Abstract
Although extensive research exists on toxic environments in the Tri-State Mining District (TSMD), there has been a lack of research on how harmful effects in TSMD could affect residents living in those areas. However, quite recently, such research regarding relationships between the health conditions of residents and toxic elements in the TSMD began to grow. The increase of empirical studies means greater complexity of the findings that require a more intricate understanding. To meet the goals of this study, an extensive, systematic review of the literature using PRISMA was conducted. This method resulted in 19 articles that define the harmful effects of the TSMD on the ecology and the physical health of residents. This research found that toxic metals not only negatively impact natural processes in the TSMD environments (fish species reduction, kidney and liver problems, and toxic diet) but also continuously affect the health of residents (high blood Pb and mortality).This study makes a vital contribution building upon the existing outcomes of the correlations between toxic elements in the TSMD areas and the health of residents. Furthermore, conclusions of this study provide updated information to policymakers and health-related professionals by providing adequate and innovative remediations and health-related services in the TSMD.
1. Introduction
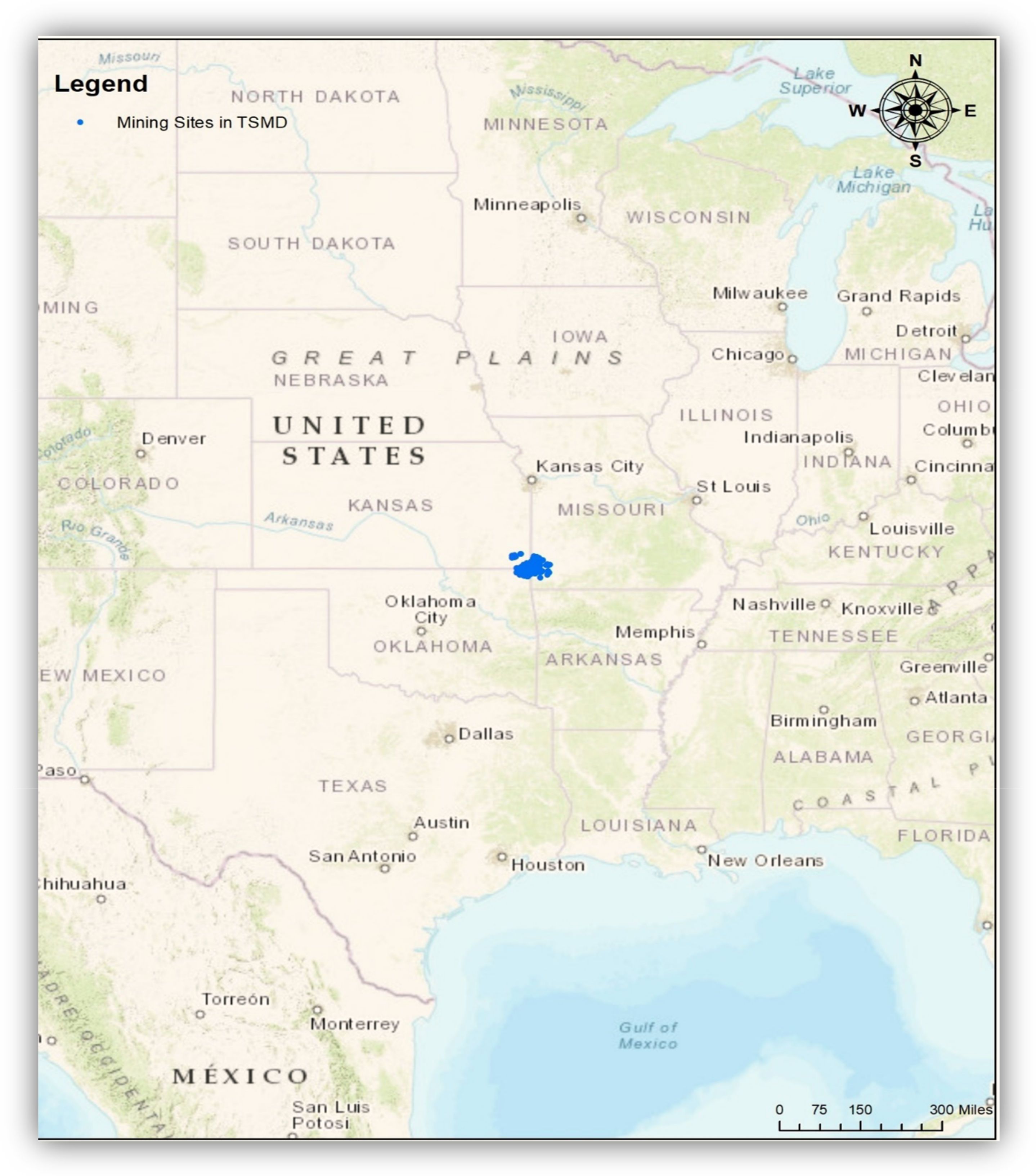
The Tri-State Mining District (hereafter TSMD) of Kansas, Missouri, and Oklahoma in the United States mined lead (Pb) and zinc (Zn) actively from 1850 to 1970 and has been shut down since 1970 [1]. (Figure 1) Even though the mining companies moved out, the TSMD groundwater, soil, and sediments have been contaminated due to the concentration of toxic metal components, such as Pb, Zn, and Cadmium (Cd), in spite of remediation efforts [1,2,3]. Studies regarding the effect of these toxic components on human health (kidney and lung cancer) and ecological systems (e.g., aquatic systems and wild birds) have been increasing for more than a decade. For instance, Beyer et al. [2] found that wild animals, such as northern cardinals and waterfowl living in the TSMD had increased Pb levels, which led to the decrease of red blood cells; Coolon and his colleagues [4] discovered that there has been a harmful impact to mammals living in the TSMD (e.g., reducing biodiversity); and Allert et al. [5] found that aquatic habitats in the TSMD with increased heavy metal levels reduced the biodiversity of the population. In addition, children living in the TSMD had elevated blood Pb levels causing neurological disorders [6], and residents in the TSMD were more likely to have a stroke, chronic kidney disease, hypertension, heart disease, skin cancer, and anemia [7,8,9]. Still many low-income households and ethnic minority people (Native Americans) living in the TSMD earn their living predominantly through agriculture (wheat, sorghum, corn, soybeans, and hay) and pastureland for livestock grazing. They look to enhance their income through hunting and fishing opportunities [10].
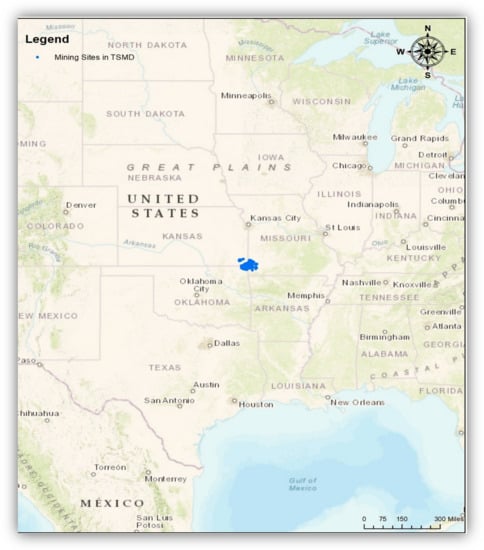
Figure 1.
Locations of the Tri-State Mining District (TSMD) in the Unites States. Note: The TSMD is located in the Tri-State area (southeast Kansas, southwest Missouri, and northeast Oklahoma). Blue indicates mine-related sites including mines, mineral deposits, and mineral regions in the TSMD. The geospatial database was obtained from the U.S. Geological Survey (USGS) (Date taken: 29 July 2020). The USGS data are publicly available from https://www.usgs.gov/.
Progress has been made by the U.S. Environmental Protection Agency (USEPA) and other stakeholders to remediate the problems of TSMD [3]. Even though the condition of Pb, Cd, and Zn in water, soil, and sediments have improved [3], living creatures, including humans, in the TSMD are still susceptible to the toxic metals. Accordingly, numerous studies have examined habitats, remediations, and toxic components in the TSMD. Unfortunately, very few studies have focused on the TSMD residents’ health conditions, even though many scholars [11,12,13] have found significant relationships between drinking water and physical conditions such as obesity. Therefore, this study addresses how the toxic components lead to degrading the physical conditions of TSMD residents by utilizing systematic review analysis.
For this purpose, first, we described the history and the characteristics of the TSMD, the conditions of habitats in the TSMD, and how toxic components would affect the TSMD ecological system and human bodies in the literature review. Secondly, for the analysis, we utilized Endnote to search and reserve articles relevant to the subject with a diverse database, as well as the Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA).
Finally, we discussed how our study findings would make a vital contribution building upon the existing outcomes of the correlations between toxic elements coming from the TSMD areas and health conditions of low-income residents by suggesting several recommendations. Additionally, this study outcome will provide updated information for policymakers and public health-related professionals who aim to promote economic and social justice by providing adequate and innovative policies and practices in TSMD rural areas.
2. Literature Review
2.1. History of the TSMD
The TSMD was initially located in Leadville near Joplin, Missouri, in 1848 and in Granby, Missouri, in 1850. This onset of mining business expanded to Galena, Kansas, in 1876; Aurora, Missouri, in 1886; Peoria, Oklahoma, in 1891; and Commerce, Oklahoma, in 1905 [3]. Mining activity in the TSMD was prosperous between 1880 and 1955. The TSMD, in particular, during this period of time had been known for its leading industry of mining Pb and Zn. The business, however, declined after 1955, and the entire mining industry was closed in the 1970s [3].
The TSMD of Kansas, Missouri, and Oklahoma had produced ore since 1850. However, by the 1970s, ore was depleted, and most mines and smelters shut down. Overall, the TSMD produced 23 million tons of Zn concentrates and four million tons of Pb concentrates [14]. Non-ore waste rock and mill wastes were piled up near production centers in masses called chat piles [15]. Emissions from smelters also accumulated to the metal content of topsoils through fugitive dust and fallout [3]. (Figure 1)
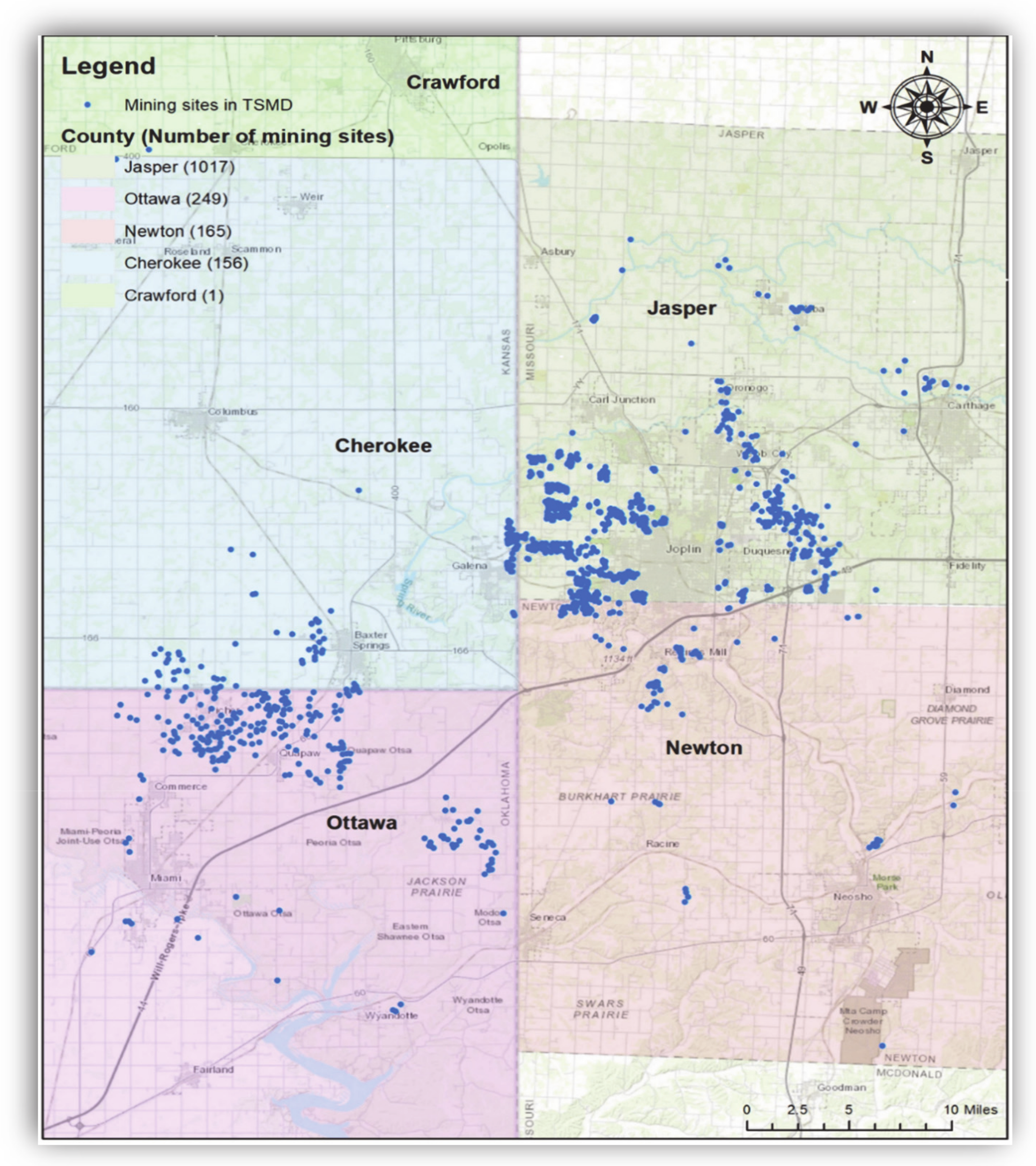
There is a large portion of toxic metal areas, which are called “Superfund” sites by the US EPA [16]. Despite remedial actions, tons of waste materials (in particular, Pb, Cd, and Zn), mining shafts, and tunnels are still left in those areas, which in turn contaminated groundwater and soil [3]. Most of all, the components of Pb and Zn are associated with the geographical location of former mining and smelting centers [3,16]. Figure 2 shows the locations and distribution of the mine-related sites in the TSMD Superfund sites.
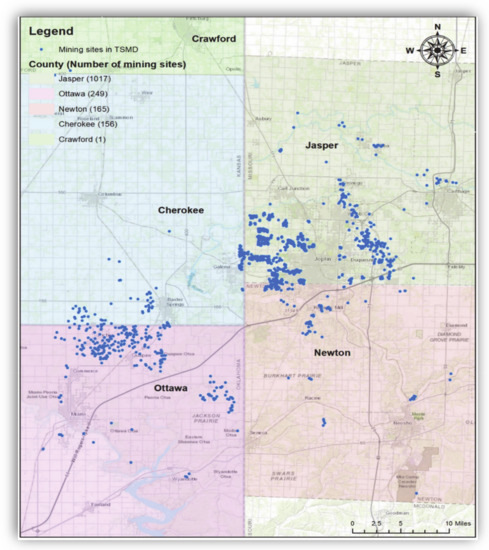
Figure 2.
Locations of TSMD Superfund Sites. Note: Blue indicates the mine-related sites in the TSMD. There were 1017 sites in Jasper County, Missouri, 249 sites in Ottawa County, Oklahoma, 165 sites in Newton County, Missouri, and 156 sites in Cherokee County, Kansas. The geospatial database was obtained from the U.S. Geological Survey (USGS) (Date taken: 29 July 2020). The USGS data are publicly available from https://www.usgs.gov/.
2.2. Characteristics of TSMD Superfund Sites and Their Contamination
In the 1980s, the USEPA designated some areas in the TSMD as Superfund sites—Tar Creek, the area of Picher in “Ottawa County” in Oklahoma, “Cherokee County” in Kansas, and the Oronogo-Duenweg Mining Belt in “Jasper County” in Missouri.
2.2.1. Cherokee County (Kansas) Superfund Site (298 km2)
After the mining operation and dewatering pumps were stopped, mines started flooding into a local aquifer. The range of concentration of Cd, Pb, and Zn in these areas was much greater than any other regions (0.6 to 469 mg/kg for Cd, 22 to 7460 mg/kg for Pb, and 100 to 45,000 mg/kg for Zn) [17]. These intensively higher concentrations were detected in mining-affected streams near the Short, Tar, and Spring Branch Creek watersheds [17]. Concentrations exceeding USEPA guidelines cause toxicological effects to aquatic life forms [17]. The U.S. government began to remediate this site in 1986, which aimed to prevent the flow of acidic waters by blocking the aquifer and plugging abandoned wells and mine shafts [3].
2.2.2. Jasper County (Missouri) Superfund Site (498 km2)
Jasper county includes Joplin, Webb City, and Carterville. Residential soils in Joplin were primarily impacted by dust and fallout from a smelter that was open until 1970, and mining wastes [3]. Contaminated metallic soil was removed from 2600 properties by 2002, water supply systems improvements were completed in 2007, and 6.06 km2 of milling waste were remediated by 2012 [3].
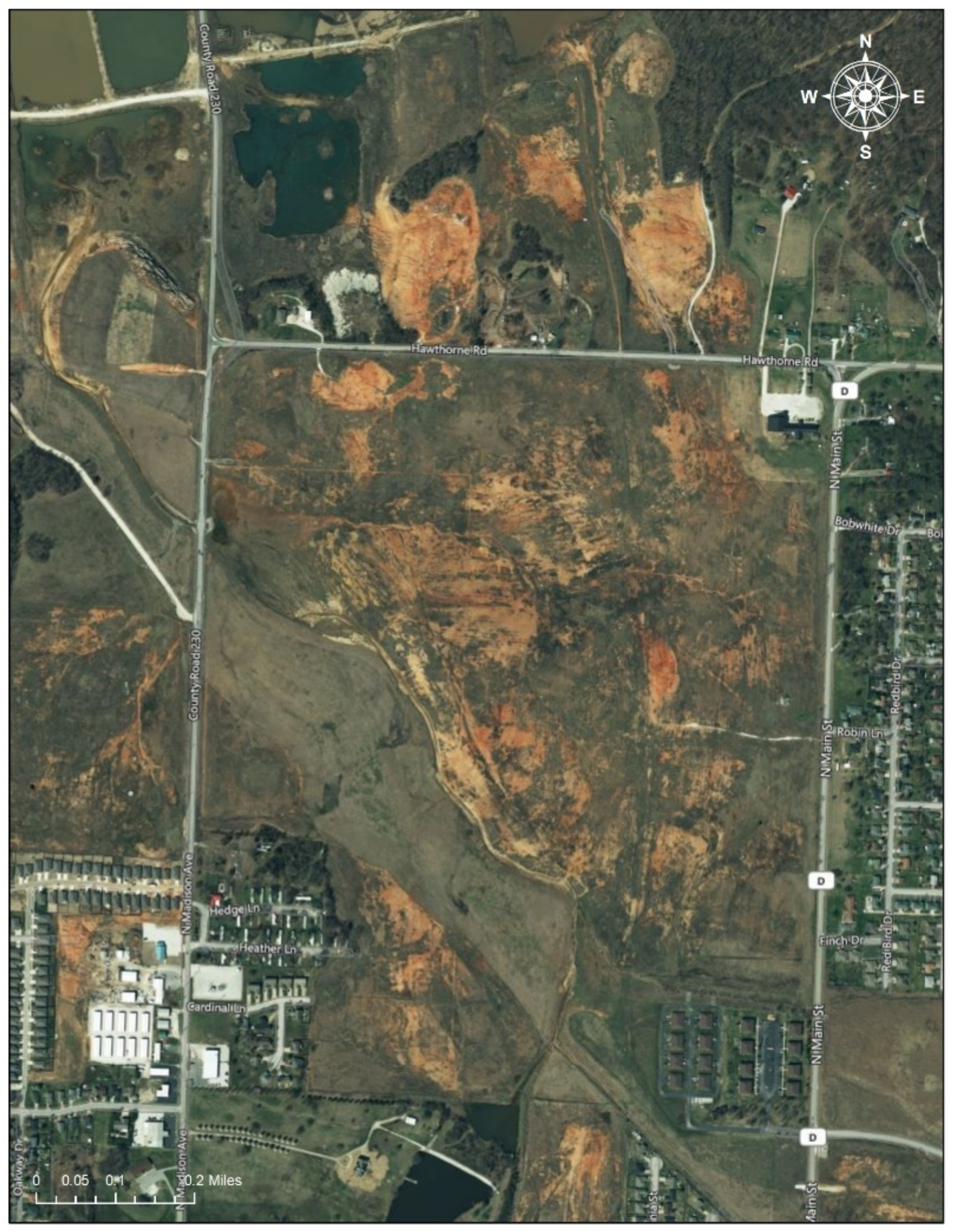
However, the 2011 tornado (EF-5) brought up the issue of the displacement of contaminated soils throughout the area, and new funding was collected for additional remediation. Consequently, various assessments to detect the dispersion of metals by the tornado in the city of Joplin have been initiated [3,18] (Figure 3).
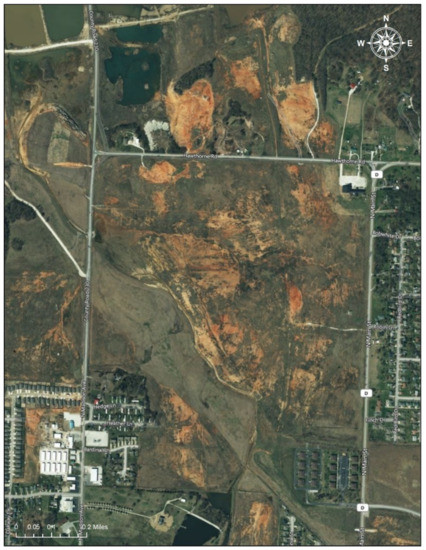
Figure 3.
Oronogo-Duenweg Mining Belt Site. Note: The Oronogo-Duenweg Mining Belt Site was one of the major mine waste areas near Joplin, Missouri. The satellite imagery, obtained from Google, shows that the site has left marks (the reddish brown areas) since it was inactive in the 1970s (Date taken: 1 August 2020). The image in the Figure 3 is eligible for the Fair Use of a copyrighted work from Google Map [19].
2.2.3. Tar Creek, Ottawa County (Oklahoma) Superfund Site (104 km2)
The Tar Creek Superfund Site covers the cities of Picher, Miami, Commerce, Cardin, and Peoria in Oklahoma. Mining wastes containing Cd, Pb, and Zn were found in 60-m-high piles and sediments of former flotation ponds. Along with the designation of the Superfund site, the USEPA implemented remediation from 1984 to 1986, including plugging wells and constructing dikes to divert water around abandoned mines and collapsed mine shafts3 Water combined with sulfide minerals to become acidic, which dissolved metals [3].
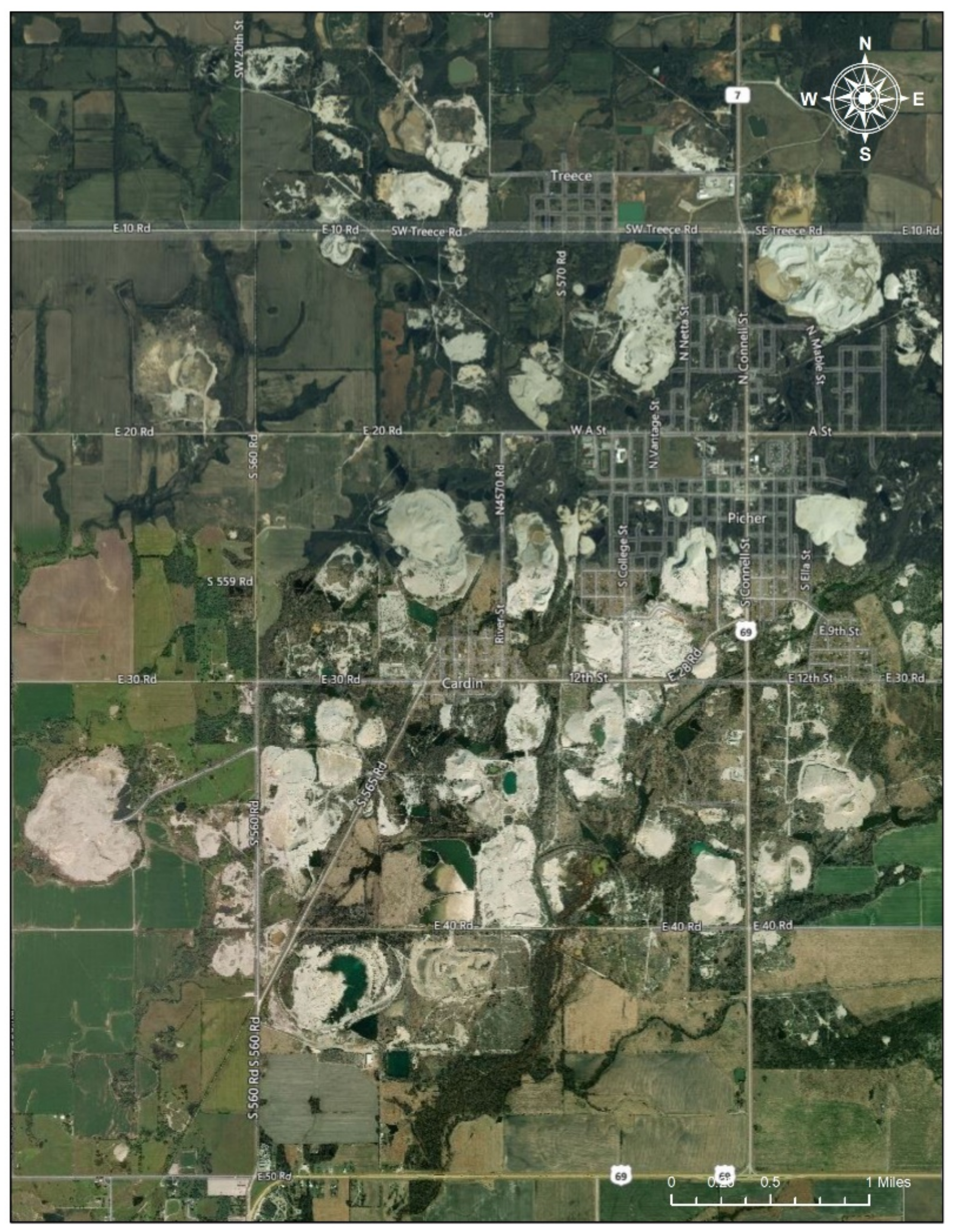
This acidic water contaminated with aqueous metals overflowed into Tar Creek [20]. The remaining chat is widespread in residential areas of Picher [21]. Nine Native Americans tribes—Cherokee, Eastern Shawnee, Miami, Modoc, Ottawa, Peoria, Quapaw, Seneca Cayuga, and Wyandotte—live near the boundaries of the Tar Creek Superfund Site areas and, due to contaminated food and water in these areas, have had to address the environmental health problems [20] (Figure 4).
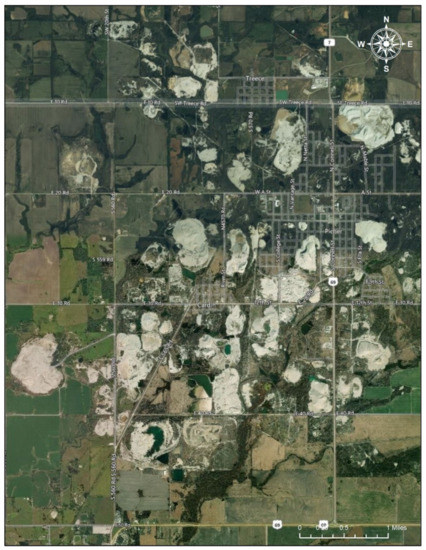
Figure 4.
Mining Sites near Treece and Picher. Note: The satellite imagery shows areas with mining sites (the white areas in the picture) clustering near Treece and Picher, Oklahoma, in the Tar Creek Superfund Site (Date taken: 1 August 2020). The image in the Figure 4 is eligible for the Fair Use of a copyrighted work from Google Map [19].
2.3. The Impact of Toxic Metal Components on Ecological Systems
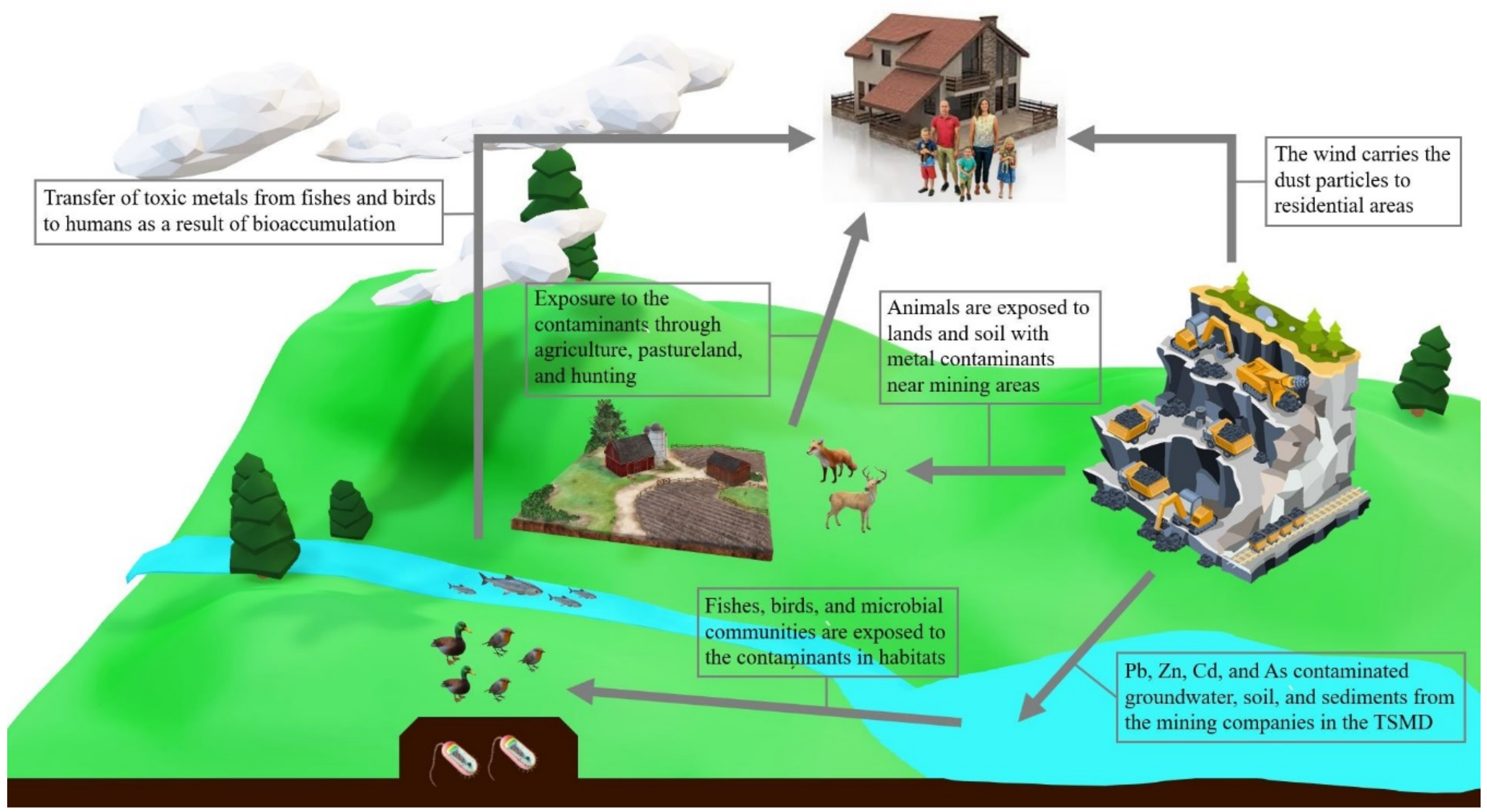
The bioaccumulation of contaminant metals in the TSMD has been detected frequently in various studies. Increased levels of Pb, Cd, and Zn in soils and water in the TSMD severely and negatively impact its environment [3] (Figure 5).
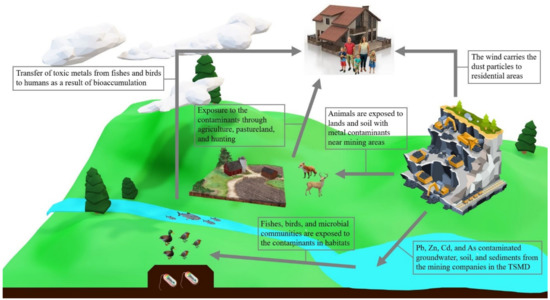
Figure 5.
Impacts of Toxic Metals on the Ecosystem. Note: The graphical abstract above shows the impacts of toxic metals on the ecosystem. The toxic metals, such as lead (Pb), zinc (Zn), cadmium (Cd), and arsenic (As), in the wastes from the mining sites and companies in the Tri-State Mining District (TSMD) contaminate the entire ecology from the nature environment to human life; the images in the Figure are eligible for the Fair Use of a copyrighted work [19].
Impacts on wildlife: Areas within and near the mining district have remnants of Cd, Pb, and Zn even after remediation efforts. Unfortunately, these metal contaminants have been harming the living organisms around them. For many wild birds within the TSMD, the metal contaminants have caused serious health effects [2]. These birds may have been exposed to the contaminants through inhalation or ingestion of Pb from chat or plants. The brown thrasher, cardinal, and robin most likely consumed Pb-contaminated soil or food because Pb usually appears on the surface of the soil where birds can accidentally ingest it. Birds can also be exposed to Zn by consumption of the freshwater vascular plants that absorb Zn-contaminated water; aquatic plants can also build up iron and Zn plaque on their roots. Therefore, birds who consume these plants are also ingesting metal contaminants. It is important to note, however, that because contamination throughout the TSMD is sporadic, the average exposure of these wild birds to the contaminants, as well as the concentration of metal within the soil, only give a partial assessment of the factors that are harming the birds [2].
In terms of the metal contaminant Pb, wild bird species—the American robin and other waterfowl in the TSMD—have been harmed by its presence. For instance, American robins, waterfowls, and northern cardinals have increased concentrations of Pb in their tissue, which cause weakened biological functions and indications of poisoning. In addition, the activity of delta-aminolevulinic acid dehydratase (ALAD), Pb-sensitive enzymes, in the red blood cells of these species of birds was reduced by over 50%. This is an indication that these wild birds did not have strong mediators to the toxicity of Pb [22]. The same results could be seen for songbirds, which also had a reduced ALAD activity of less than 10%, which is an indicator of heavy exposure to Pb. Metal contaminants can also be very lethal to wild birds. Many mallards, teals, and pintails have died along the Spring River near Riverton, Kansas, because of Pb poisoning [2].
Within the TSMD, songbirds and swallows had a high concentration level of Cd in their kidneys and livers, and these concentrations are much greater than those birds outside of the TSMD [2]. The waterfowl and trumpeter swan also had Zn in their livers and kidneys. Waterfowls within the district also experienced pancreatitis, the inflation of the pancreas due to the Zn. Overall, the mean metal concentrations of Pb, Cd, and Zn that affected the digestive system of these birds were much higher than their concentrations in wild birds outside of the TSMD with these comparable values (units in milligrams per kg) [2]. In addition, the runoff of metals that were extracted during the mining process contaminated water sources, which caused a decline of fish populations; in particular, the Neosho madtom that lives in these waters is currently listed by the federal government as one of the most severely threatened species of fish [2].
Impacts on microbial communities: Although bacteria are often overlooked, the effect of metal contaminants on microbial communities influences the health of other organisms and the environment. Metal contaminants often decrease bacterial diversity, bacterial biomass, and the richness of an environment. This can cause serious disruptions in the ecosystem by harming the health of other organisms and biochemical elements in soil [4]. Particularly for other organisms, the reduced bacterial diversity and biomass result in less bacterial by-products, which are important for nutrient absorption for animals by assisting the intestinal epithelial cells. Therefore, many rats that live within remediated sites have reduced microbiota diversity, meaning that metal contamination is a factor in their declining health. There is currently no research published specifically on the effects of metal contaminants of the TSMD on bacterial communities and other organisms. However, it can be assumed that the same circumstances in other heavily contaminated sites would occur within the TSMD [4].
2.4. The Impact of Toxic Meta Components on Human Health
A study by Zota et al. [13] examined the ways in which children could be exposed to metal contaminants. Within the study, researchers collected yard soil, house dust, and particulate matter of homes near chat sources to test for Zn, Pb, Cd, manganese (Mn), and arsenic (As). They discovered that the dust within the homes had a higher concentration of Zn, Pb, Cd, and As than the dust concentrations discovered in the nearby soil, and Zn had the highest concentration. The metal particles from chat sources can easily enter the home as the wind can carry the particles right outside someone’s home.
Therefore, the metal particles can travel indoors and mix with a house’s dust particles. Even those who do not live near a mining site can be exposed to metal contaminants if they are close to secondary uses of chat. This study by Zota et al. [13], overall, shows that even decades after the TSMD closed, it can still affect various families living within the district. Especially for children who live inside these homes, metals can easily enter their system through ingestion, inhalation, or dermal absorption because many children crawl and frequently put their hands in their mouths [13].
Furthermore, water with Pb contamination has been seriously challenging for utility companies to deal with and affects the potential health of residents who consume the water [11]. There are metal ores present in the shallow aquifers in the TSMD, which are a source of drinking water for residents of the area with private wells [23]. Overall, these studies show there are numerous ways in which residents of the TSMD are highly likely to be exposed to metal contaminants (e.g., Pb poisoning) from past mining activity and end up experiencing adverse health effects. Another study conducted by the Agency for Toxic Substances and Disease Registry in 1995 discovered by examining blood levels of children in Jasper County, MO, that around 14% of children aged 6–71 months had blood Pb levels ≥ 10 μg/dL, compared to no children with elevated blood Pb levels for children in a control group. Average blood Pb levels of adults were also higher than those of people in the control group [16]. A recent study has found that toxic metals can have an effect on obesity rates. In particular, Cd and As influence a person’s reproduction and growth. Cd, an endocrine disrupting chemical (EDC), influences a person’s chances of developing obesity [12]. The study showed that children from seven to nine years old exposed to EDC became overweight or obese in adulthood. For instance, children who had lived in the environment exposed to Cd were likely to become obese/overweight in adulthood [12]. Another study also examined if blood Pb levels influenced obesity in Chinese residents [24]. This study identified that Pb is an endocrine-disrupting chemical, or EDC, meaning that Pb also influences the development of obesity. These studies overall suggest the possibility that the toxic environment of the TSMD could contribute to increased obesity of the people living there.
Faulk et al. [25] discovered that mice, who have similar biological functions to humans, could experience increased body weight and fat as well as greater food intake if they were prenatally exposed to Pb. On the other hand, for humans, this prenatal exposure has been linked with low weight at birth, but these children were more susceptible to becoming obese in the future. In addition, Leasure [26] emphasized that exposure to Pb during the period of gestation could also lead to a child’s increase in body weight. However, after birth, children could continuously be exposed to Pb during childhood, which means they could more easily develop late-onset obesity. This was because Pb was found to alter the hypothalamic-pituitary-adrenal axis, resulting in obesity. Specifically, in a study by Wang et al. [24], there was a positive relationship between high blood Pb levels and a high BMI for women. Another possible explanation for this correlation was that Pb caused oxidative stress and irregularity in a person’s fat metabolism, which could be influencers of obesity. Pb and Cd are some of the common metal contaminants that resulted from the TSMD. Overall, abundant studies indicated that exposure to the toxic contaminants before and after birth could make a person much more susceptible to developing obesity.
Several studies have indicated that toxic heavy metals may play an important role in the development of diabetes mellitus [27,28]. They found a higher incidence of diabetes in arsenic-exposed areas from drinking water, compared to non-exposed areas. Afridi et al. [27] reported that the mean blood Cd concentrations of male non-smoker and smoker diabetic patients were significantly higher than controls. However, a study using the general population in examining the relationship between toxic heavy metals and diabetes found no association between toxic metals and diabetes [29].
3. Methods
3.1. Search Strategy and Selection Procedure
This study first systematically searched articles from the electronic literature databases Academic Search Premier, American Chemical Society Legacy Archives, PubMed Central (PMC), GreenFILE, JSTOR, and ELSEVIER pertinent to literature, using the terms “TSMD, toxic elements, and negative effects.” Specifically, the search terms were divided into five groups: (1) Tri-State Mining District AND toxicity AND wildlife OR animals OR human health, (2) superfund sites AND toxic contamination AND microbial community OR ecology OR environment, (3) USA AND mining areas AND heavy metal AND physical health OR well-being OR fish OR plants OR birds, (4) USA AND mining AND toxic contamination AND natural system OR plants OR bacteria, and (5) USA AND mining AND arsenic OR zinc OR lead OR cadmium AND environment OR community OR humans.
After locating relevant studies, we exported them into the Covidence software. Once Covidence removed duplicates from the listings, titles, and abstracts were screened to identify potentially related articles by two authors independently. Secondly, these two authors uploaded PDF articles to Covidence to thoroughly review each single article following screen criteria. The third author performed as a mediator when discrepancy took place. Finally, the 19 reviewed articles were created as extraction forms.
3.2. Study Selection
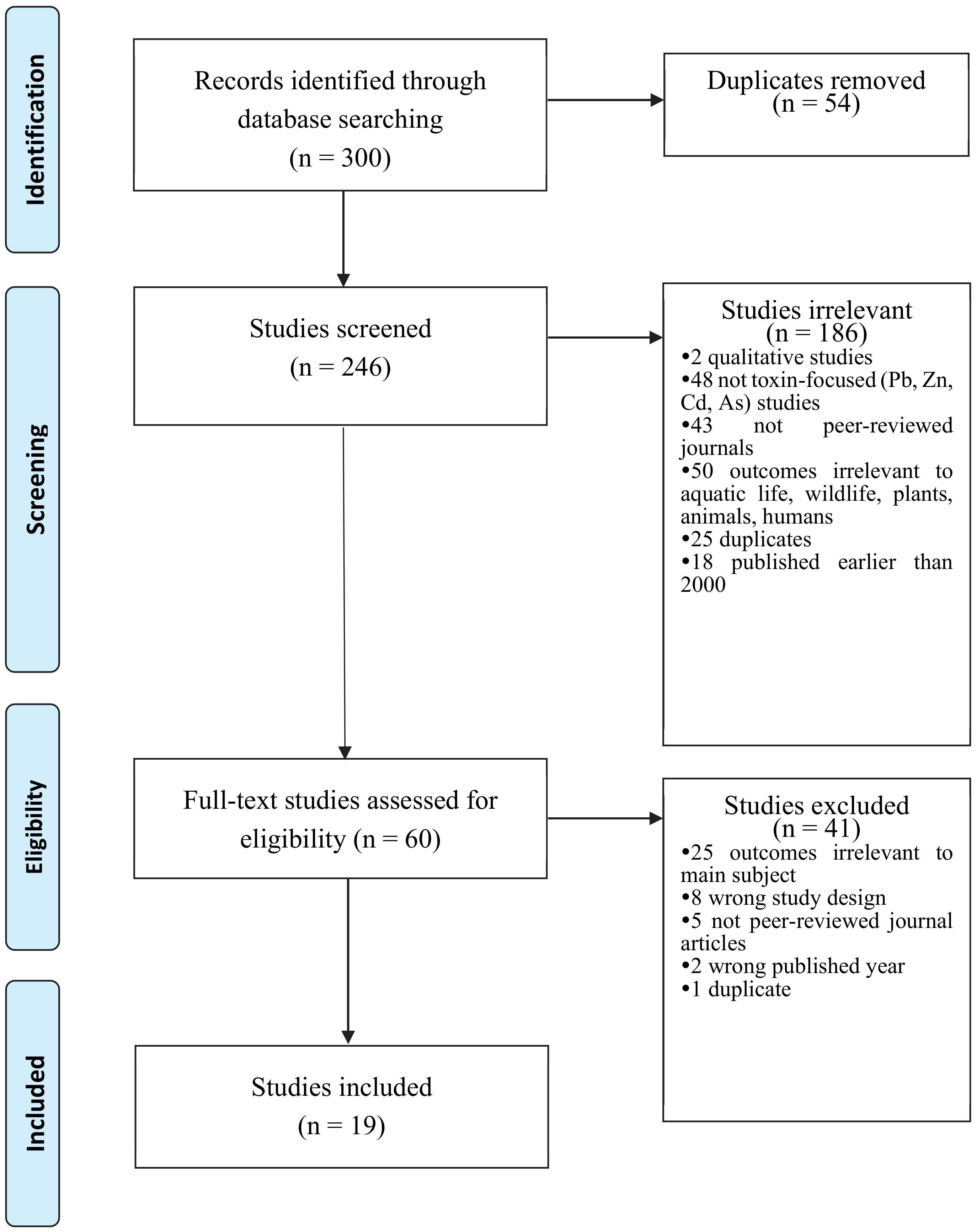
Out of 300 articles identified through database searching, 246 studies were screened after removing duplicates (n = 54). The screened 246 studies initially excluded several articles based on the following reasons: (a) qualitative studies, (b) studies where the main focus is not toxic/toxin, (c) not peer-reviewed journals, (d) outcomes irrelevant to the main subject (e.g., aquatic life, wildlife, plants, animals, humans, ecology), (e) outdated publication (i.e., earlier than 2000), and (f) duplication. After the excluding process, 60 studies were considered eligible. However, the articles underwent another screening process after detecting irrelevant material. The second process of exclusion criteria was (a) wrong outcomes (e.g., remediation effects), (b) wrong study design (e.g., case studies), (c) not peer-reviewed journal articles, (d) wrong published year, and (e) duplication. As a result, 19 studies remained in the sample. Figure 6 shows the creation of the sample of this study.
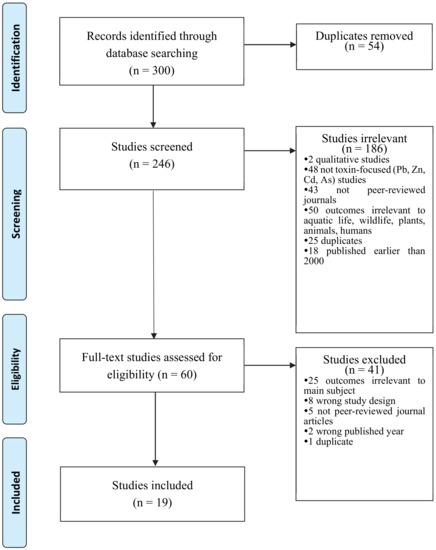
Figure 6.
PRISMA Flowchart of Study Selection Process.
Table 1 shows the characteristics of the 19 most eligible studies included in the final sample of this review. For the final process of the sample, the first and second author extracted the following information from each included study: author(s), the purpose of the study, research design/sampling method, key predictor (Independent Variable) measurement, key outcome (Dependent Variable) measurement, sample subject and location, statistical methods, and main findings.

Table 1.
Characteristics of the Studies on the Effects of Toxic Contaminations in the TSMD on the Ecological Communities and Human Health.
3.3. Quality Assessment
The quality of the included studies was assessed using Standard Quality Assessment Criteria for Evaluating Primary Research Projects from a Variety of Fields designed by Kmet et al. [30]. This assessment was developed using two scoring systems to evaluate the quality of the studies potentially eligible for inclusion in the review [30]. This tool includes 14 items to capture the quality of a study, such as study design, sample, methodology, data analysis, results, and conclusion [31]. Out of two main types of assessment—quantitative and qualitative—we employed a quantitative type for our study assessment. This assessment tool was also used in previous studies for their systematic reviews [31,32,33].
The 14 items were scored by the degree to which the specific criteria were met, yes = 2, partial = 1, and no = 0. Items not applicable to a particular study design were marked “N/A” and were excluded from the calculation of the summary score [30]. The final score of each study was achieved by the formula, ((“yes” × 2) + (“partial” × 1)/28 − not applicable numbers × 2). The quality of the study was expressed by percentage from 0% to 100% [30]. For this study, the first and second authors assessed the quality of each study independently, and any discrepancies were resolved by a third author. The final score in Table 2 represents the consensus between all three authors.

Table 2.
Manuscripts Quality Assessment.
4. Results
4.1. Risk of Bias
Table 2 presents the result of the risk of bias assessment of the included 19 articles. For two items, “Blinding Investigations” and “Blinding Subjects,” no selected articles received points because of the non-intervention nature of the studies. There was no need, and it was not possible for participants/subjects and researchers to be blinded. Hence all articles received “N/A” for the two categories.
Assessment bias due to method. Some studies such as Coolon [4], Allert et al. [5], Beyer et al. [34], Ettinger [35], Lynch et al. [6], Malcoe et al. [36], Neuberger et al. [37], and Merwe et al. [38] were not able to receive full scores, due to the failure of explanation of the validity of specific samples. However, other studies, Brumbaugh et al. [39], Garvin et al. [40], Schmitt et al. [41], and Struckhoff et al. [42] showed accurately represented population (species) selecting different types of species or selecting them by different locations. Additionally, the study by Yoo and Janz [43] selected different types of female fish (black bullhead and bluegill sunfish) and compared their outcomes seasonally (spring vs. winter).
Assessment bias due to subject: The assessment looks at the clear demographic/characteristic information provided and/or precise description/categorization of participants/samples [30]. Two studies, Beattie et al. [44] and Merwe et al. [38], were not able to obtain the full score since they did not describe the characteristics of their samples to make readers understand the reasons for their subject criteria. On the other hand, Neuberger et al. [37], for instance, described the specific conditions of samples (e.g., health problems) due to the environmental exposures, and Phelps and McBee [45] described the mammal communities’ conditions for the lives of the comparison groups. Additionally, the study by Malcoe et al. [36] hired phlebotomists, interviewers, canvassers, and project coordinators to collect baseline information (e.g., home environment) from the samples’ (children) caregivers.
Assessment bias due to randomization: Due to the characteristics of the study subjects (toxicity, chemistry, and environment), a majority of studies did not conduct randomization except for the two studies conducted by Lynch et al. [6] and Malcoe et al. [36]. The first study clearly addressed that they performed a population-based random sampling as part of a blood lead screening project. By obtaining an overall response rate of over 60%, Lynch et al. [6] collected blood samples from a total of 144 residents out of 550 eligible family representatives of Native American and white households residing within 31 contiguous census blocks in Northeastern Ottawa County, Oklahoma. The study by Malcoe et al. [36] also addressed that their sample consisted of a population-based, representative sample of Native American and white children living within 31 contiguous census block groups in Northeastern Ottawa County, Oklahoma. To recruit eligible families, city blocks were randomly selected within each block group, proportional to the estimated number of households with young children in each block group. At least three visits were made to each residence to determine eligibility, and if there was more than one eligible child in the household, the youngest child was selected to participate.
Furthermore, compared to the rest of the studies that received “N/A,” two studies, Ettinger et al. [35] and Neuberger et al. [37], received a score of zero due to employment of a convenient sampling of the secondary dataset.
Assessment bias due to sample size. This assessment is to see whether the study achieved outcomes from the appropriate sample size. Some studies, Beattie et al. [44], Beyer et al. [2], Beyer et al. [34], and Brumbaugh et al. [39] received a full score because they were able to achieve sizable and comparable samples, other studies, Coolon et al. [4], Allert et al. [5], Struckhoff et al. [42], and Yoo and Janz [43] consisted of a small sample size and other studies; Besser et al. [46], Neuberger et al. [46], and Schmitt et al. [41] showed unbalanced sample group sizes for comparing outcomes.
Assessment bias due to analytic methods. A majority of the selected studies employed advanced and proper analytic methods except for the study by Hays and Mcbee [47], which mostly presented least-square means and relative frequency.
Assessment bias due to variance. A majority of the studies presented an estimate of variance (e.g., confidence intervals, standard errors, and standard deviation) to explain the main results and outcomes except for a study by Garvin et al. [40], which only addressed the sample group differences using ANOVA (2-tailed).
4.2. Characteristics of Included Studies
4.2.1. TSMD Toxic Effects on Plants
Two studies—Garvin et al. [40] and Struckhoff et al. [42] did research about the correlations between metal concentrations (Cd, Pb, and Zn) and plants in the TSMD. Specifically, the study by Garvin et al. [40] focusing on 36 species of edible plants and soil from floodplain areas in southwestern Missouri (MO), southeastern Kansas (KS), and northeastern Oklahoma (OK), found that there was a significantly positive association between metal concentrations in plant tissues and soil, and a significant difference in metal concentration distribution between reference plants and impacted plant samples. In particular, most plants included a higher degree of exceedance of Cd and Pb.
The study by Struckhoff et al. [42] used two standard floristic quality measures, mean coefficient of conservatism (Mean C) and floristic quality index (FQI), and examined the concentration of Pb and Zn, soil nutrients, other soil characteristics, and plant communities near mine waste and a Pb smelter in two subregions known as the Old Lead Belt and the Viburnum Trend in the Southeast Missouri mining district (SEMO). They also found that exotic species had the highest Pb concentration (3464 mg/kg) and second-highest Zn concentration (851 mg/kg), while Pb and Zn soil concentration had the highest correlation values. Their findings of lower Mean C and FQI, as well as abundance of non-native species with higher Pb and Zn soil concentrations, supported the adverse effects of elevated metals concentrations on plant community composition and structure [42].
4.2.2. TSMD Toxic Effects on Aquatic Life
We found several articles that examined the effects of TSMD toxic metals on living creatures in the water. First, Allert et al. [5] examined the characteristics of physical habitat and water quality, focusing on toxic metals (Pb, Zn, and Cd) and crayfish. By collecting subsamples of water, sediments, detritus, and crayfish from three metal-contaminated sites in Spring River of southwestern MO and southeastern KS, they found that there were differences in groups of sites (reference, mining, and downstream), there were significant correlations between surface water toxic units and aquatic biota, and toxic metals in crayfish at several sites exceeded concentrations considered harmful to carnivorous wildlife. In addition, Schmitt et al. [48] and Brumbaugh et al. [39] also found a similar outcome that Pb, Zn, and Cd in fish in TSMD rivers were high compared to those in reference sites. Additionally, by testing the blood and livers of their study samples (carp, bass, and catfish), Brumbaugh et al. [39] highlighted that for Zn, differences among sites for both blood and carcass were significant between catfish and bass and between bass and crappie. There were also significant differences among sites for Pb in both blood and carcass.
Two studies—Schmitt et al. [41] as well as Yoo and Janz [43] discovered a direct biochemical effect of Pb, Cd, and Zn on fish living in the TSMD water. First, Schmitt et al. [41] found that delta-aminolevulinic acid dehydratase (ALAD) activity in catfish was more sensitive to blood Pb than in the other species, and catfish from TSMD sites had more problems with enzyme activity than those in reference sites. Overall, Pb was both bioavailable and active biochemically in the Spring–Neosho River system. Secondly, Yoo and Janz [43] observed that aqueous concentrations of Cd and Zn and liver concentrations of Cd and Zn in their study samples, bluegill sunfish, and black bullhead, were significantly greater at Tar Creek compared to the reference site, and the 70-kDa stress protein family (HSP70) expression was consistently elevated in the head kidney of both fish species at Tar Creek compared to fish collected from the reference creek. However, there were no consistent differences observing HSP70 expression in liver, gill, or ovarian tissues between sites.
4.2.3. TSMD Toxic Effects on Wildlife Animals
Five articles, Beyer et al. [2], Beyer et al. [34], Hays and McBee [47], Phelps and McBee [45], and Merwe et al. [38] identified TSMD toxic exposure as relevant to animals Observing wild birds (American robins, cardinals, and waterfowl), Beyer et al. [2] uncovered that Pb tissue concentrations of their study samples from chat environments had increased Pb tissue concentrations (p < 0.05) compared to those of reference site birds; mean activities of Pb-sensitive ALAD were decreased by >50% in the red blood cells of these sample birds (p < 0.05); Zn concentrations in the liver and kidneys of waterfowl from chat environments were significantly higher (p < 0.05) than reference birds; and the increased Zn concentrations in the chat appeared to cause pancreatitis in waterfowl.
Another study from Beyer [34] discovered that earthworms from Southeast MO contributed to high Pb exposure in songbirds which prey on soil organisms. Mean tissue Pb concentrations in songbirds from the sites were greater (p < 0.05) than those in songbirds in the reference sites by factors of 8 in blood, 13 in the liver, and 23 in the kidney, and mean activity of the enzyme ALAD in red blood cells was lower by 58–82% in songbirds from the mining sites. Examining bird species (Canada Geese), Merwe et al. [38] detected similar outcomes with toxic metals. Adverse effects associated with Zn poisoning were found from the examination of pancreas tissue, and elevated tissue Pb concentrations and inhibited blood ALAD enzyme activities were consistently found in birds from the TSMD.
Finally, looking at the ecological characteristics of small mammal communities (white-footed mouse in Tar Creek Superfund Site), Phelps and McBee [45] found that the Tar Creek Superfund Site has experienced reduced species diversity, including richness and evenness compared to the non-mining sites. Additionally, species composition was different between contaminated sites and reference sites, as a detrended correspondence analysis indicated that, in terms of species diversity, contaminated sites were quite similar to each other but had fewer similarities when compared to either reference.
4.2.4. TSMD Toxic Effects on Microbial Communities
The two studies by Beattie et al. [44] and Coolon et al. [4] examined bacteria to understand the microbial community structure in the TSMD. First, analyzing 16S r(ribosomal)RNA gene sequences and q(quantitative)PCR of bacteria and archaea, Beattie et al. [44] discovered that bacteria were negatively and significantly correlated with Pb, Cd, Zn, and Mg levels. However, archaea were only significantly and positively correlated with pH. Illumina sequencing of 16S rRNA genes showed significant differences in microbial communities near chat environments and west transect samples, and soil chemistry with community structure indicated that Al, Pb, Cd, and Zn significantly impacted community composition and distribution of individual operational taxonomic units (OTUs). Additionally, Coolon et al. [4] found that the assemblage of bacterial communities differed with respect to contamination in remediated sites. Both studies emphasized that it is vital to provide ecosystem services to chat environments and understand the effects of long-term heavy metal contamination on microbial populations.
4.2.5. TSMD Effects on Human Health
Four studies, Ettinger et al. [35], Lynch et al. [6], Malcoe et al. [36], and Neuberger et al. [37], observed the effect of TSMD toxic contaminations on human health. First, using convenient sampling—recruiting 532 pregnant women from a health center near the Tar Creek Superfund Site—Ettinger et al. [35] discovered that blood As concentrations ranged from 0.2 to 24.1 ug/L (ppb) and in hair, it ranged from 1.1 to 724.4 ng/g (ppb); impaired glucose tolerance was observed in 11.9% of women when all participants had the glucose screen test; and after controlling for covariates, women in the highest quartile of blood As exposure had 2.8 higher odds of impaired glucose tolerance test (GTT) than women in the lowest quartile of exposure (95% CI, 1.1–6.9) (p = 0.008).
Examining blood lead concentrations (BPbs) from 245 residents in the TSMD, Lynch et al. [6] and Malcoe et al. [36] also found that BPbs were significantly associated with floor dust and yard soil. Malcoe et al. [36] additionally discovered that soil Pb levels and poverty were strongly correlated (p = 0.005). All three studies supported the result of the study by Neuberger et al. [37] that the health of the TSMD residents, specifically mortality related to stroke and heart disease, is still affected by ongoing heavy metal exposure even after remediation involvement, and that the presence of toxic concentrations in the TSMD should not be ignored.
5. Discussion
In this systematic review, we evaluated 19 studies on the effects of toxic elements in the TSMD on the ecological community and physical health of residents. Harmful metal components from the TSMD mining sites which mined Pb, Zn, Cd, and As were found to affect the surrounding environments and residents negatively through both water and soil. Despite the remediation effort by the U.S. government, we were still able to find the prevalence of harmful effects of the metal components produced from the mining sites. Fifteen studies among the 19 studies in this review focused on the results of toxic metal contaminants on the ecological community, such as plants, aquatic life, wildlife animals, and microbial communities. In all of these studies, heavy-toxic metals were concentrated in natural habitats and the internal organs of animals themselves. The effects of the heavy-toxic metal contaminants, needless to say, are harmful to biodiversity. Their harmful effects on human health should not be overlooked either.
Toxic metals, such as Pb, Zn, and Cd, in mine wastes have been found to environmentally travel between primary sources and other ecological communities. This environmental mobility of toxic metals makes them pose a considerable risk to human health [20,49]. In addition to the included studies in this review, other studies also found that blood Pb levels in children who lived near the toxic metal exposed areas (i.e., the TSMD) exceeded the maximum level recommended by the Center for Disease Control [50,51,52,53].
The TSMD was one of the leading producers of heavy metals in the country. The smelting process in the TSMD left byproducts called chat and slag. Chat can easily harm the local soil along with slag. The soil was not only affected by this waste but also experienced sinkholes that resulted from the mining process. After discovering the high concentrations of Pb in the TSMD, the USEPA recognized the Pb poisoning of local residents. However, the remediation efforts were not completely successful as the mines continue to harm the environment to this day [23]. For instance, many piles of chat remain in Oklahoma, particularly in Tar Creek (south of Picher, OK). The runoff of these piles of waste and mine-water discharge harm the environment. Jasper County, MO, is also experiencing poor water quality as a result of seepage, metal particles traveling to the water, and mining runoff [23]. Other water sources such as watersheds in the TSMD are contaminated with heavy metals because groundwater filtered those metals into the watersheds. However, in general, a high concentration of >1000 ppm of metals was discovered in soil and water sources in areas within the TSMD. It is important to note, however, that the metal contaminants are not just a threat to the local area as contaminants can spread at least 20 km from their original location [44]. In addition, despite remediation efforts, metal contamination continues to affect the local environment, wildlife, and daily life of residents in the TSMD.
This systematic review, however, has some limitations. First, although we rigorously employed a broad search strategy to find related journal articles on this subject matter, there were only a few published studies on the effects of metal contamination in the TSMD on the ecological community and human health. This may be due to a relatively limited number of databases that were available to us for potentially eligible studies.
Second, to assess the included studies, we relied on a single quality assessment tool, Standard Quality Assessment Criteria for Evaluating Primary Research Projects from a Variety of Fields [30] instead of multiple tools. We were not able to find other quality assessment tools that could be universally applied to studies with a lack of homogeneity in terms of study outcomes and designs.
The third limitation is also related to the issue with a lack of homogeneity in study outcomes and designs. It was difficult to synthesize the included studies in this review for a meta-analysis because each of the included studies in this review had an inconsistent study outcome and subject. As seen in the results, the number of included studies for each area of its effects (i.e., plants, aquatic life, wild animals, etc.) ranged between two and five. Conducting a meta-analysis on this small number of studies would not produce meaningful and reliable results.
Fourth, although there have been studies on the effects of heavy toxic metals on the insect community which found that insects were good bioindicators of metal pollution [54,55], no studies on the insect community were included in our review. We could not find such studies from our database searching process, perhaps because such studies were conducted outside the TSMD.
6. Recommendations
There are possible and cost-effective remediations recommended by many scholars. The first one is recovering the contaminated areas to reuse and recycle the mining wastes [56]. For example, repurposing mine wastes might produce economic benefits while addressing environmental concerns. Waste rocks can be used as filling for mine shafts and cavities, and benign tailings can be mixed with cement to cover less benign tailings [56]. Rock waste can also be used for pavement, buildings, and other structures through mineralogy [3].
Second, phytoremediation utilization can be practical. This assists in bioaccumulated plant revival through roots, stems, or shoots [57]. Another possible solution is the application of the methodology used in Oklahoma to other Superfund sites. The comprehensive remediation plan for Oklahoma consists of hydrology, the reactivity of contaminants, and bedrock geology. Since the hydrology and geology of Oklahoma and the TSMD are similar, it will be an evidence-based methodology that can be safely and economically utilized in other parts of TSMD areas [3].
Even though several studies relevant to humans’ physical health are smaller than other study foci, it is obvious that the recommended remediation would be not only helpful for habitats in the TSMD environment but also effectively boost the physical well-being of residents in those areas. Additionally, public health policymakers need to make careful applications for the overall health of low-income TSMD households in terms of repaving playground sand or surface materials in play areas, avoiding the use of mine waste piles for the construction of residential homes or public use areas like parks, replacing fill material that comes in contact with free-standing water or with surface water, and using an agricultural soil amendment to adjust soil alkalinity [23]. Additionally, providing regular health checkups by community clinics or health care providers to TSMD residents would be beneficial to prevent the acceleration of health problems related to toxic materials.
7. Conclusions
Despite the limitations of the systematic review process that we had, this review clearly shows that the local flora, fauna, and residents of the TSMD are still suffering from the lingering effects of the area’s mining legacy. While remediation efforts by the government have made some progress, they have not gone nearly far enough to ensure the well-being of the people and environment of the region.
One possible impediment to remediation is the fact that a significant portion of the area lies within a karst terrain [58]. The USEPA uses excavation of contaminated chat and sediment followed by disposal in subsidence pits as its preferred remediation strategy in the area [23]. The excavation and disposal technique relies on the isolation of contaminants within the pit to prevent them from entering nearby aquifers. If these subsidence pits are part of a karst topography, the porous and fractured nature of the karst would render this method ineffective. A study of a similar superfund site in Puerto Rico showed that the fractures and conduits within the karst aquifer were responsible for spreading contaminants to nearby streams, wells, and wetlands [59]. In fact, most of the case studies that the USEPA categorizes as impossible to remediate, or technical impracticability, were in areas of karst terrain [60].
Overall, this review provides details about the mechanisms through which heavy metal contaminants from mining industry waste are dispersed and absorbed into the surrounding soil and water, the effects of those metal contaminants on microbe, fish, plant, and bird populations, and the increased health risks to lower-income children and adults living in the area. Policymakers can use the data presented here to influence the decision-making process to show that more remediation needs to be conducted. Furthermore, this review provides several techniques that can be implemented to make sure the land and people no longer have to be impacted by an industry that abandoned the area decades ago.
Author Contributions
Formal analysis, H.P. and K.N.; funding acquisition, H.P.; investigation, H.P. and J.J.M.; methodology, H.P. and K.N.; resources, H.P.; software, H.P.; supervision, C.R.; validation, K.N. and J.J.M.; visualization, K.N.; Research question initiator, J.J.M.; writing—original draft, H.P., K.N., and J.J.M.; and writing—review and editing, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pittsburg State University and the APC was funded by Pittsburg State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Juracek, K.E.; Drake, K.D. Mining-Related Sediment and Soil Contamination in a Large Superfund Site: Characterization, Habitat Implications, and Remediation. Environ. Manag. 2016, 58, 721–740. [Google Scholar] [CrossRef]
- Beyer, W.N.; Dalgarn, J.; Dudding, S.; French, J.B.; Mateo, R.; Miesner, J.; Sileo, L.; Spann, J. Zinc and lead poisoning in wild birds in the tri-state mining district (Oklahoma, Kansas, and Missouri). Arch. Environ. Contam. Toxicol. 2005, 48, 108–117. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gutiérrez, M.; Gouzie, D.; McAliley, L.R. State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef]
- Coolon, J.D.; Jones, K.L.; Narayanan, S.; Wisely, S.M. Microbial ecological response of the intestinal flora of Peromyscus maniculatus and P. leucopus to heavy metal contamination. Mol. Ecol. 2010, 19 (Suppl. S1), 67–80. [Google Scholar] [CrossRef]
- Allert, A.L.; DiStefano, R.J.; Schmitt, C.J.; Fairchild, J.F.; Brumbaugh, W.G. Effects of mining-derived metals on riffle-dwelling crayfish in southwestern Missouri and southeastern Kansas, USA. Arch. Environ. Contam. Toxicol. 2012, 63, 563–573. [Google Scholar] [CrossRef][Green Version]
- Lynch, R.A.; Malcoe, L.H.; Skaggs, V.J.; Kegler, M.C. The Relationship between Residential Lead Exposures and Elevated Blood Lead Levels in a Rural Mining Community. J. Environ. Health 2000, 63, 9–15. [Google Scholar]
- Gulson, B.L. Tooth Analyses of Sources and Intensity of Lead Exposure in Children. Environ. Health Perspect. 1996, 104, 306–312. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Weitzman, M.; Winter, N.L.; Eberly, S.; Yakir, B.; Tanner, M.; Emond, M.; Matte, T.D. Lead-contaminated house dust and urban children’s blood lead levels. Am. J. Public Health 1996, 86, 1416–1421. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Roghmann, K.J. Pathways of lead exposure in urban children. Environ. Res. 1997, 74, 67. [Google Scholar] [CrossRef]
- The Tar Creek Trustee Council. Natural Resource Progrmmatic Restoration Plan and Environmental Assessment; U.S. Fish & Wildlife Service, Department of the Interior: Washington, DC, USA, 2017. Available online: https://www.fws.gov/southwest/es/oklahoma/Documents/Contaminants/Final%20draft_TarCreek%20ProgrammaticRP_EA.pdf (accessed on 30 June 2020).
- Gagnon, G. Distribution system water quality. J. Am. Water Work. Assoc. 2012, 104, 6668. [Google Scholar]
- Delvaux, I.; Van Cauwenberghe, J.; Den Hond, E.; Schoeters, G.; Govarts, E.; Nelen, V.; Baeyens, W.; Van Larebeke, N.; Sioen, I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ. Res. 2014, 132, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Schaider, L.A.; Ettinger, A.S.; Wright, R.O.; Shine, J.P.; Spengler, J.D. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.R.; Brady, L.L.; Wilson, F.W. Study of Stability Problems and Hazard Evaluation of the Kansas Portion of the Tri-state Mining Area (Contract 10100131 Report); U.S. Bureau of Mines Open-file Report 75–83, 193. P. (Also Kansas Geological Survey Open-file Report 83-2); United States Bureau of Mines: Washington, DC, USA, 1983.
- Gibson, A.M. Wilderness Bonanza: The Tri-State District of Missouri, Kansas, and Oklahoma; University of Oklahoma: Norman, OK, USA, 1972. [Google Scholar]
- Perry, P.M.; Pavlik, J.W.; Sheets, R.W.; Biagioni, R.N. Lead, cadmium, and zinc concentrations in plaster and mortar from structures in Jasper and Newton Counties, Missouri (Tri-State Mining District). Sci. Total Environ. 2005, 336, 275–281. [Google Scholar] [CrossRef]
- Pope, L.M. Assessment of Contaminated Streambed Sediment in the Kansas Part of the Historic Tri-State Lead and Zinc Mining District, Cherokee County, 2004; Scientific Investigation Report 2005–5201; U.S. Fish & Wildlife Service and the Kansas Department of Health and Environment: Manhattan, KS, USA, 2005. Available online: https://pubs.usgs.gov/sir/2005/5251/report.pdf (accessed on 20 May 2020).
- Peebles, J. Special analysis of mining related sediment contamination of Turkey Creek Watershed in the Tri-state mining district, Joplin (MS Thesis), Missouri State University, Springfield MO. In Proceedings of the 2013 GSA Annual Meeting in Denver, Springfield, MO, USA, 27–30 December 2013. [Google Scholar]
- United State Code. Chapter 1: Subject Matter and Scope of Copyright; USA Government: Washington, DC, USA, 2020. [Google Scholar]
- Hu, H.; Shine, J.; Wright, R.O. The challenge posed to children’s health by mixtures of toxic waste: The Tar Creek superfund site as a case-study. Pediatr. Clin. N. Am. 2007, 54, 155–175. [Google Scholar] [CrossRef]
- Robertson, D. Hard as the Rock Itself; University Press of Colorado: Louisville, CO, USA, 2006. [Google Scholar]
- Fowler, B.A. Molecular Biological Markers for Toxicology and Risk Assessment; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Pierzynski, G.M.; Vaillant, G.C. Remediation to reduce ecological risk from trace element contamination: A decion case study. J. Nat. Resour. Life Sci. Educ. 2006, 35, 85–94. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; et al. Blood lead level and its association with body mass index and obesity in China-Results from SPECT-China study. Sci. Rep. 2015, 5, 18299. [Google Scholar] [CrossRef]
- Faulk, C.; Barks, A.; Liu, K.; Goodrich, J.M.; Dolinoy, D.C. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene gene regulation in weanling mice. Epigenomics 2014, 5, 487–500. [Google Scholar] [CrossRef]
- Leasure, J.L.G.A.; Chaney, S.; Johnson, J.E., Jr.; Pothakos, K.; Lau, Y.S.; Fox, D. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008, 116, 355–361. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Baig, J.A.; Sarfraz, R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2014, 1, 169–176. [Google Scholar] [CrossRef]
- Moon, S.S. Association of lead, mercury and cadmium with diabetes in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabetes Med. 2013, 30, e143–e148. [Google Scholar] [CrossRef]
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2004; Available online: https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e/view/a1cffdde-243e-41c3-be98-885f6d4dcb29/standard_quality_assessment_criteria_for_evaluating_primary_research_papers_from_a_variety_of_fields.pdf (accessed on 11 June 2020).
- Fernández-Espínola, C.; Abad Robles, M.T.; Giménez Fuentes-Guerra, F.J. Small-Sided Games as a Methodological Resource for Team Sports Teaching: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1884. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Buck, C.; Freiberger, E.; Murphy, M.; Brug, J.; Cardon, G.; O’Donoghue, G.; Pigeot, I.; Oppert, J.-M.; DEDIPAC Consortium. Systematic literature review of determinants of sedentary behaviour in older adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 127. [Google Scholar] [CrossRef]
- Serra-Olivares, J.; González-Víllora, S.; García-López, L.M.; Araújo, D. Game-Based Approaches’ Pedagogical Principles: Exploring Task Constraints in Youth Soccer. J. Hum. Kinet. 2015, 46, 251–261. [Google Scholar] [CrossRef]
- Beyer, W.N.; Franson, J.C.; French, J.B.; May, T.; Rattner, B.A.; Shearn-Bochsler, V.I.; Warner, S.E.; Weber, J.; Mosby, D. Toxic Exposure of Songbirds to Lead in the Southeast Missouri Lead Mining District. Arch. Environ. Contam. Toxicol. 2013, 65, 598–610. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Zota, A.R.; Amarasiriwardena, C.J.; Hopkins, M.R.; Schwartz, J.; Hu, H.; Wright, R.O. Maternal Arsenic Exposure and Impaired Glucose Tolerance during Pregnancy. Environ. Health Perspect. 2009, 117, 1059–1064. [Google Scholar] [CrossRef]
- Malcoe, L.H.; Lynch, R.A.; Kegler, M.C.; Skaggs, V.J. Lead Sources, Behaviors, and Socioeconomic Factors in Relation to Blood Lead of Native American and White Children: A Community-Based Assessment of a Former Mining Area. Environ. Health Perspect. 2002, 110, 221–231. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Hu, S.C.; Drake, K.D.; Jim, R. Potential health impacts of heavy-metal exposure at the Tar Creek Superfund site, Ottawa County, Oklahoma. Environ. Geochem. Health 2009, 31, 47. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Carpenter, J.W.; Nietfeld, J.C.; Miesner, J.F. Adverse health effects in Canada geese (Branta canadensis) associated with waste from zinc and lead mines in the Tri-State Mining District (Kansas, Oklahoma, and Missouri, USA). J. Wildl. Dis. 2011, 47, 650–660. [Google Scholar] [CrossRef]
- Brumbaugh, W.G.; Schmitt, C.J.; May, T.W. Concentrations of cadmium, lead, and zinc in fish from mining-influenced waters of northeastern Oklahoma: Sampling of blood, carcass, and liver for aquatic biomonitoring. Arch. Environ. Contam. Toxicol. 2005, 49, 76–88. [Google Scholar] [CrossRef]
- Garvin, E.M.; Bridge, C.F.; Garvin, M.S. Edible wild plants growing in contaminated floodplains: Implications for the issuance of tribal consumption advisories within the Grand Lake watershed of northeastern Oklahoma, USA. Environ. Geochem. Health 2018, 40, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.J.; Whyte, J.J.; Brumbaugh, W.G.; Tillitt, D.E. Biochemical effects of lead, zinc, and cadmium from mining on fish in the Tri-States District of northeastern Oklahoma, USA. Environ. Toxicol. Chem. 2005, 24, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Struckhoff, M.A.; Stroh, E.D.; Grabner, K.W. Effects of mining-associated lead and zinc soil contamination on native floristic quality. J. Environ. Manag. 2013, 119, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.L.; Janz, D.M. Tissue-specific HSP70 levels and reproductive physiological responses in fishes inhabiting a metal-contaminated creek. Arch. Environ. Contam. Toxicol. 2003, 45, 110–120. [Google Scholar] [CrossRef]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H.; Oak Ridge National Lab; Lawrence Berkeley National Lab. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef]
- Phelps, K.L.; McBee, K. Ecological Characteristics of Small Mammal Communities at a Superfund Site. Am. Midl. Nat. 2009, 161, 57–68. [Google Scholar] [CrossRef]
- Besser, J.M.; Ingersoll, C.G.; Brumbaugh, W.G.; Kemble, N.E.; May, T.W.; Wang, N.; MacDonald, D.D.; Roberts, A.D. Toxicity of sediments from lead-zinc mining areas to juvenile freshwater mussels (Lampsilis siliquoidea) compared to standard test organisms. Environ. Toxicol. Chem. 2015, 34, 626–639. [Google Scholar] [CrossRef]
- Hays, K.A.; McBee, K. Population Demographics of Red-Eared Slider Turtles (Trachemys scripta) from Tar Creek Superfund Site. South Am. J. Herpetol. 2010, 44, 441–446. [Google Scholar] [CrossRef]
- Schmitt, C.J.; Brumbaugh, W.G.; Linder, G.L.; Hinck, J.E. A screening-level assessment of lead, cadmium, and zinc in fish and crayfish from Northeastern Oklahoma, USA. Environ. Geochem. Health 2006, 28, 445–471. [Google Scholar] [CrossRef]
- Gasser, U.G.; Walker, W.J.; Dahlgren, R.A.; Borch, R.S.; Burau, R.G. Lead Release from Smelter and Mine Waste Impacted Materials under Simulated Gastric Conditions and Relation to Speciation. Environ. Sci. Technol. 1996, 30, 761–769. [Google Scholar] [CrossRef]
- Cook, M.; Chappell, W.R.; Hoffman, R.E.; Mangione, E.J. Assessment of blood lead levels in children living in a historic mining and smelting community. Am. J. Epidemiol. 1993, 137, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, T.; Sokołowska, D.; Kulka, E. Health risk assessment in children exposed to lead compounds in the vicinity of mine-smelter plant “Orzeł Biały”. Pol. J. Occup. Med. Environ. Health 1993, 6, 71. [Google Scholar] [PubMed]
- Gulson, B.L.; Davis, J.J.; Mizon, K.J.; Korsch, M.J.; Law, A.J.; Howarth, D. Lead Bioavailability in the Environment of Children: Blood Lead Levels in Children Can Be Elevated in a Mining Community. Arch. Environ. Health Int. J. 1994, 49, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Murgueytio, A.M.; Evans, R.G.; Roberts, D. Relationship between soil and dust lead in a lead mining area and blood lead levels. J. Expo. Anal. Environ. Epidemiol. 1998, 8, 173. [Google Scholar]
- Kozlov, M.W.T. Population densities and diversity of Calliphoridae (Diptera) around a nickel-copper smelter at Monchegorsk, Northwestern Russia. Èntomol. Fenn. 2002, 13, 98–104. [Google Scholar] [CrossRef]
- Verneaux, J.; Schmitt, A.; Verneaux, V.; Prouteau, C. Benthic insects and fish of the Doubs River system: Typological traits and the development of a species continuum in a theoretically extrapolated watercourse. Hydrobiologia 2003, 490, 63–74. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Recycling, Reuse and Rehabilitation of Mine Wastes. Entomol. Fenn. 2011, 13, 98–104. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals--concepts and applications. Chemosphere 2013, 91, 869. [Google Scholar] [CrossRef]
- Bansah, K.J.A. Factors Contributing to Karst Development in Southwestern Missouri, USA. In Proceedings of the Symposium on the Application of Geophysics to Engineering and Environmental Problems 2017, Denver, CO, USA, 19–23 March 2017; p. 399. [Google Scholar]
- Steele-Valentín, K.M.; Padilla, I.Y. Assessment of potential exposure pathways in karst groundwater systems in Vega Alta, Puerto Rico using geographic information systems. In Proceedings of the Seventh LACCEI Latin American and Caribbean Conference for Engineering and Technology (LACCEI’2009)—Energy and Technology for the Americas: Education, Innovation, Technology and Practice, San Cristóbal, Venezuela, 2–5 June 2009; Available online: http://www.laccei.org/LACCEI2009-Venezuela/p223.pdf (accessed on 30 August 2020).
- Deeb, R.; Hawley, E.; Kell, L.; O’Laskey, R. Assessing Alternative Endpoints for Groundwater Remediation at Contaminated Sites; ESTCP Project Report ER-200832 Environmental Security Technology Certification Program (ESTCP); ARCADIS/Malcolm Pirnie, Inc.: Arlington, VA, USA, 2011; p. 233. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a544571.pdf (accessed on 1 September 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).