USA), which was monitored at 214 nm. The column was eluted with a mobile phase of acetonitrile:H2O:trifluoroacetic acid = 40:60:0.1 at a flow rate of 1 mL min−1. The ACE-inhibitory rate (R) was calculated using the following formula:
where A and B are the peak areas of hippuric acid in blank and experimental samples, respectively. A 100% ACE activity was defined as the amount of hippuric acid released from HHL hydrolysis by ACE in the absence of inhibitors. The IC50 of a sample was defined as the amount of sample required to inhibit ACE activity by 50%.
R (%) = (1 − B)/A × 100,
3.8. LC-MS/MS Analysis of the Prepared PPs
The prepared PPs were analyzed by mass spectrometry using Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) and a sequence database search was performed in Beijing biotech-pack Scientific Co., Ltd. (Beijing, China). A total of 1 g of PPs powder was dissolved in 100 μL of 50 mM NH4HCO3. After being reduced by 1 μL of 10 mM DTT at 56 °C for 1 h and alkylated by 10 μL of 0.5 M IAM at room temperature, in the dark, for 40 min, the peptides were desalted using a self-priming desalting column, and the solvent was evaporated in a vacuum centrifuge at 45 °C. Then, the peptides were dissolved in 100 μL of 0.1% formic acid for LC-MS/MS analysis. The peptides in the sample were separated on LC with an inorganic phase A (0.1% formic acid) and an organic phase B (80% acetonitrile and 0.1% formic acid) by Reprosil-Pur 120 C18-AQ 3 μm (1.9 μm, 100 Å, Dr. Maisch GmbH, Tübingen, BW, Germany) on the Easy-nLC 1200 system (ThermoFisher Scientific, Waltham, MA, USA). The flow rate was 600 nL min−1. The LC linear gradient program used was: from 4% to 8% B for 3 min, from 8% to 28% B for 86 min, from 28% to 40% B for 20 min, from 40% to 95% B for 1 min, and from 95% to 95% B for 10 min. Then, mass spectrometry was performed using Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer. The raw MS files were analyzed and searched against protein databases based on the species in the sample using Byonic. The parameters were set as follows: the protein modifications were carbamidomethylation (C) (fixed), oxidation (M) (variable), and acetyl (N-term) (variable), the enzyme specificity was set to non-specific, the maximum missed cleavages were set to 3, the precursor ion mass tolerance was set to 20 ppm, and MS/MS tolerance was 0.02 Da. Only peptides identified with high confidence were chosen for downstream protein identification analysis.Based on the determined peptide sequences, the residue frequencies of the P1 and P1’ sites of the peptide bonds hydrolyzed by A69 were calculated. The peptide sequences with ACE-inhibitory activity deposited in the AHTPDB database (https://webs.iiitd.edu.in/raghava/ahtpdb/, accessed on 1 June 2024) were searched in the determined peptide sequences of the prepared PPs. The peptide sequences with antioxidant activity deposited in the AODB database (https://aodb.idruglab.cn/, accessed on 1 June 2024) were searched in the determined peptide sequences of the prepared PPs.
4. Conclusions
In the present study, a process to prepare PPs using the marine bacterial metalloprotease A69 was established by optimizing the hydrolysis parameters. With this process, the hydrolysis efficiency of peanut protein powder reached 86.15%, and the prepared PPs powder was fine and had good solubility. The PPs contained 17 kinds of amino acids and exhibited a high content of peptides with a molecular weight lower than 1000 Da (>80%) and 3000 Da (>95%). The PPs showed remarkable ACE-inhibitory activity, with an IC50 of 0.71 mg mL−1, and showed a great scavenging ability with respect to •OH and DPPH•, with IC50 values of 1.50 mg mL−1 and 1.66 mg mL−1, respectively. Moreover, 19 ACE-inhibitory peptides and 15 antioxidant peptides were identified in the PPs. These results indicate that the PPs prepared with A69 have a good nutritional value as well as antioxidant and antihypertensive effects, and may be used as a functional food additive. The findings in this study suggest that the marine bacterial metalloprotease A69 has potential in the preparation of bioactive peptides from peanut protein.
Author Contributions
Conceptualization, X.-L.C. and Y.-Q.Z.; investigation, W.-J.C., R.L., W.-X.Z., J.L., X.-J.Y. and H.-L.W.; methodology, W.-J.C., W.-X.Z. and Y.W.; data curation, W.-J.C. and W.-X.Z.; writing—original draft preparation, W.-J.C. and W.-X.Z.; writing—review and editing, X.-L.C. and Y.-Q.Z.; supervision, X.-L.C.; project administration, X.-L.C. and Y.-Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation of China (grant U2006205 and 32330001), the Marine S&T Fund of Shandong Province for Qingdao Marine Science and Technology Center (grant 2022QNLM030004-3), and the SKLMT Frontiers and Challenges Project (grant SKLMTFCP-2023-06).
Data Availability Statement
The original data presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Haiyan Sui from the State Key Laboratory of Microbial Technology of Shandong University for her help and guidance in the amino acid analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Gutiérrez-López, G.F.; Dávila-Ortiz, G. Use of Proteomics and Peptidomics Methods in Food Bioactive Peptide Science and Engineering. Food Eng. Rev. 2012, 4, 224–243. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Sato, H.H. Biologically active peptides: Processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res. Int. 2015, 74, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Biochemical Properties of Peptides Encrypted in Bovine Milk Proteins. Curr. Med. Chem. 2005, 12, 1905–1919. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Rizzello, C.G. Angiotensin I-converting-enzyme-inhibitory and antimicrobial bioactive peptides. Int. J. Dairy Technol. 2004, 57, 173–188. [Google Scholar] [CrossRef]
- Bamdad, F.; Chen, L. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol. Nutr. Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yang, X.; Li, Y.; Liang, Q.; Wang, Y.; Lu, F.; Owusu, J.; Huang, S.; Ren, X.; Ma, H. The milk macromolecular peptide: Preparation and evaluation of antihypertensive activity in rats. Food Funct. 2020, 11, 4403–4415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Zhang, X.-Y.; Wang, Z.; Zhang, X.; Liu, S.-C.; Song, X.-Y.; Zhang, Y.-Z.; Ding, J.-M.; Chen, X.-L.; Xu, F. Potential of Thermolysin-like Protease A69 in Preparation of Bovine Collagen Peptides with Moisture-Retention Ability and Antioxidative Activity. Mar. Drugs 2021, 19, 676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.X.; Wang, Y.; Cheng, J.H.; Bao, K.; He, J.; Chen, X.L. Production of marine bacterial metalloprotease A69 and evaluation of its potential in preparing soybean peptides with angiotensin-converting enzyme-inhibitory activity. J. Sci. Food Agric. 2023, 103, 7153–7163. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.G.; Chavan, J.K.; Kadam, S.S.; Salunkhe, D.K. Effects of dry heat treatments to peanut kernels on the functional properties of the defatted meal. Plant Foods Hum. Nutr. 1993, 43, 157–162. [Google Scholar] [CrossRef]
- Damame, S.V.; Chavan, J.K.; Kadam, S.S. Effects of roasting and storage on proteins and oil in peanut kernels. Plant Foods Hum. Nutr. 1990, 40, 143–148. [Google Scholar] [CrossRef]
- Tang, L.; Sun, J.; Zhang, H.C.; Zhang, C.S.; Yu, L.N.; Bi, J.; Zhu, F.; Liu, S.F.; Yang, Q.L. Evaluation of Physicochemical and Antioxidant Properties of Peanut Protein Hydrolysate. PLoS ONE 2012, 7, e37863. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Bai, J.; Huang, Y.; Wang, Z.; Xu, Y.; Huang, Y.; Zhong, K.; Huang, Y.; Gao, H.; Bu, Q. Purification and Identification of Novel Antioxidant Peptides from Hydrolysates of Peanuts (Arachis hypogaea) and Their Neuroprotective Activities. J. Agric. Food Chem. 2023, 71, 6039–6049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Ma, L.; Wang, Q. Bioactive Peptides from Low Denatured Peanut Dregs: Production and Antihypertensive Activity. Adv. Mater. Res. 2011, 343–344, 698–706. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D. Purification, Activity and Sequence of Angiotensin I Converting Enzyme Inhibitory Peptide from Alcalase Hydrolysate of Peanut Flour. J. Agric. Food Chem. 2009, 57, 10102–10106. [Google Scholar] [CrossRef] [PubMed]
- Al-Bukhaiti, W.Q.; Al-Dalali, S.; Li, H.; Yao, L.; Abed, S.M.; Zhao, L.; Qiu, S.-X. Identification and in vitro Characterization of Novel Antidiabetic Peptides Released Enzymatically from Peanut Protein. Plant Foods Hum. Nutr. 2023, 79, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2011, 5, 2342–2352. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Iwaniak, A.; Minkiewicz, P. Ex vivo digestion of carp muscle tissue—ACE inhibitory and antioxidant activities of the obtained hydrolysates. Food Funct. 2015, 6, 210–217. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Guo, X.; Wang, X.; Yao, H. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010, 119, 226–234. [Google Scholar] [CrossRef]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Ren, X.-J.; Deng, S.-G.; Wu, C.-W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-B.; Kim, J.-G.; Je, J.-Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; FitzGerald, R. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity and Production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Jeong, S.-C.; Kim, J.-H.; Lee, Y.-H.; Ju, Y.-C.; Lee, J.-S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Coppey, L.J.; Davidson, E.P.; Rinehart, T.W.; Gellett, J.S.; Oltman, C.L.; Lund, D.D.; Yorek, M.A. ACE Inhibitor or Angiotensin II Receptor Antagonist Attenuates Diabetic Neuropathy in Streptozotocin-Induced Diabetic Rats. Diabetes 2006, 55, 341–348. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, J. LC–MS/MS coupled with QSAR modeling in characterising of angiotensin I-converting enzyme inhibitory peptides from soybean proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef]
- Yang, Y.; Marczak, E.D.; Yokoo, M.; Usui, H.; Yoshikawa, M. Isolation and Antihypertensive Effect of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Spinach Rubisco. J. Agric. Food Chem. 2003, 51, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, B.G.; Pauly, A.; Celus, I.; Brijs, K.; Delcour, J.A. Inhibition of angiotensin I-converting enzyme by wheat gliadin hydrolysates. Food Chem. 2011, 127, 1653–1658. [Google Scholar] [CrossRef]
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.-I.; Sung, W.-C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.-C.; Wang, T.-C.; Hsu, J.-L. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J. Proteom. 2015, 128, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, Y.; Hou, X.; Lu, Y.; Meng, H.; Pei, S.; Dai, Z.; Wu, S. Separation and identification of ACE inhibitory peptides from lizard fish proteins hydrolysates by metal affinity-immobilized magnetic liposome. Protein Expr. Purif. 2022, 191, 106027. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, R.; Vizovišek, M.; Turk, D.; Turk, B.; Fonović, M. Protease cleavage site fingerprinting by label-free in-gel degradomics reveals pH-dependent specificity switch of legumain. EMBO J. 2017, 36, 2455–2465. [Google Scholar] [CrossRef]
- Thayer, M.M.; Flaherty, K.M.; McKay, D.B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J. Biol. Chem. 1991, 266, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Biochemical properties of regulatory peptides derived from mil proteins. Biopolymers 1997, 43, 119–128. [Google Scholar] [CrossRef]
- Iroyukifujita, H.; Eiichiyokoyama, K.; Yoshikawa, M. Classification and Antihypertensive Activity of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Food Proteins. J. Food Sci. 2008, 65, 564–569. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, B. Characterization of structure–antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Ye, R.; Wu, Y.; Xia, W. Comparison of analytical methods to assay inhibitors of angiotensin I-converting enzyme. Food Chem. 2013, 141, 3329–3334. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: Structure–activity relationship. Peptides 2010, 31, 1165–1176. [Google Scholar] [CrossRef]
- Motta, A.; Shi, A.; Liu, H.; Liu, L.; Hu, H.; Wang, Q.; Adhikari, B. Isolation, Purification and Molecular Mechanism of a Peanut Protein-Derived ACE-Inhibitory Peptide. PLoS ONE 2014, 9, e111188. [Google Scholar]
- Liu, L.-N.; Zhang, S.-H.; He, D.-P. Detection of an angiotensin converting enzyme inhibitory peptide from peanut protein isolate and peanut polypeptides by western blot and dot blot hybridization. Eur. Food Res. Technol. 2009, 230, 89–94. [Google Scholar] [CrossRef]
- Ji, N.; Sun, C.; Zhao, Y.; Xiong, L.; Sun, Q. Purification and identification of antioxidant peptides from peanut protein isolate hydrolysates using UHR-Q-TOF mass spectrometer. Food Chem. 2014, 161, 148–154. [Google Scholar] [CrossRef]
- Zheng, L.; Su, G.; Ren, J.; Gu, L.; You, L.; Zhao, M. Isolation and Characterization of an Oxygen Radical Absorbance Activity Peptide from Defatted Peanut Meal Hydrolysate and Its Antioxidant Properties. J. Agric. Food Chem. 2012, 60, 5431–5437. [Google Scholar] [CrossRef]
- Cavazos, A.; Gonzalez de Mejia, E. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef]
- Castellano, P.; Aristoy, M.-C.; Sentandreu, M.Á.; Vignolo, G.; Toldrá, F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J. Proteom. 2013, 89, 183–190. [Google Scholar] [CrossRef]
- Dziuba, M.; Dziuba, B.; Iwaniak, A. Milk proteins as precursors of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2009, 8, 71–90. [Google Scholar]
- Puchalska, P.; Marina Alegre, M.L.; García López, M.C. Isolation and Characterization of Peptides with Antihypertensive Activity in Foodstuffs. Crit. Rev. Food Sci. Nutr. 2014, 55, 521–551. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Matsui, T.; Seki, E.; Osajima, K.; Nakashima, M.; Osajima, Y. Angiotensin I-converting Enzyme Inhibitory Peptides in an Alkaline Protease Hydrolyzate Derived from Sardine Muscle. Biosci. Biotechnol. Biochem. 2014, 58, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Terashima, M.; Oe, M.; Ogura, K.; Matsumura, S. Inhibition Strength of Short Peptides Derived from an ACE Inhibitory Peptide. J. Agric. Food Chem. 2011, 59, 11234–11237. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.-G.; Kim, S.-K. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Alaskan Pollack Skin. BMB Rep. 2002, 35, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Van Camp, J.; Morel, N.; Rougé, P.; Herregods, G.; Smagghe, G. Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats. Peptides 2010, 31, 482–488. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.H.A.; El-Shibiny, S. Bioactive Peptides of Buffalo, Camel, Goat, Sheep, Mare, and Yak Milks and Milk Products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-Converting-Enzyme-Inhibitory and Antibacterial Peptides from Lactobacillus helveticus PR4 Proteinase-Hydrolyzed Caseins of Milk from Six Species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides from Sake and Sake Lees. Biosci. Biotechnol. Biochem. 2014, 58, 1767–1771. [Google Scholar] [CrossRef]
- He, H.-L.; Liu, D.; Ma, C.-B. Review on the Angiotensin-I-Converting Enzyme (ACE) Inhibitor Peptides from Marine Proteins. Appl. Biochem. Biotechnol. 2012, 169, 738–749. [Google Scholar] [CrossRef]
- Matoba, N.; Yamada, Y.; Yoshikawa, M. Design of a Genetically Modified Soybean Protein Preventing Hypertension Based on an Anti-Hypertensive Peptide Derived from Ovalbumin. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2003, 1, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Jin, D.-H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative Properties of Tripeptide Libraries Prepared by the Combinatorial Chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Xiong, Y.L. Chromatographic Separation and Tandem MS Identification of Active Peptides in Potato Protein Hydrolysate That Inhibit Autoxidation of Soybean Oil-in-Water Emulsions. J. Agric. Food Chem. 2010, 58, 8825–8832. [Google Scholar] [CrossRef] [PubMed]
- Sadat, L.; Cakir-Kiefer, C.; N’Negue, M.-A.; Gaillard, J.-L.; Girardet, J.-M.; Miclo, L. Isolation and identification of antioxidative peptides from bovine α-lactalbumin. Int. Dairy J. 2011, 21, 214–221. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.d.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Tian, M.; Fang, B.; Jiang, L.; Guo, H.; Cui, J.; Ren, F. Structure-activity relationship of a series of antioxidant tripeptides derived from β-Lactoglobulin using QSAR modeling. Dairy Sci. Technol. 2015, 95, 451–463. [Google Scholar] [CrossRef]
- Chen, X.-L.; Zhang, Y.-Z.; Gao, P.-J.; Luan, X.-W. Two different proteases produced by a deep-sea psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913. Mar. Biol. 2003, 143, 989–993. [Google Scholar] [CrossRef]
- GB 5009.168-2016; Determination of Protein in Foods. Standards Press of China: Beijing, China, 2016.
- Wang, J.; Hu, J.; Cui, J.; Bai, X.; Du, Y.; Miyaguchi, Y.; Lin, B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008, 111, 302–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).