The Impact of Water Intrusion on Pathogenic Vibrio Species to Inland Brackish Waters of China
Abstract
:1. Introduction
2. Materials and Methods
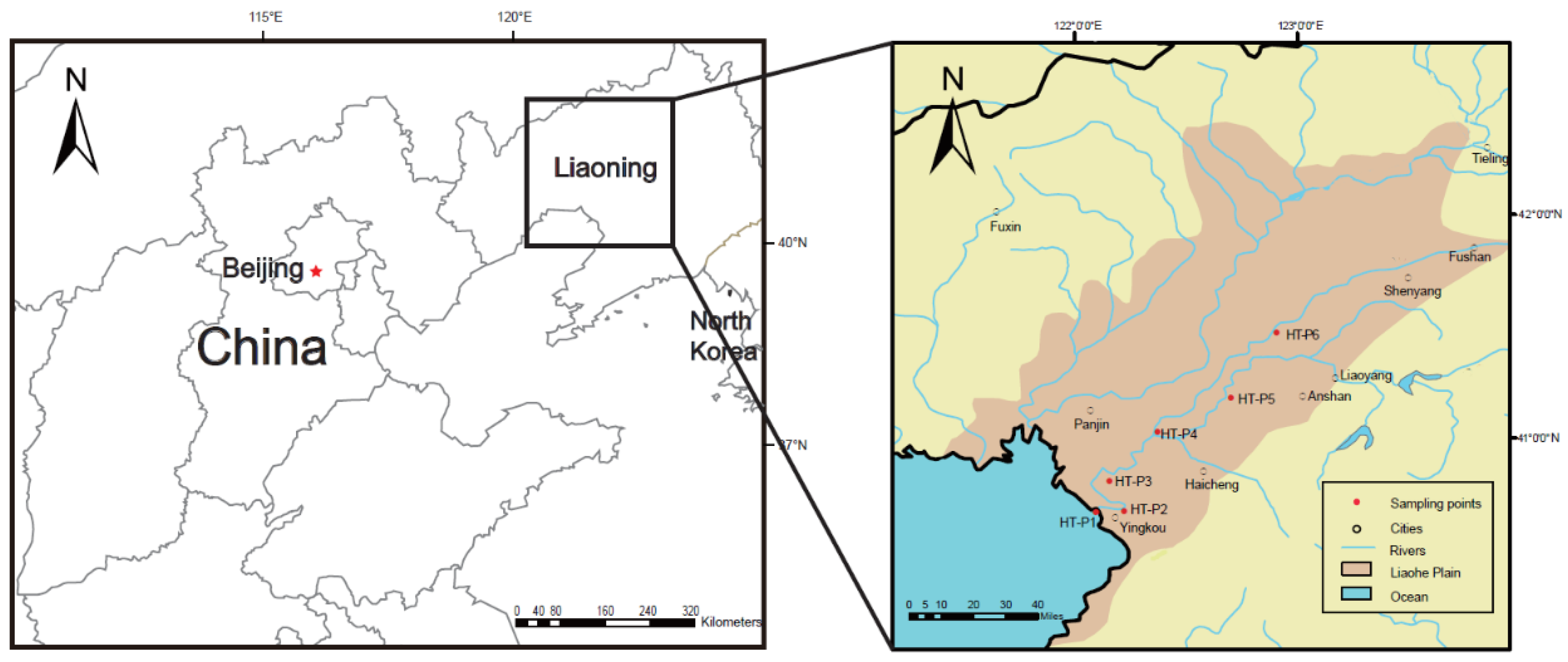
2.1. Study Area and Sample Collection
2.2. Abundance Analysis of V. cholerae, V. parahaemolyticus, and V. vulnificus
2.3. MLST Typing of V. parahaemolyticus and V. cholerae
2.4. Detection of Virulence Genes
2.5. Statistical Analysis
3. Results
3.1. Characterization of Seawater Intrusion in the HT River
3.2. The Abundance of V. cholerae, V. parahaemolyticus, and V. vulnificus
3.3. Relationship between Pathogenic Vibrio Species and Environmental Parameters
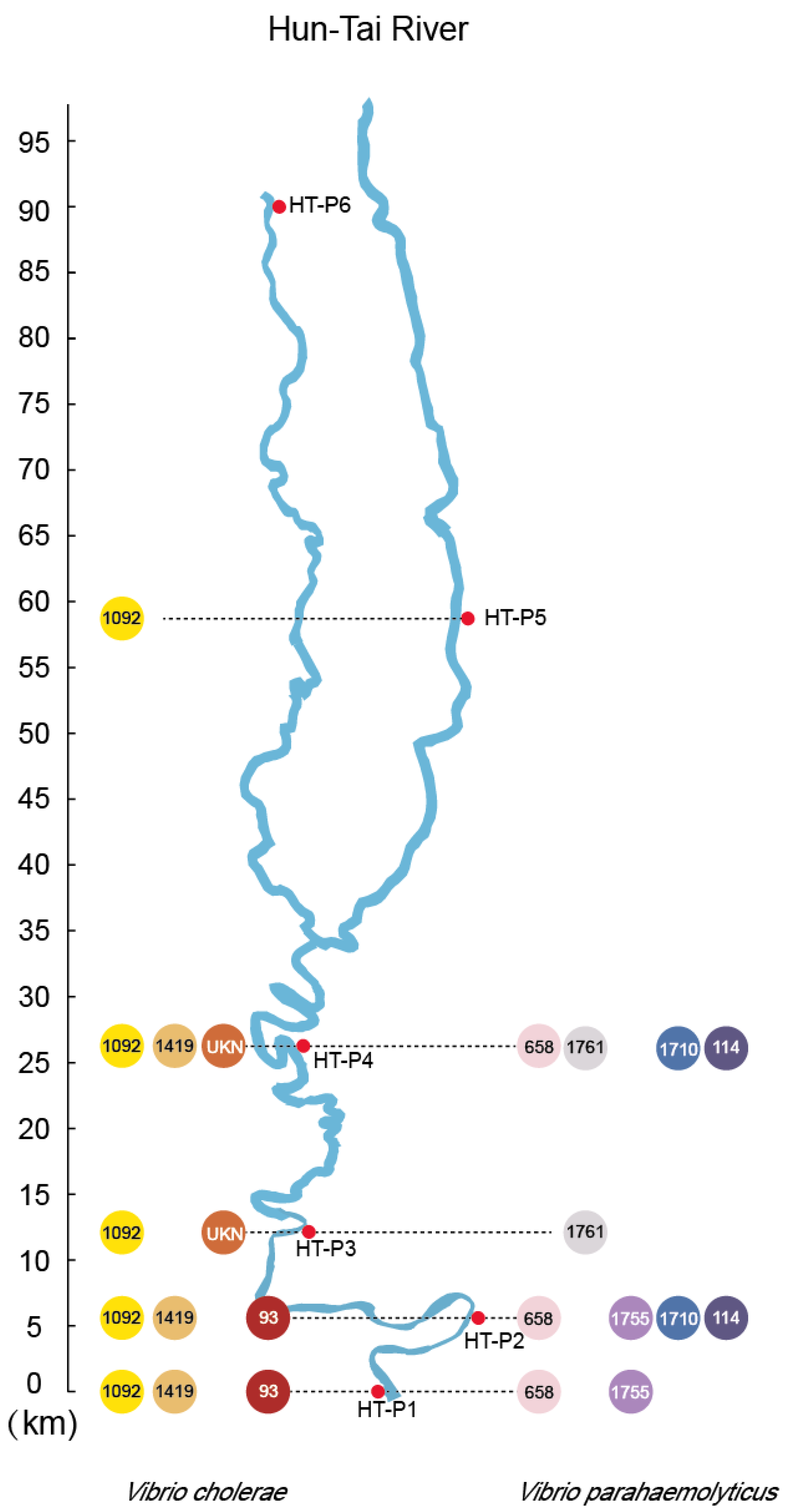
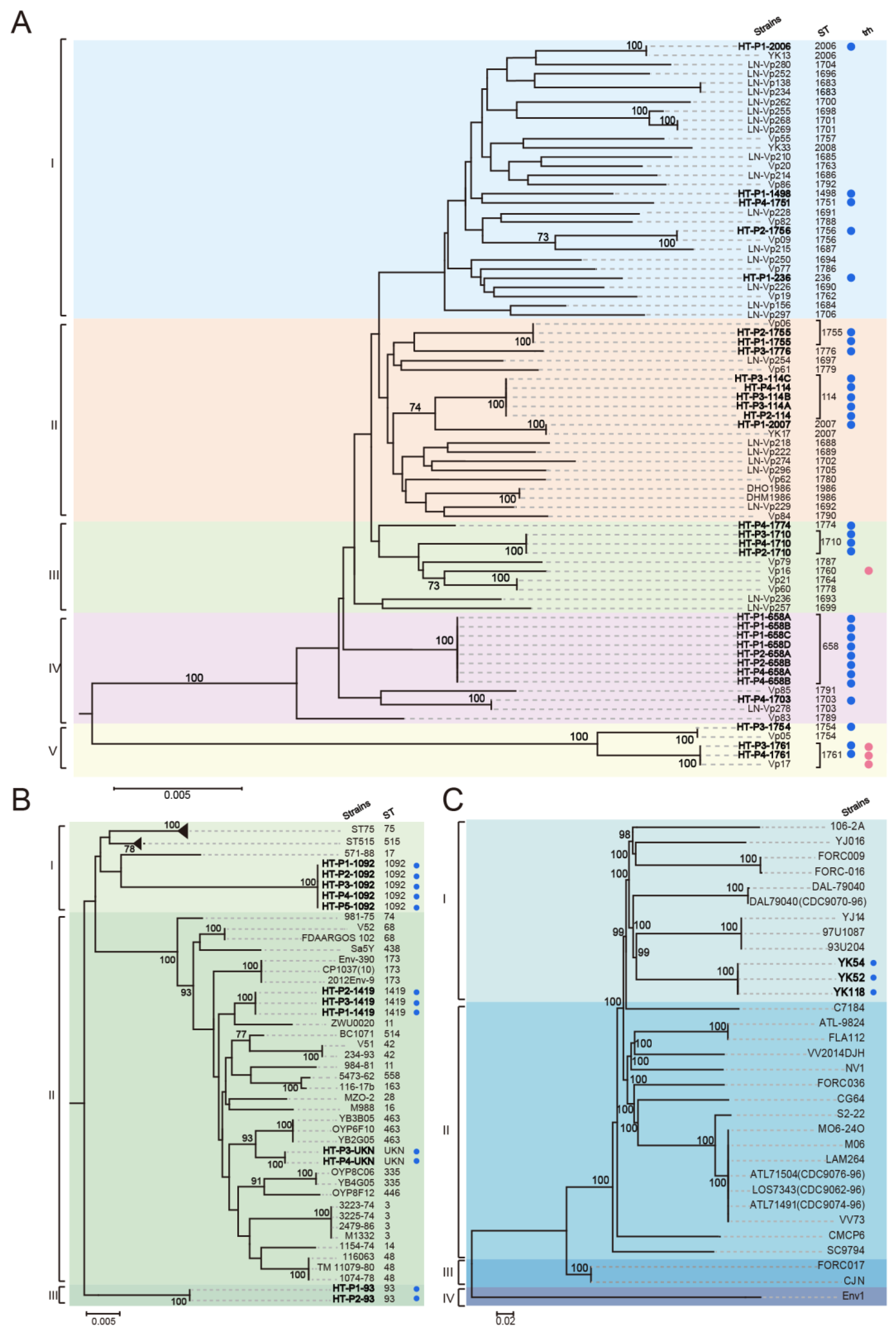
3.4. Detection and MLST of Pathogenic Vibrio spp. in the HT River
3.5. Characterization of Virulence Genes and VSP-I/II Clusters
3.6. Antibiotic Resistance Profiles
4. Discussion
4.1. Seawater Intrusion Potentially Impacts on the Distribution of Pathogenic Vibrio Species
4.2. Identification of Virulence Factors in Pathogenic Vibrio spp. and Its Implication for Risk Management of the River
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, D.; Colwell, R.R. Vibrio ecology, pathogenesis, and evolution. Front. Microbiol. 2014, 5, 256. [Google Scholar] [CrossRef] [PubMed]
- Didelot, X.; Pang, B.; Zhou, Z.; McCann, A.; Ni, P.; Li, D.; Achtman, M.; Kan, B. The role of China in the global spread of the current cholera pandemic. PLoS Genet. 2015, 11, e1005072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letchumanan, V.; Yin, W.F.; Lee, L.H.; Chan, K.G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Froelich, B.; Oliver, J. Orientation of mannitol related genes can further differentiate strains of Vibrio vulnificus possessing the vcgC allele. Adv. Stud. Biol. 2011, 3, 151–160. [Google Scholar]
- Ceccarelli, D.; Chen, A.; Hasan, N.A.; Rashed, S.M.; Huq, A.; Colwell, R.R. Non-O1/non-O139 Vibrio cholerae carrying multiple virulence factors and V. cholerae O1 in the Chesapeake Bay, Maryland. Appl. Environ. Microbiol. 2015, 81, 1909–1918. [Google Scholar] [CrossRef] [Green Version]
- Esteves, K.; Hervio-Heath, D.; Mosser, T.; Rodier, C.; Tournoud, M.G.; Jumas-Bilak, E.; Colwell, R.R.; Monfort, P. Rapid proliferation of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae during freshwater flash floods in French Mediterranean coastal lagoons. Appl. Environ. Microbiol. 2015, 81, 7600–7609. [Google Scholar] [CrossRef] [Green Version]
- Voynova, Y.G.; Brix, H.; Petersen, W.; Weigelt-Krenz, S.; Scharfe, M. Extreme flood impact on estuarine and coastal biogeochemistry: The 2013 Elbe flood. Biogeosciences 2017, 14, 541–557. [Google Scholar] [CrossRef] [Green Version]
- Torresi, M.; Sperandii, A.; Ricci, L.; Prencipe, V.; Migliorati, G.; Pomilio, F. Detection and characterisation of potentially pathogenic species of Vibrio in the Vibrata river, Abruzzo Region, Italy. Vet. Ital. 2018, 54, 125–135. [Google Scholar]
- Mo, Q.H.; Wang, H.B.; Tan, H.; An, S.L.; Feng, Z.L.; Wang, Q.; Lin, J.C.; Yang, Z. Optimization and head-to-head comparison of MISSR-PCR, ERIC-PCR, RAPD and 16S rRNA evolutionary clock for the genotyping of Vibrio cholerae isolated in China. Indian. J. Med. Microbiol. 2015, 33, 516–523. [Google Scholar]
- Chahorm, K.; Prakitchaiwattana, C. Application of Reverse Transcriptase-PCR-DGGE as a rapid method for routine determination of Vibrio spp. in foods. Int. J. Food Microbiol. 2018, 264, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Escalona, N.; Martinez-Urtaza, J.; Romero, J.; Espejo, R.T.; Jaykus, L.A.; DePaola, A. Determination of molecular phylogenetics of Vibrio parahaemolyticus strains by multilocus sequence typing. J. Bacteriol. 2008, 190, 2831–2840. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Escalona, N.; Jolley, K.A.; Reed, E.; Martinez-Urtaza, J. Defining a Core Genome Multilocus Sequence Typing Scheme for the Global Epidemiology of Vibrio parahaemolyticus. J. Clin. Microbiol. 2017, 55, 1682–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Hao, J.; Jin, S.; Wu, K.; Wang, Y.; Ye, S.; Liu, Y.; Li, R. A Human Intestinal Infection Caused by a Novel Non-O1/O139 Vibrio cholerae Genotype and Its Dissemination Along the River. Front. Public Health 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Ren, Z.; Zhang, M.; Liu, X.; Peng, W. Sediment heavy metals and benthic diversities in Hun-Tai River, northeast of China. Environ. Sci. Pollut.Res. Int. 2017, 24, 10662–10673. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Abad, V.; Ansede-Bermejo, J.; Rodriguez-Castro, A.; Martinez-Urtaza, J. Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. Int. J. Food Microbiol. 2009, 129, 229–236. [Google Scholar] [CrossRef]
- Neogi, S.B.; Chowdhury, N.; Asakura, M.; Hinenoya, A.; Haldar, S.; Saidi, S.M.; Kogure, K.; Lara, R.J.; Yamasaki, S. A highly sensitive and specific multiplex PCR assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. Lett. Appl. Microbiol. 2010, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Natividad-Bonifacio, I.; Fernandez, F.J.; Quinones-Ramirez, E.I.; Curiel-Quesada, E.; Vazquez-Salinas, C. Presence of virulence markers in environmental Vibrio vulnificus strains. J. Appl. Microbiol. 2013, 114, 1539–1546. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Santini, Y.; Gruter, D.; Stettler, R.; Bachofen, R. Bacterial diversity and community composition in the chemocline of the meromictic alpine Lake Cadagno as revealed by 16S rDNA analysis. FEMS Microbiol. Ecol. 2000, 31, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Han, K.H.; Choi, S.Y.; Lucas, M.; Mondlane, C.; Ansaruzzaman, M.; Nair, G.B.; Sack, D.A.; von Seidlein, L.; Clemens, J.D.; et al. Multilocus sequence typing (MLST) analysis of Vibrio cholerae O1 El Tor isolates from Mozambique that harbour the classical CTX prophage. J. Med. Microbiol. 2006, 55, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Reynaud, Y.; Pitchford, S.; De Decker, S.; Wikfors, G.H.; Brown, C.L. Molecular typing of environmental and clinical strains of Vibrio vulnificus isolated in the northeastern USA. PLoS ONE 2013, 8, e83357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, J.; Ohashi, T.; Nishimura, N.; Shirasaki, Y.; Ozaki, H.; Fukushima, S.; Takano, J.; Nishibuchi, M.; Takeda, Y. Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell. Probes. 1992, 6, 477–487. [Google Scholar] [CrossRef]
- Tarr, C.L.; Patel, J.S.; Puhr, N.D.; Sowers, E.G.; Bopp, C.A.; Strockbine, N.A. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J. Clin. Microbiol. 2007, 45, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noriea, N.R.; Johnson, C.N.; Griffitt, K.J.; Grimes, D.J. Distribution of type III secretion systems in Vibrio parahaemolyticus from the northern Gulf of Mexico. J. Appl. Microbiol. 2010, 109, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Nandi, B.; Nandy, R.K.; Mukhopadhyay, S.; Nair, G.B.; Shimada, T.; Ghose, A.C. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 2000, 38, 4145–4151. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.N.; Chun, J.; Huq, A.; Sack, R.B.; Colwell, R.R. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. J. Appl. Microbiol. 2001, 67, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Ghosh, K.; Raychoudhuri, A.; Chowdhury, G.; Bhattacharya, M.K.; Mukhopadhyay, A.K.; Ramamurthy, T.; Bhattacharya, S.K.; Klose, K.E.; Nandy, R.K. Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J. Clin. Microbiol. 2009, 47, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Y.; Yang, W.M.; Li, S.S. Analysis of virulence and drug resistance of clinical isolates of non-O1/O139 Vibrio cholerae. Euro Surveill. 2016, 31, 517–521. [Google Scholar]
- O’Shea, Y.A.; Reen, F.J.; Quirke, A.M.; Boyd, E.F. Evolutionary genetic analysis of the emergence of epidemic Vibrio cholerae isolates on the basis of comparative nucleotide sequence analysis and multilocus virulence gene profiles. J. Clin. Microbiol. 2004, 42, 4657–4671. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, H.; Seki, R. Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol. Ecol. 2004, 48, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.S.; Hite, M.F.; Oliver, J.D. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. J. Appl. Microbiol. 2003, 69, 3526–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Roberts, N.; Singleton, F.L.; Attwell, R.W.; Grimes, D.J.; Colwell, R.R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 1982, 8, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chu, Y.; Xie, G.; Li, F.; Wang, L.; Huang, J.; Zhai, Y.; Yao, L. Antimicrobial resistance, virulence and genetic relationship of Vibrio parahaemolyticus in seafood from coasts of Bohai Sea and Yellow Sea, China. Int. J. Food Microbiol. 2019, 290, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Nishibuchi, M.; Fasano, A.; Russell, R.G.; Kaper, J.B. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect. Immun. 1992, 60, 3539–3545. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef] [Green Version]
- Okada, N.; Iida, T.; Park, K.S.; Goto, N.; Yasunaga, T.; Hiyoshi, H.; Matsuda, S.; Kodama, T.; Honda, T. Identification and characterization of a novel type III secretion system in trh-positive Vibrio parahaemolyticus strain TH3996 reveal genetic lineage and diversity of pathogenic machinery beyond the species level. Infect. Immun. 2009, 77, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Yu, P.; Zheng, H.; Gu, W.; He, W.; Tang, Y.; Wang, Y.; Dong, Y.; Peng, X.; She, Q.; et al. Comparative genomics for non-O1/O139 Vibrio cholerae isolates recovered from the Yangtze River Estuary versus V. cholerae representative isolates from serogroup O1. Mol. Genet. Genomic Med. 2019, 294, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Arredondo, P.; Heuser, J.E.; Akopyants, N.S.; Morisaki, J.H.; Giono-Cerezo, S.; Enriquez-Rincon, F.; Berg, D.E. Cell vacuolation caused by Vibrio cholerae hemolysin. Infect. Immun. 2001, 69, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Taviani, E.; Grim, C.J.; Choi, J.; Chun, J.; Haley, B.; Hasan, N.A.; Huq, A.; Colwell, R.R. Discovery of novel Vibrio cholerae VSP-II genomic islands using comparative genomic analysis. FEMS Microbiol. Lett. 2010, 308, 130–137. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Wang, Y.; Jin, S.; Jiang, Z.; Ye, S.; Li, R. Delineating the origins of the multidrug-resistant pathogens in ornamental fish farms by multilocus sequence typing and identification of a novel multidrug-resistant plasmid. Can. J. Microbiol. 2019, 65, 551–562. [Google Scholar] [CrossRef]


| Site | Spring | Summer | Autumn | |||
|---|---|---|---|---|---|---|
| WT (°C) | Salinity (‰) | WT (°C) | Salinity (‰) | WT (°C) | Salinity (‰) | |
| HT-P1 | 13.4 ± 3.3 | 26.8 ± 1.7 | 27.8 ± 1.3 | 24.7 ± 2.4 | 15.25 ± 5.1 | 32.3 ± 1.5 |
| HT-P2 | 13.4 ± 7.6 | 8.78 ± 1.7 | 27.5 ± 1.4 | 4.67 ± 1.6 | 14.4 ± 4.3 | 8.08 ± 1.8 |
| HT-P3 | 12.57 ± 1.8 | 4.3 ± 0.78 | 26.8 ± 1.3 | 1.1 ± 0.8 | 15.47 ± 5.6 | 3.1 ± 1.21 |
| HT-P4 | 13.67 ± 3.1 | 1.05 ± 0.65 | 24.9 ± 3.1 | 0.45 ± 0.2 | 14.4 ± 5.8 | 0.41 ± 0.09 |
| HT-P5 | 13.27 ± 3.2 | 0.53 ± 0.25 | 27 ± 1.2 | 0.12 ± 0.21 | 13.6 ± 3.1 | 0.23 ± 0.12 |
| HT-P6 | 13.1 ± 3.2 | 0 | 26.4 ± 3.2 | 0 | 13.1 ± 3.2 | 0 |
| Variables | V. cholerae | V. parahaemolyticus | |
|---|---|---|---|
| Salinity | rs | 0.499 ** | 0.332 * |
| p | <0.01 | 0.038 | |
| Temperature | rs | 0.667 ** | 0.698 ** |
| p | <0.01 | <0.01 | |
| V. cholerae | rs | / | 0.855 ** |
| p | / | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Fu, S.; Yang, Q.; Hao, J.; Zhou, C.; Liu, Y. The Impact of Water Intrusion on Pathogenic Vibrio Species to Inland Brackish Waters of China. Int. J. Environ. Res. Public Health 2020, 17, 6781. https://doi.org/10.3390/ijerph17186781
Wang Q, Fu S, Yang Q, Hao J, Zhou C, Liu Y. The Impact of Water Intrusion on Pathogenic Vibrio Species to Inland Brackish Waters of China. International Journal of Environmental Research and Public Health. 2020; 17(18):6781. https://doi.org/10.3390/ijerph17186781
Chicago/Turabian StyleWang, Qingyao, Songzhe Fu, Qian Yang, Jingwei Hao, Can Zhou, and Ying Liu. 2020. "The Impact of Water Intrusion on Pathogenic Vibrio Species to Inland Brackish Waters of China" International Journal of Environmental Research and Public Health 17, no. 18: 6781. https://doi.org/10.3390/ijerph17186781




