A 15 Year Evaluation of West Nile Virus in Wisconsin: Effects on Wildlife and Human Health
Abstract
:1. Introduction
2. Methods
2.1. Study Location
2.2. WNV Case Data
2.3. Final Case Definition
2.4. Climate Data
2.5. Elevation and Land Cover
2.6. Statistical Methods
2.7. Geospatial Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Environmental Parameter | Month | Year | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | ||
| Average Accumulation (in.) | Jan | 0.45 | 0.48 | 0.33 | 0.30 | 0.39 | 0.55 | 0.42 | 0.36 | 0.24 | 0.34 | 0.30 | 0.44 | 0.41 | 0.27 | 0.35 | 0.37 |
| Feb | 0.39 | 0.58 | 0.37 | 0.47 | 0.53 | 0.42 | 0.32 | 0.39 | 0.47 | 0.80 | 0.44 | 0.54 | 0.43 | 0.28 | 0.28 | 0.48 | |
| Mar | 0.55 | 0.56 | 0.64 | 0.70 | 0.59 | 0.66 | 0.71 | 0.54 | 0.65 | 0.74 | 0.62 | 0.87 | 0.54 | 0.48 | 0.63 | 0.81 | |
| Apr | 0.99 | 0.92 | 0.89 | 0.92 | 0.94 | 1.02 | 0.89 | 0.93 | 0.91 | 1.01 | 0.89 | 0.92 | 0.82 | 0.89 | 0.93 | 0.87 | |
| May | 1.13 | 1.01 | 1.13 | 1.20 | 1.02 | 1.15 | 1.12 | 1.03 | 1.07 | 1.10 | 1.04 | 1.20 | 1.12 | 1.08 | 1.13 | 1.11 | |
| Jun | 1.25 | 1.31 | 1.19 | 1.23 | 1.27 | 1.22 | 1.25 | 1.30 | 1.19 | 1.32 | 1.23 | 1.24 | 1.27 | 1.34 | 1.23 | 1.30 | |
| Jul | 1.27 | 1.32 | 1.27 | 1.26 | 1.33 | 1.38 | 1.28 | 1.29 | 1.15 | 1.41 | 1.35 | 1.36 | 1.24 | 1.20 | 1.25 | 1.34 | |
| Aug | 1.31 | 1.27 | 1.28 | 1.19 | 1.24 | 1.28 | 1.38 | 1.19 | 1.25 | 1.31 | 1.23 | 1.24 | 1.25 | 1.30 | 1.22 | 1.33 | |
| Sept | 1.12 | 1.21 | 1.13 | 1.16 | 1.22 | 1.07 | 1.14 | 1.12 | 1.12 | 1.16 | 1.09 | 1.10 | 1.14 | 1.11 | 1.21 | 1.25 | |
| Oct | 0.89 | 0.84 | 0.90 | 0.95 | 0.96 | 0.86 | 1.03 | 0.89 | 0.88 | 0.96 | 0.94 | 0.92 | 0.91 | 0.91 | 0.93 | n/A | |
| Nov | 0.85 | 0.60 | 0.69 | 0.71 | 0.75 | 0.69 | 0.60 | 0.63 | 0.73 | 0.67 | 0.72 | 0.67 | 0.63 | 0.53 | 0.82 | n/A | |
| Dec | 0.54 | 0.49 | 0.51 | 0.46 | 0.41 | 0.55 | 0.43 | 0.42 | 0.44 | 0.40 | 0.52 | 0.52 | 0.33 | 0.47 | 0.68 | n/A | |
| Maximum Accumulation (in.) | Jan | 0.99 | 0.66 | 0.70 | 0.57 | 0.81 | 1.02 | 0.94 | 0.80 | 0.51 | 0.89 | 0.48 | 0.70 | 0.89 | 0.59 | 0.59 | 0.69 |
| Feb | 1.01 | 0.97 | 0.70 | 0.81 | 0.88 | 0.62 | 0.90 | 0.80 | 0.89 | 1.42 | 0.97 | 1.29 | 0.93 | 1.01 | 0.52 | 0.84 | |
| Mar | 0.70 | 1.05 | 1.03 | 1.35 | 1.19 | 1.07 | 1.26 | 0.83 | 1.29 | 1.04 | 1.39 | 1.17 | 0.97 | 0.99 | 0.89 | 1.99 | |
| Apr | 1.58 | 1.37 | 1.67 | 1.42 | 1.39 | 1.35 | 1.31 | 1.63 | 1.57 | 1.36 | 1.45 | 1.56 | 1.66 | 1.64 | 1.44 | 1.22 | |
| May | 1.47 | 1.30 | 1.69 | 1.87 | 1.38 | 1.62 | 1.50 | 1.71 | 1.42 | 1.47 | 1.50 | 1.73 | 1.69 | 1.81 | 1.90 | 1.72 | |
| Jun | 2.20 | 1.95 | 1.55 | 1.79 | 1.55 | 1.51 | 1.75 | 2.47 | 1.69 | 1.84 | 1.82 | 1.81 | 2.04 | 1.98 | 1.88 | 2.16 | |
| Jul | 1.61 | 1.91 | 1.64 | 1.86 | 2.10 | 1.72 | 2.02 | 1.99 | 1.48 | 2.74 | 1.76 | 1.72 | 1.75 | 1.61 | 2.08 | 1.90 | |
| Aug | 2.00 | 1.94 | 1.57 | 1.56 | 1.64 | 1.95 | 2.45 | 1.52 | 2.09 | 2.13 | 1.56 | 1.49 | 1.74 | 1.97 | 1.81 | 1.79 | |
| Sept | 2.02 | 2.25 | 1.70 | 1.82 | 1.58 | 1.53 | 1.55 | 1.33 | 1.43 | 2.08 | 1.59 | 1.54 | 1.53 | 1.67 | 1.91 | 2.29 | |
| Oct | 1.35 | 1.73 | 1.33 | 1.48 | 1.99 | 1.48 | 1.64 | 1.43 | 1.46 | 1.53 | 1.49 | 1.50 | 1.46 | 1.90 | 1.54 | n/A | |
| Nov | 1.26 | 0.88 | 1.60 | 1.02 | 1.43 | 1.13 | 0.78 | 1.05 | 0.93 | 0.95 | 1.14 | 1.31 | 1.26 | 1.07 | 1.57 | n/A | |
| Dec | 1.07 | 0.86 | 0.99 | 0.81 | 0.65 | 0.98 | 1.30 | 0.75 | 1.22 | 0.93 | 0.97 | 0.88 | 0.67 | 0.94 | 1.91 | n/A | |
| Total Accumulation (in.) | Jan | 13.86 | 14.73 | 10.23 | 9.23 | 12.06 | 16.98 | 13.00 | 11.10 | 7.53 | 10.13 | 9.32 | 13.74 | 12.82 | 8.22 | 10.72 | 11.44 |
| Feb | 10.86 | 16.14 | 10.42 | 13.67 | 14.79 | 11.79 | 9.00 | 10.88 | 13.03 | 23.88 | 12.27 | 15.00 | 12.10 | 7.76 | 7.73 | 14.28 | |
| Mar | 17.16 | 17.21 | 19.72 | 21.67 | 18.17 | 20.61 | 22.11 | 16.87 | 20.16 | 23.07 | 19.08 | 26.86 | 16.82 | 14.90 | 19.51 | 25.14 | |
| Apr | 29.72 | 27.52 | 26.65 | 27.54 | 28.12 | 30.48 | 26.63 | 28.02 | 27.22 | 30.30 | 26.65 | 27.66 | 24.65 | 27.59 | 28.03 | 26.11 | |
| May | 35.13 | 31.35 | 35.11 | 37.26 | 31.62 | 35.68 | 34.67 | 31.82 | 33.04 | 34.11 | 32.16 | 37.08 | 34.67 | 33.53 | 34.90 | 34.54 | |
| Jun | 37.42 | 39.18 | 35.69 | 36.94 | 38.13 | 36.63 | 37.47 | 39.11 | 35.65 | 39.47 | 37.01 | 37.27 | 38.07 | 40.23 | 36.91 | 38.92 | |
| Jul | 39.30 | 40.98 | 39.23 | 39.21 | 41.10 | 42.66 | 39.76 | 40.05 | 35.77 | 43.73 | 41.80 | 42.11 | 38.51 | 37.09 | 38.85 | 41.51 | |
| Aug | 40.50 | 39.39 | 39.58 | 36.81 | 38.37 | 39.77 | 42.82 | 36.74 | 38.74 | 40.75 | 38.25 | 38.49 | 38.68 | 40.26 | 37.76 | 41.16 | |
| Sept | 33.84 | 36.21 | 33.82 | 34.74 | 36.46 | 32.16 | 34.07 | 33.58 | 33.47 | 34.82 | 32.76 | 33.08 | 34.23 | 33.38 | 36.44 | 37.56 | |
| Oct | 27.65 | 25.96 | 27.76 | 29.49 | 29.65 | 26.56 | 31.84 | 27.55 | 27.13 | 29.65 | 29.16 | 28.61 | 28.35 | 28.10 | 28.92 | n/A | |
| Nov | 25.55 | 17.93 | 20.57 | 21.24 | 22.35 | 20.68 | 18.12 | 19.04 | 21.78 | 19.99 | 21.74 | 20.16 | 18.93 | 15.88 | 24.45 | n/A | |
| Dec | 16.74 | 15.06 | 15.67 | 14.36 | 12.77 | 17.14 | 13.38 | 13.07 | 13.78 | 12.26 | 16.04 | 16.11 | 10.26 | 14.53 | 21.01 | n/A | |
| Mean Temperature (°F) | Jan | 18.38 | 22.83 | 13.27 | 10.76 | 13.71 | 26.38 | 19.29 | 14.03 | 6.21 | 14.32 | 11.79 | 19.97 | 16.26 | 5.91 | 14.72 | 15.85 |
| Feb | 14.69 | 25.04 | 13.33 | 19.84 | 23.26 | 17.33 | 11.03 | 12.80 | 19.03 | 18.55 | 17.39 | 24.51 | 16.04 | 7.09 | 7.78 | 21.29 | |
| Mar | 26.35 | 23.86 | 27.56 | 31.49 | 25.91 | 29.46 | 32.60 | 23.85 | 28.23 | 34.46 | 26.27 | 42.00 | 22.54 | 20.67 | 28.77 | 34.52 | |
| Apr | 43.78 | 39.75 | 39.49 | 41.58 | 44.67 | 45.37 | 39.88 | 40.06 | 40.37 | 46.16 | 38.96 | 42.11 | 36.02 | 37.10 | 41.51 | 39.90 | |
| May | 52.78 | 47.03 | 49.94 | 49.28 | 48.81 | 51.97 | 54.97 | 48.46 | 50.48 | 53.83 | 50.31 | 55.49 | 51.39 | 51.23 | 52.51 | 51.51 | |
| Jun | 60.28 | 61.93 | 57.56 | 56.99 | 64.72 | 60.43 | 61.92 | 59.69 | 59.23 | 60.78 | 59.27 | 62.52 | 59.70 | 61.29 | 59.64 | 61.37 | |
| Jul | 65.17 | 67.21 | 63.76 | 61.79 | 66.36 | 67.73 | 64.64 | 64.09 | 59.37 | 66.27 | 68.57 | 70.36 | 63.91 | 61.44 | 63.12 | 66.17 | |
| Aug | 65.55 | 62.68 | 64.70 | 58.00 | 63.40 | 63.05 | 64.56 | 62.14 | 60.73 | 65.96 | 63.44 | 62.84 | 63.80 | 62.55 | 61.42 | 65.06 | |
| Sept | 53.48 | 57.83 | 55.20 | 58.22 | 59.44 | 53.20 | 57.37 | 56.89 | 57.47 | 53.04 | 54.06 | 53.66 | 56.17 | 54.49 | 60.92 | 58.48 | |
| Oct | 43.52 | 39.63 | 44.16 | 45.36 | 46.78 | 39.88 | 49.67 | 44.07 | 39.30 | 46.11 | 46.20 | 42.21 | 44.19 | 42.26 | 44.61 | n/A | |
| Nov | 40.59 | 30.02 | 30.97 | 34.87 | 32.99 | 34.62 | 30.88 | 31.04 | 37.43 | 32.76 | 34.38 | 32.55 | 28.93 | 24.11 | 36.80 | n/A | |
| Dec | 25.85 | 23.28 | 23.76 | 19.78 | 17.54 | 25.72 | 16.69 | 12.56 | 17.84 | 16.11 | 24.95 | 23.75 | 11.84 | 23.12 | 30.01 | n/A | |
| Maximum Temperature (°F) | Jan | 37.71 | 47.94 | 47.75 | 37.63 | 38.75 | 46.68 | 44.05 | 43.21 | 29.43 | 37.56 | 37.89 | 49.18 | 45.62 | 37.59 | 38.79 | 39.78 |
| Feb | 37.76 | 47.45 | 45.81 | 47.23 | 48.07 | 40.23 | 45.63 | 36.65 | 47.01 | 38.04 | 49.72 | 43.55 | 39.39 | 41.83 | 33.4 | 51.06 | |
| Mar | 47.55 | 51.11 | 62.72 | 58.04 | 62.63 | 56.98 | 75.06 | 47.59 | 62.68 | 67.36 | 54.6 | 78.49 | 48.81 | 55.32 | 63.04 | 63.02 | |
| Apr | 76.13 | 81.61 | 80.03 | 75.45 | 75.37 | 74.4 | 79.84 | 71.96 | 76.58 | 78.08 | 71.97 | 71.65 | 72.41 | 68.88 | 73.05 | 73.95 | |
| May | 81.26 | 81.05 | 74.28 | 75.8 | 74.42 | 85.6 | 84.24 | 75.81 | 81.02 | 88.19 | 81.44 | 86.97 | 83.84 | 83.56 | 80.33 | 79.27 | |
| Jun | 85.08 | 85.64 | 84.03 | 82.42 | 88.47 | 84.83 | 87.25 | 82.42 | 89.65 | 84.39 | 90.85 | 89.88 | 83.88 | 84.06 | 83.64 | 83.96 | |
| Jul | 88.08 | 89.65 | 84.42 | 82.09 | 90.08 | 91.94 | 89.97 | 84.9 | 81.06 | 86.47 | 92.82 | 96.3 | 90.95 | 86.48 | 86.42 | 85.52 | |
| Aug | 91.09 | 85.24 | 88.32 | 78.8 | 87.75 | 91.09 | 87.81 | 84.34 | 84.08 | 87.18 | 86.91 | 89.33 | 90.94 | 83.51 | 88.9 | 83.07 | |
| Sept | 78.87 | 85.46 | 84.32 | 81.09 | 85.56 | 78.1 | 85.96 | 85.41 | 80.33 | 79.1 | 85.99 | 86.73 | 88.29 | 80.23 | 86.25 | 77.82 | |
| Oct | 73.73 | 76.26 | 75.39 | 72.21 | 79.13 | 75.47 | 81.91 | 75.51 | 62.63 | 78.8 | 79.78 | 72.41 | 75.62 | 67.08 | 76.95 | n/A | |
| Nov | 62.98 | 57.75 | 55.83 | 59.19 | 59.65 | 62.6 | 55.08 | 70.13 | 66.11 | 63.12 | 58.86 | 62.55 | 55.33 | 54.6 | 69.39 | n/A | |
| Dec | 58.31 | 46.4 | 45.98 | 46.9 | 38.73 | 45.84 | 38.04 | 43.29 | 44.89 | 43.45 | 46.35 | 56.57 | 41.79 | 45.07 | 51.34 | n/A | |
| Source | DF | F | P |
|---|---|---|---|
| Average Daily Accumulation for State (in.) | |||
| By: Month/Year | |||
| Model | 185 | 5.58 | 0.164 |
| Error | 2 | ||
| Month/Year | 185 | 5.58 | 0.164 |
| By: Year | |||
| Model | 15 | 0.16 | 0.999 |
| Error | 172 | ||
| Year | 15 | 0.30 | 0.020 |
| Maximum Daily Accumulation for State (in.) | |||
| By: Month/Year | |||
| Model | 185 | 3.28 | 0.263 |
| Error | 2 | ||
| Date | 185 | 3.28 | 0.263 |
| By: Year | |||
| Model | 15 | 0.34 | 0.991 |
| Error | 172 | ||
| Year | 15 | 0.34 | 0.991 |
| Average Total Accumulation for State (in.) | |||
| By: Month/Year | |||
| Model | 185 | 5.92 | 0.155 |
| Error | 2 | ||
| Date | 185 | 5.92 | 0.155 |
| By: Year | |||
| Model | 15 | 0.16 | 0.999 |
| Error | 172 | ||
| Year | 15 | 0.16 | 0.999 |
| Average Temperature for State (°F) | |||
| By: Month/Year | |||
| Model | 185 | 8 | 0.117 |
| Error | 2 | ||
| Date | 185 | 8 | 0.117 |
| By: Year | |||
| Model | 15 | 0.16 | 0.999 |
| Error | 172 | ||
| Year | 15 | 0.16 | 0.999 |
| Maximum Temperature for State (°F) | |||
| By: Month/Year | |||
| Model | 185 | 4.74 | 0.190 |
| Error | 2 | ||
| Date | 185 | 4.74 | 0.190 |
| By: Year | |||
| Model | 15 | 0.15 | 0.999 |
| Error | 172 | ||
| Year | 15 | 0.15 | 0.999 |
References
- Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. B 2015, 370, 20130561. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef] [Green Version]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [Green Version]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef] [Green Version]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. J. Am. Med Assoc. 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Centers for Disease Control. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD); Centers for Disease Control: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/westnile/index.html (accessed on 12 September 2019).
- American Society for Microbiology. West Nile Virus Frequently Asked Questions; American Academy of Microbiologist: Washington, DC, USA, 2013; Available online: https://www.asmscience.org/content/report/faq/faq.7 (accessed on 11 August 2019).
- Hughes, T.; Irwin, P.; Hofmeister, E.; Paskewitz, S.M. Occurrence of avian Plasmodium and West Nile virus in Culex species in Wisconsin. J. Am. Mosq. Control Assoc. 2010, 26, 24–31. [Google Scholar] [CrossRef]
- Abdelrazec, A.; Lenhart, S.; Zhu, H. Transmission dynamics of West Nile virus in mosquitoes and corvids and non-corvids. J. Math. Biol. 2014, 68, 1553–1582. [Google Scholar] [CrossRef]
- Guptill, S.C.; Julian, K.G.; Campbell, G.L.; Price, S.D.; Marfin, A.A. Early-season avian deaths from West Nile virus as warnings of human infection. Emerg. Infect. Dis. 2003, 9, 483–484. [Google Scholar] [CrossRef]
- Karki, S.; Hamer, G.L.; Anderson, T.K.; Goldberg, T.L.; Kitron, U.D.; Krebs, B.L.; Walker, E.D.; Ruiz, M.O. Effect of trapping methods, weather, and landscape on estimates of the Culex vector mosquito abundance. Environ. Health Insights 2016, 10, 93–103. [Google Scholar] [CrossRef]
- Tedesco, C.; Ruiz, M.O.; Mclafferty, S. Mosquito politics: Local vector control policies and the spread of West Nile Virus in the Chicago region. Health Place 2010, 16, 1188–1195. [Google Scholar] [CrossRef]
- Sugumaran, R.; Larson, S.R.; DeGroote, J.P. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. Int. J. Health Geogr. 2009, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J. West Nile Virus Hits California—Drought Triggers Skyrocketing Number of Cases—238 in Humans—So Far This Year; The Wall Street Journal: New York, NY, USA, 2014. [Google Scholar]
- Haines, A.; Patz, J.A. Health effects of climate change. J. Am. Med. Assoc. 2004, 291, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Grossi-Soyster, E.N.; Cook, E.A.J.; de Glanville, W.A.; Thomas, L.F.; Krystosik, A.R.; Wamae, C.N.; Kariuki, S.; Fevre, E.M.; LaBeaud, A.D. Serological and spatial analysis of alphavirus and flavivirus prevalence and risk factors in a rural community in western Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005998. [Google Scholar] [CrossRef] [Green Version]
- Anyamba, A.; Small, J.L.; Britch, S.C.; Tucker, C.J.; Pak, E.W.; Reynolds, C.A.; Crutchfield, J.; Linthicum, K.J. Recent weather extremes and impacts on agricultural production and vector-borne disease outbreak patterns. PLoS ONE 2014, 9, e92538. [Google Scholar] [CrossRef]
- Shuman, E.K. Global climate change and infectious diseases. Int. J. Occup. Environ. Med. 2010, 2, 11–19. [Google Scholar] [CrossRef]
- Ivers, L.C.; Ryan, E.T. Infectious diseases of severe weather-related and flood-related natural disasters. Curr. Opin. Infect. Dis. 2006, 19, 408–414. [Google Scholar] [CrossRef]
- Chevalier, V.; Tran, A.; Durand, B. Predictive modeling of West Nile virus transmission risk in the mediterranean basin: How far from landing? Int. J. Environ. Res. Public Health 2014, 11, 67–90. [Google Scholar] [CrossRef] [Green Version]
- Talbot, B.; Ardis, M.; Kulkarni, M.A. Influence of demography, land use, and urban form on West Nile virus risk and human West Nile virus incidence in Ottawa, Canada. Vector-Borne Zoonotic Dis. 2019, 19, 533–539. [Google Scholar] [CrossRef]
- Brown, H.E.; Childs, J.E.; Diuk-Wasser, M.A.; Fish, D. Ecological factors associated with West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2008, 14, 1539–1545. [Google Scholar] [CrossRef]
- Illinois Department of Public Health (IDPH). West Nile Virus (WNV) Fact Sheet; Illinois Department of Public Health: Springfield, IL, USA, 2019. Available online: http://dph.illinois.gov/topics-services/diseases-and-conditions/west-nile-virus (accessed on 26 February 2019).
- Morin, C.W.; Comrie, A.C. Regional and seasonal response of a West Nile virus vector to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 15620–15625. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control. One Health Basics; Centers for Disease Control: Atlanta, GA, USA, 2017. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 26 September 2017).
- United States Census Bureau. Quick Facts Data Sheet: Wisconsin; United States Census Bureau: Suitland, MD, USA, 2017. Available online: https://www.census.gov/quickfacts/fact/table/WI/PST045216 (accessed on 12 June 2017).
- SAS Institute Inc. Version 9.4.; SAS Institute Inc.: Cary, NC, USA, 2017. [Google Scholar]
- United States Geological Survey. West Nile Virus Case Definition; Unpublished work; 2017.
- MRCC. Midwestern Regional Climate Center cli-MATE Tool; MRCC: Mahwah, NJ, USA, 2017; Available online: http://mrcc.isws.illinois.edu/CLIMATE/ (accessed on 12 August 2017).
- Larson, S.R.; DeGroote, J.P.; Bartholomay, L.C.; Sugumaran, R. Ecological niche modeling of potential West Nile virus vector mosquito species in Iowa. J. Insect Sci. 2010, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Brown, H.E.; Andreadis, T.G.; Fish, D. Modeling the spatial distribution of mosquito vectors for West Nile virus in Connecticut, USA. J. Vector-Borne Zoonotic Dis. 2006, 6, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Dutch, S. Wisconsin Bedrock Elevation Map; University of Wisconsin: Green Bay, WI, USA, 2000; Available online: http://www.uwgb.edu/dutchs/GeologyWisconsin/WisBrkElevLgNoCty.htm (accessed on 11 December 2018).
- State Cartographer’s Office. University of Wisconsin: Madison, WI, USA. 2017. Available online: https://www.sco.wisc.edu/2016/09/23/wiscland-2-project-complete-data-now-available/ (accessed on 10 December 2018).
- Centers for Disease Control. Arboviral Diseases, Neuroinvasive and Non-Neuroinvasive 2015 Case Definition; Centers for Disease Control: Atlanta, GA, USA, 2015. Available online: https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ (accessed on 30 November 2018).
- Sallam, M.F.; Fizer, C.; Pilant, A.N.; Whung, P.-Y. Systematic review: Land cover, meteorological, and socioeconomic determinants of Aedes mosquito habitat for risk mapping. Int. J. Environ. Res. Public Health 2017, 14, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop: Release 10.5.1; Environmental Systems Research Institute: Redlands, CA, USA, 2017; Available online: http://desktop.arcgis.com/en/arcmap/ (accessed on 22 September 2018).
- Giordano, B.V.; Kaur, S.; Hunter, F.F. West Nile virus in Ontario, Canada: A twelve-year analysis of human case prevalence, mosquito surveillance, and climate data. PLoS ONE 2017, 12, e0183568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehringer-Ingelheim. Vetera®. Available online: https://www.boehringer-ingelheim.com/animal-health/companion-animals-products/vetera? (accessed on 26 February 2019).
- DVM360. Merial Unveils Equine WNV Vaccine; DVM360: Lenexa, KS, USA, 2004; Volume 35, p. 92. [Google Scholar]
- Gates, M.C.; Boston, R.C. Irrigation linked to a greater incidence of human and veterinary West Nile virus cases in the United States from 2004 to 2006. Prev. Vet. Med. 2009, 89, 134–137. [Google Scholar] [CrossRef] [PubMed]
- DeGroote, J.P.; Sugumaran, R.; Brend, S.M.; Tucker, B.J.; Bartholomay, L.C. Landscape, demographic, entomological, and climatic associations with human disease incidence of West Nile virus in the state of Iowa, USA. Int. J. Health Geogr. 2008, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wimberly, M.C.; Hildreth, M.B.; Boyte, S.P.; Lindquist, E.; Kightlinger, L. Ecological niche of the 2003 West Nile virus epidemic in the northern Great Plains of the United States. PLoS ONE 2008, 3, e3744. [Google Scholar] [CrossRef]
- Harrigan, R.J.; Thomassen, H.A.; Buermann, W.; Smith, T.B. A continental risk assessment of West Nile virus under climate change. Glob. Chang. Biol. 2014, 20, 2417–2425. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 2007, 447, 710–714. [Google Scholar] [CrossRef]
- VanDalen, K.K.; Hall, J.S.; Clark, L.; McLean, R.G.; Smeraski, C. West Nile virus infection in American robin: New insights on dose response. PLoS ONE 2013, 8, e68537. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Chaves, L.F.; Anderson, T.K.; Kitron, U.D.; Brawn, J.D.; Ruiz, M.O.; Loss, S.R.; Walker, E.D.; Goldberg, T.L. Fine-scale variation in vector host use and force of infection drive localized patterns of West Nile virus transmission. PLoS ONE 2011, 6, e23767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, M.A. Globalization, land use, and the invasion of West Nile virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janousek, W.M.; Marra, P.P.; Kilpatrick, A.M. Avian roosting behavior influences vector-host interactions for West Nile virus hosts. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meece, J.K.; Henkel, J.S.; Glaser, L.; Reed, K.D. Mosquito surveillance for West Nile virus in Southeastern Wisconsin—2002. Clin. Med. Res. 2003, 1, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Brown, W.M.; Uelmen, J.A.; Ruiz, M.O.; Smith, R.L. The Drives of West Nile Virus Human Illness: Fine Scale Dynamic Effects of Weather, Mosquito Infection, Social, and Biological Conditions; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2019. [Google Scholar]
- Hahn, M.B.; Monaghan, A.J.; Hayden, M.H.; Eisen, R.J.; Delorey, M.J.; Lindsey, N.P.; Nasci, R.S.; Fischer, M. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am. J. Trop. Med. Hyg. 2015, 92, 1013–1022. [Google Scholar] [CrossRef]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasites Vectors 2010, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Westcott, N.E.; Muturi, E.J.; Brown, W.M.; Ruiz, M.O. Assessing human risk of illness with West Nile virus mosquito surveillance data to improve public health preparedness. Zoonoses Public Health 2018, 65, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.K.; Vincent, G.; Hildreth, M.B.; Kightlinger, L.; Carlson, C.; Wimberly, M.C. Integrating environmental monitoring and mosquito surveillance to predict vector-borne disease: Prospective forecasts of a West Nile virus outbreak. PLoS Curr. Outbreaks 2017. [Google Scholar] [CrossRef] [Green Version]
- Wimberly, M.C.; Giacomo, P.; Kightlinger, L.; Hildreth, M.B. Spatio-temporal epidemiology of human West Nile virus disease in South Dakota. Int. J. Environ. Res. Public Health 2013, 10, 5584–5602. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. IPCC Fourth Assessment Report: Climate Change; IPCC: Geneva, Switzerland, 2007; Available online: https://www.ipcc.ch/assessment-report/ar4/ (accessed on 5 January 2019).
- Chung, W.M.; Buseman, C.M.; Joyner, S.N.; Hughes, S.M.; Fomby, T.B.; Luby, J.P.; Haley, R.W. The 2012 West Nile encephalitis epidemic in Dallas, Texas. J. Am. Med. Assoc. 2013, 3, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Paz, S.; Semenza, J.C. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia: A review. Int. J. Environ. Res. Public Health 2013, 10, 3543–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisen, W.K.; Thiemann, T.; Barker, C.M.; Lu, H.; Carroll, B.; Fang, Y.; Lothrop, H.D. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J. Med. Entomol. 2010, 47, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001, 3, 747–754. [Google Scholar] [CrossRef]
- Uelmen, J.A.; Lindroth, R.L.; Tobin, P.C.; Reich, P.B.; Schwartzberg, E.G.; Raffa, K.F. Effects of winter temperatures, spring degree-day accumulation, and insect population source on phenological synchrony between forest tent caterpillar and host trees. For. Ecol. Manag. 2016, 362, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Frumkin, H.; Holloway, T.; Vimont, D.J.; Haines, A. Climate change: Challenges and opportunities for global health. J. Am. Med. Assoc. 2014, 312, 1565–1580. [Google Scholar] [CrossRef]
- Fischer, D.; Thomas, S.M.; Suk, J.E.; Sudre, B.; Hess, A.; Tjaden, N.B.; Beierkuhnlein, C.; Semenza, J.C. Climate change effects on Chikungunya transmission in Europe: Geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 2013, 12, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.-W.; Wimberly, M.C. Remote sensing of climatic anomalies and West Nile virus incidence in the Northern Great Plains of the United States. PLoS ONE 2012, 7, e46882. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Kilpatrick, M.A.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Deichmester, J.M.; Telang, A. Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J. Vector Ecol. 2011, 36, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2009, 213, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherst, R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, A.B.; Slooff, R. Vector-borne disease problems in rapid urbanization: New approaches to vector control. Bull. World Health Organ. 1992, 70, 1–6. [Google Scholar]
- Myer, M.H.; Campbell, S.R.; Johnston, J.M. Spatiotemporal modeling of ecological and sociological predictors of West Nile virus in Suffolk County, NY, mosquitoes. Ecosphere 2017, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wimberly, M.C.; Lamsal, A.; Giacomo, P.; Chuang, T.-W. Regional variations of climatic influences on West Nile virus outbreaks in the United States. Am. J. Trop. Med. Hyg. 2014, 91, 677–684. [Google Scholar] [CrossRef]
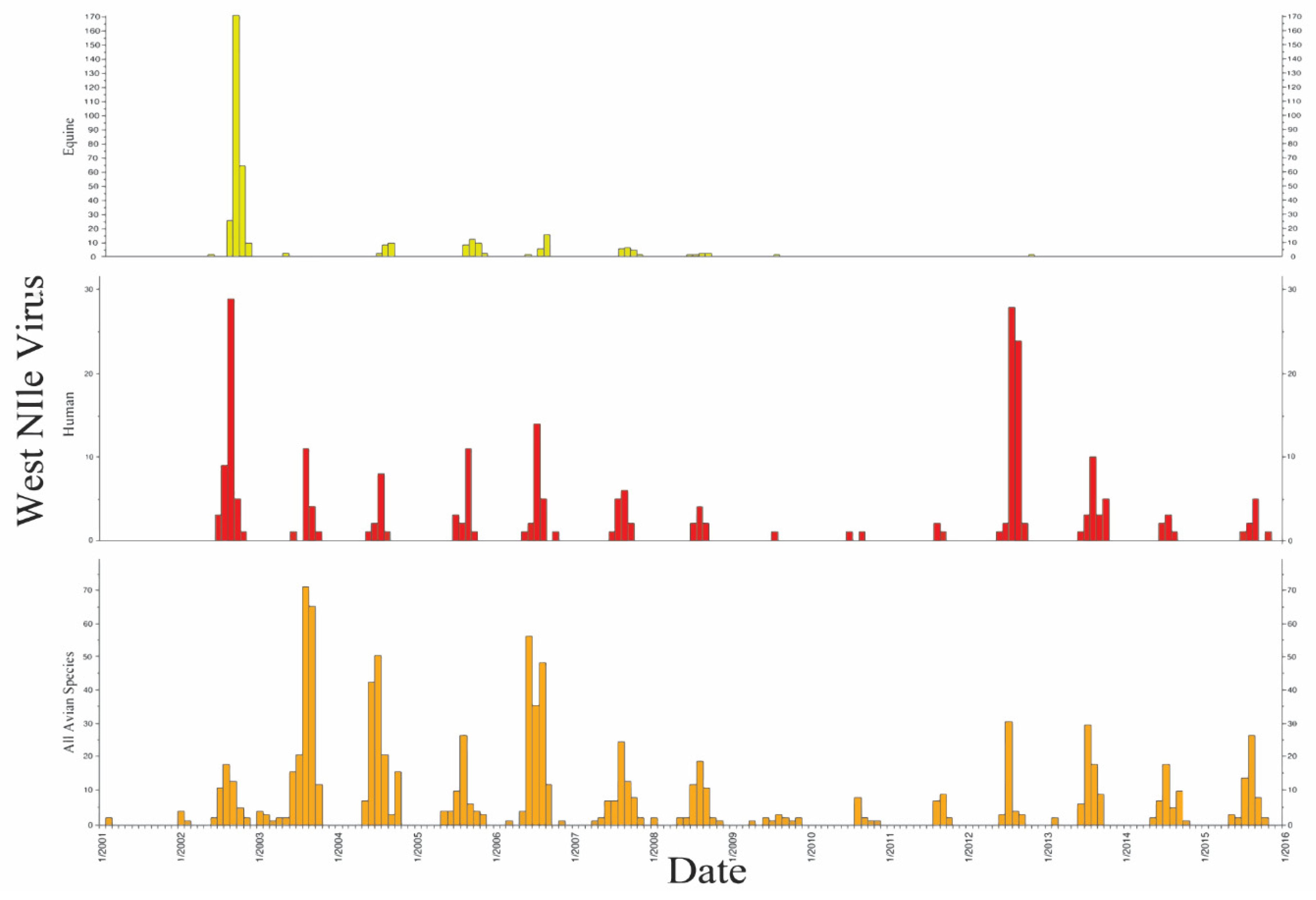



| Avian | Mammal | Unknown | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Negative | Positive | Probable | Suspect | Undetermined | Negative | Positive | Probable | Suspect | N/A | Negative |
| 2000 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2001 | 238 | 2 | 0 | 1 | 129 | 11 | 1 | 0 | 4 | 0 | 0 |
| 2002 | 157 | 56 | 0 | 1 | 143 | 583 | 323 | 1 | 23 | 0 | 0 |
| 2003 | 2023 | 190 | 6 | 8 | 28 | 200 | 31 | 0 | 1 | 0 | 0 |
| 2004 | 1706 | 127 | 16 | 4 | 25 | 200 | 33 | 0 | 0 | 0 | 1 |
| 2005 | 1510 | 58 | 0 | 1 | 12 | 156 | 56 | 0 | 2 | 2 | 0 |
| 2006 | 3612 | 156 | 5 | 6 | 5 | 135 | 45 | 5 | 2 | 0 | 0 |
| 2007 | 1436 | 64 | 1 | 0 | 3 | 86 | 29 | 1 | 4 | 0 | 0 |
| 2008 | 1306 | 49 | 2 | 0 | 0 | 53 | 14 | 0 | 0 | 0 | 0 |
| 2009 | 619 | 10 | 2 | 6 | 0 | 43 | 2 | 0 | 0 | 0 | 0 |
| 2010 | 631 | 11 | 1 | 9 | 6 | 6 | 2 | 0 | 0 | 0 | 0 |
| 2011 | 331 | 18 | 0 | 0 | 3 | 14 | 3 | 0 | 0 | 0 | 0 |
| 2012 | 1228 | 41 | 0 | 3 | 11 | 19 | 58 | 1 | 0 | 0 | 0 |
| 2013 | 66 | 64 | 1 | 940 | 7 | 20 | 18 | 4 | 0 | 0 | 0 |
| 2014 | 56 | 43 | 0 | 403 | 1 | 4 | 6 | 1 | 0 | 0 | 0 |
| 2015 | 84 | 56 | 0 | 691 | 4 | 8 | 7 | 2 | 0 | 0 | 0 |
| 2016 1 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 3 | 3 | 0 | 0 | 0 | 15 | 8 | 3 | 1 | 0 | 0 |
| Total | 15012 | 948 | 34 | 2073 | 377 | 1554 | 636 | 18 | 37 | 2 | 1 |
| Species | Number WNV Positive | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Common Name | Scientific Name | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Unk. |
| Avian | Blackbird, Unidentified | Icteridae | 1 | |||||||||||||||
| Bluebird, Eastern | Sialia sialis | 2 | 1 | |||||||||||||||
| Cardinal, Northern | Cardinalis cardinalis | 1 | 2 | 1 | 1 | |||||||||||||
| Chickadee, Black-capped | Poecile atricapillus | 2 | ||||||||||||||||
| Chicken, Greater Prairie | Tympanuchus cupido | 1 | 1 | |||||||||||||||
| Cormorant, Double-crested | Phalacrocorax auritus | 3 | 1 | 2 | 6 | |||||||||||||
| Corvid, Unidentified | Corvidae | 2 | 1 | |||||||||||||||
| Crane, Blue (Stanley or Paradise) | Anthropoides paradiseus | 1 | ||||||||||||||||
| Crane, Hooded | Grus monacha | 2 | ||||||||||||||||
| Crane, Indian Sarus | Grus antigone antigone | 2 | ||||||||||||||||
| Crane, Red-crowned (Japanese) | Grus japonensis | 2 | ||||||||||||||||
| Crane, Sandhill | Grus canadensis | 3 | 6 | 3 | ||||||||||||||
| Crane, Sarus, Unidentified | Grus antigone | 1 | ||||||||||||||||
| Crane, Siberian | Grus leucogeranus | 1 | 3 | |||||||||||||||
| Crane, Wattled | Bugeranus carunculatus | 5 | 2 | |||||||||||||||
| Crane, White-naped | Grus vipio | 1 | ||||||||||||||||
| Crane, Whooping | Grus americana | 1 | 6 | 6 | 3 | 6 | 1 | 3 | 1 | 4 | 3 | 8 | ||||||
| Crow, American | Corvus brachyrhynchos | 14 | 146 | 109 | 38 | 108 | 43 | 30 | 5 | 3 | 15 | 33 | 53 | 33 | 40 | |||
| Dove, Mourning | Zenaida macroura | 1 | 1 | |||||||||||||||
| Eagle, Bald | Haliaeetus leucocephalus | 2 | 2 | 10 | 1 | 2 | 3 | 2 | 1 | 1 | ||||||||
| Emu | Dromaius novaehollandiae | 1 | ||||||||||||||||
| Finch, Unidentified | Ardeidae | 1 | ||||||||||||||||
| Goshawk, Northern | Accipiter gentilis | 3 | 1 | |||||||||||||||
| Grackle, Common | Quiscalus quiscula | 1 | ||||||||||||||||
| Hawk, Cooper’s | Accipiter cooperii | 1 | 2 | 2 | ||||||||||||||
| Hawk, Red-tailed | Buteo jamaicensis | 2 | 2 | 2 | 1 | 1 | 1 | |||||||||||
| Hawk, Sharp-shinned | Accipiter striatus | 1 | 2 | |||||||||||||||
| Hawk, Unidentified | Accipitridae | 1 | ||||||||||||||||
| Jay, Blue | Cyanocitta cristata | 18 | 9 | 9 | 28 | 6 | 15 | 2 | 1 | 3 | 3 | 5 | 9 | |||||
| Avian | Loon, Common | Gavia immer | 1 | |||||||||||||||
| Merlin | Falco columbarius | 1 | ||||||||||||||||
| Owl, Horned, Great | Bubo virginianus | 1 | 1 | |||||||||||||||
| Pelican, Unidentified | Pelecanus | 1 | ||||||||||||||||
| Pelican, White, American | Pelecanus erythrorhynchos | 8 | 1 | |||||||||||||||
| Raven, Common | Corvus corax | 3 | 1 | 1 | 1 | |||||||||||||
| Robin, American | Turdus migratorius | 2 | ||||||||||||||||
| Sora | Porzana carolina | 1 | ||||||||||||||||
| Sparrow, Unidentified | Passeridae | 1 | 1 | |||||||||||||||
| Starling, European | Sturnus vulgaris | 1 | ||||||||||||||||
| Swan, Trumpeter | Cygnus buccinator | 16 | 2 | |||||||||||||||
| Swan, Tundra | Cygnus columbianus | 1 | ||||||||||||||||
| Thrush, Unidentified | Turdidae | 1 | ||||||||||||||||
| Turkey, Wild | Meleagris gallopavo | 1 | ||||||||||||||||
| Waxwing, Cedar | Bombycilla cedrorum | 2 | ||||||||||||||||
| Woodpecker, Downy | Dryobates pubescens | 1 | ||||||||||||||||
| Woodpecker, Hairy | Picoides villosus | 1 | ||||||||||||||||
| Mammal | Bat, Brown, Big | Eptesicus fuscus | ||||||||||||||||
| Bat, Brown, Little | Myotis lucifugus | |||||||||||||||||
| Coyote | Canis latrans | |||||||||||||||||
| Elk | Cervus canadensis | 7 | 1 | 2 | 1 | |||||||||||||
| Horse, Domestic | Equus ferus caballus | 270 | 2 | 19 | 31 | 21 | 16 | 6 | 1 | 1 | ||||||||
| Human | Homo sapien | 46 | 18 | 12 | 17 | 23 | 14 | 8 | 1 | 2 | 3 | 57 | 22 | 6 | 9 | 1 | ||
| Squirrel, Gray, Eastern | Sciurus carolinensis | 6 | 1 | 1 | ||||||||||||||
| Unknown | Unknown | 1 | ||||||||||||||||
| Wolf, Gray | Canis lupus | 1 | 1 | 3 | 5 | 5 | ||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Unknown | Total Cases (n) | Annual Incidence (%) b | Cumulative Incidence (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams | 1 | 1 | 0.35 | 4.88 | ||||||||||||||
| Ashland | 1 | 1 | 0.45 | 6.24 | ||||||||||||||
| Barron | 5 | 1 | 6 | 0.94 | 13.14 | |||||||||||||
| Brown | 4 | 1 | 1 | 1 (1) | 1 | 1 | 10 | 0.28 | 3.93 | |||||||||
| Buffalo | 1 | 1 | 0.53 | 7.49 | ||||||||||||||
| Burnett | 1 | 1 | 0.47 | 6.52 | ||||||||||||||
| Calumet | 1 | 1 | 0.14 | 2.02 | ||||||||||||||
| Chippewa | 1 | 1 | 1 | 1 | 4 | 0.45 | 6.34 | |||||||||||
| Clark | 1 | 1 | 0.21 | 2.89 | ||||||||||||||
| Columbia | 1 | 1 | 0.13 | 1.77 | ||||||||||||||
| Dane | 1 | 2 | 1 | 3 * | 3 | 3 (1) | 1 | 5 | 4 | 1 | 24 | 0.34 | 4.71 | |||||
| Dodge | 1 | 1 | 2 | 2 | 6 | 0.49 | 6.79 | |||||||||||
| Douglas | 1 | 1 | 0.16 | 2.28 | ||||||||||||||
| Eau Claire | 1 * (1) | 2 | 0.14 | 1.97 | ||||||||||||||
| Fond du Lac | 1 | 1 | 2 | 0.14 | 1.96 | |||||||||||||
| Grant | 2 | 1 | 1 | 4 | 0.56 | 7.83 | ||||||||||||
| Green | 1 | 1 | 2 | 0.39 | 5.39 | |||||||||||||
| Iowa | 1 | 1 | 2 | 0.60 | 8.42 | |||||||||||||
| Jefferson | 2 | 1* | 2 | 2 | 1 | 1 | 9 | 0.76 | 10.65 | |||||||||
| Kenosha | 2 | 3 | 1 | 6 | 0.26 | 3.58 | ||||||||||||
| La Crosse | 1 | 3 | 4 | 0.24 | 3.43 | |||||||||||||
| Lafayette | 2 | 2 | 0.85 | 11.93 | ||||||||||||||
| Langlade | 1* | 1 | 0.36 | 5.11 | ||||||||||||||
| Lincoln | 1 | 1 | 0.25 | 3.49 | ||||||||||||||
| Manitowoc | 1 | 1 | 1 | 3 | 0.27 | 3.72 | ||||||||||||
| Marathon | 1 | 1 | 1 | 1 | 4 | 0.21 | 2.95 | |||||||||||
| Marinette | 1 | 1 | 0.17 | 2.4 | ||||||||||||||
| Marquette | 1 | 1 | 0.47 | 6.59 | ||||||||||||||
| Milwaukee | 9 | 8 * | 7 * | 1 | 1 | 1 | 27 *** | 3 * | 2 | 3 * | 62 | 0.46 | 6.49 | |||||
| Oconto | 1 | 1 | 0.19 | 2.68 | ||||||||||||||
| Oneida | 1 | 1 | 0.2 | 2.8 | ||||||||||||||
| Outagamie | 1 | 1 | 1 | 1 | 1 * | 1 | 1 * | 7 | 0.28 | 3.88 | ||||||||
| Ozaukee | 1 | 1 | 0.08 | 1.15 | ||||||||||||||
| Polk | 1 | 1 | 1 | 3 | 0.49 | 6.9 | ||||||||||||
| Portage | 1 | 1 | 1 | 3 | 0.3 | 4.27 | ||||||||||||
| Racine | 4 | 1 | 2 | 1 | 8 | 0.29 | 4.1 | |||||||||||
| Richland | 1 | 1 | 0.4 | 5.64 | ||||||||||||||
| Rock | 1 | 1 | 2 | 2 | 1 | 7 | 0.31 | 4.35 | ||||||||||
| Rusk | 1 | 1 | 1 | 3 | 1.49 | 20.84 | ||||||||||||
| Shawano | 1 | 1 | 0.17 | 2.4 | ||||||||||||||
| Sheboygan | 1 | 1 | 0.06 | 0.87 | ||||||||||||||
| St. Croix | 1 | 1 | 2 | 0.17 | 2.33 | |||||||||||||
| Vernon | 1 | 1 | 0.24 | 3.3 | ||||||||||||||
| Walworth | 1 | 1 | 2 * | 2 (2) | 1 | 9 | 0.62 | 8.74 | ||||||||||
| Washington | 1 | 1 | 1 | 3 | 0.16 | 2.26 | ||||||||||||
| Waukesha | 5 | 1 | 7 * | 1 | 14 | 0.25 | 3.55 | |||||||||||
| Winnebago | 1 | 2 | 1 | 1 | 1 | 6 | 0.25 | 3.54 | ||||||||||
| Wood | 2 | 2 | 0.19 | 2.7 | ||||||||||||||
| Unknown | 1 * | 1 | 0.09 c | 1.28 | ||||||||||||||
| Total Cases (n) | 46 | 18 | 12 | 17 | 23 | 14 | 8 | 1 | 2 | 3 | 57 | 18 | 6 | 9 | 1 | 239 | 0.3 | 4.24 |
| Total Deaths (n) | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 5 | 2 | 1 | 1 | 0 | 17 | 0.02 | 0.3 |
| Annual Incidence | 0.8 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.1 | 0 | 0 | 0.1 | 1 | 0.31 | 0.1 | 0.2 | 0.018 a | 0.3 | - | - |
| Cumulative Incidence | 0.8 | 1.2 | 1.4 | 1.7 | 2.1 | 2.4 | 2.5 | 2.5 | 2.6 | 2.6 | 3.6 | 3.92 | 4 | 4.2 | 4.19 | 4.24 | - | - |
| Parameter | Moran’s I | p | Spatial Interpretation | Referring Figure |
|---|---|---|---|---|
| 2002 Avians | 0.0449 | 0.486 | Random | Figure S1(A1) |
| * 2002 Corvids | 0.159 | 0.0364 | Clustered | Figure S1(B1) |
| 2002 Equines | –0.0212 | 0.938 | Random | Figure S1(C1) |
| 2002 Humans | –0.00128 | 0.872 | Random | Figure S1(D1) |
| * 2012 Avians | 0.201 | 0.00288 | Clustered | Figure S1(B2) |
| * 2012 Corvids | 0.171 | 0.0264 | Clustered | Figure S1(B2) |
| * 2012 Equines | –0.0463 | 0.0104 | Dispersed | Figure S1(C2) |
| 2012 Humans | 0.0914 | 0.194 | Random | Figure S1(D2) |
| * All Years Avians | 0.256 | 0.00149 | Clustered | Figure 3A |
| * All Years Corvids | 0.217 | 0.00748 | Clustered | Figure 3B |
| * All Years Equines | 0.178 | 0.024 | Clustered | Figure 3C |
| All Years Humans | 0.081 | 0.256 | Random | Figure 3D |
| Linear Regression—Statewide | Logistic Regression—Statewide | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | All Avian Species | Corvids | Equines | Humans | All Avian Species | Corvids | Equines | Humans | |
| Date (mm/yyyy) | + | − | Can Not Assess a | ||||||
| County Population (n) | − | − | + | − | − | ||||
| % of County | Agriculture | + | + | ||||||
| Forest | + | − | |||||||
| Grassland | + | + | + | ||||||
| Shrubland | + | + | |||||||
| Urban | + | + | − | + | + | + | |||
| Water | + | + | − | ||||||
| Wetland | + | + | + | + | |||||
| Average Elevation (ft.) | + | + | − | ||||||
| Maximum County Temperature (°F) | − | − | + | − | |||||
| Total County Accumulation (in.) | + | − | − | − | |||||
| Maximum County Daily Accumulation (in.) | − | + | |||||||
| Mean County Temperature (°F) | + | + | + | + | + | ||||
| Average County Daily Accumulation (in.) | − | + | − | − | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uelmen, J.A.; Brokopp, C.; Patz, J. A 15 Year Evaluation of West Nile Virus in Wisconsin: Effects on Wildlife and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1767. https://doi.org/10.3390/ijerph17051767
Uelmen JA, Brokopp C, Patz J. A 15 Year Evaluation of West Nile Virus in Wisconsin: Effects on Wildlife and Human Health. International Journal of Environmental Research and Public Health. 2020; 17(5):1767. https://doi.org/10.3390/ijerph17051767
Chicago/Turabian StyleUelmen, Johnny A., Charles Brokopp, and Jonathan Patz. 2020. "A 15 Year Evaluation of West Nile Virus in Wisconsin: Effects on Wildlife and Human Health" International Journal of Environmental Research and Public Health 17, no. 5: 1767. https://doi.org/10.3390/ijerph17051767
APA StyleUelmen, J. A., Brokopp, C., & Patz, J. (2020). A 15 Year Evaluation of West Nile Virus in Wisconsin: Effects on Wildlife and Human Health. International Journal of Environmental Research and Public Health, 17(5), 1767. https://doi.org/10.3390/ijerph17051767





