Reconnaissance of Surface Water Estrogenicity and the Prevalence of Intersex in Smallmouth Bass (Micropterus Dolomieu) Inhabiting New Jersey
Abstract
:1. Introduction
2. Methods
2.1. Site Selection
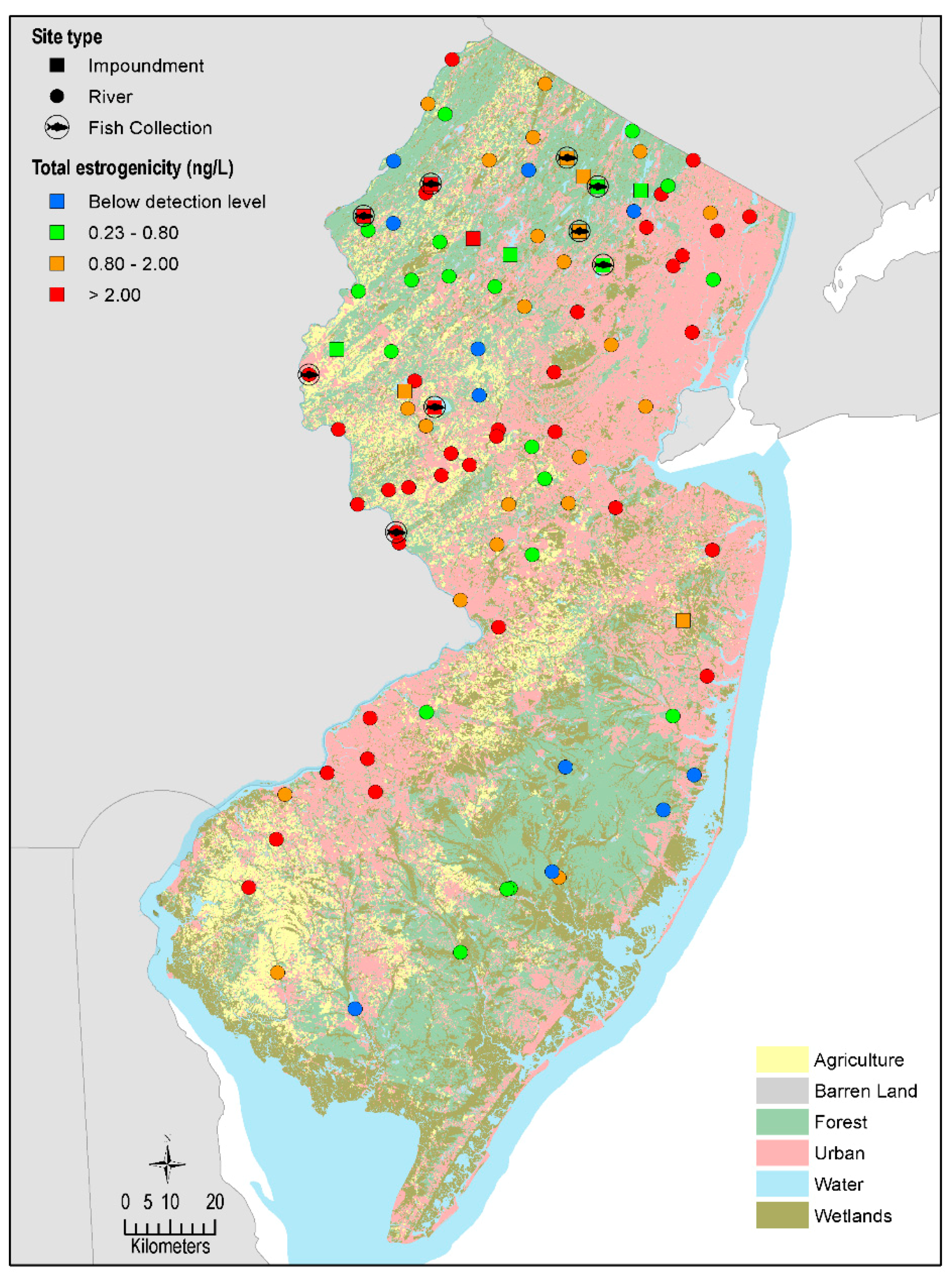
2.1.1. Sampling Locations
2.1.2. Water Sampling and Estrogenicity Bioassay
2.1.3. Fish Collection and Processing
2.1.4. Reproductive Endpoints
2.1.5. Transcript Abundance Analysis
2.1.6. Land Use Summary
2.2. Statistical Analyses
3. Results
3.1. Surface Water Estrogenicity
3.2. Prevalence and Severity of Testicular Oocytes
3.3. Plasma Vitellogenin and Differential Expression of Hepatic Transcripts
3.4. Associations with Land Use
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solecki, R.; Kortenkamp, A.; Bergman, A.; Chahoud, I.; Degen, G.H.; Dietrich, D.; Greim, H.; Hakansson, H.; Hass, U.; Husoy, T.; et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch. Toxicol. 2017, 91, 1001–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, C.R.; Jobling, S.; Sumpter, J.P. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 1998, 28, 319–361. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, P.; Wheeler, J.R.; Weltje, L. A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Crit. Rev. Toxicol. 2018, 48, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Conley, J.M.; Evans, N.; Cardon, M.C.; Rosenblum, L.; Iwanowicz, L.R.; Hartig, P.C.; Schenck, K.M.; Bradley, P.M.; Wilson, V.S. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ. Sci. Technol. 2017, 51, 4781–4791. [Google Scholar] [CrossRef]
- Stavreva, D.A.; George, A.A.; Klausmeyer, P.; Varticovski, L.; Sack, D.; Voss, T.C.; Schiltz, R.L.; Blazer, V.S.; Iwanowicz, L.R.; Hager, G.L. Prevalent glucocorticoid and androgen activity in US water sources. Sci. Rep. 2012, 2, 937. [Google Scholar] [CrossRef] [Green Version]
- Stavreva, D.A.; Varticovski, L.; Levkova, L.; George, A.A.; Davis, L.; Pegoraro, G.; Blazer, V.; Iwanowicz, L.; Hager, G.L. Novel cell-based assay for detection of thyroid receptor beta-interacting environmental contaminants. Toxicology 2016, 368–369, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Bradley, P.M.; Iwanowicz, L.; Journey, C.A.; Walsh, H.L.; Blazer, V.S. Pharmaceuticals, hormones, pesticides, and other bioactive contaminants in water, sediment, and tissue from Rocky Mountain National Park, 2012–2013. Sci. Total Environ. 2018, 643, 651–673. [Google Scholar] [CrossRef]
- Bradley, P.M.; Journey, C.A.; Romanok, K.M.; Barber, L.B.; Buxton, H.T.; Foreman, W.T.; Furlong, E.T.; Glassmeyer, S.T.; Hladik, M.L.; Iwanowicz, L.R.; et al. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. streams. Environ. Sci. Technol. 2017, 51, 4792–4802. [Google Scholar] [CrossRef]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; de Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, P.; Noyes, P.D.; Casey, W.M.; Dix, D.J. Application of adverse outcome pathways to U.S. EPA’s endocrine disruptor screening program. Environ. Health Perspect. 2017, 125, 096001. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Martin, M.T.; Tan, S.W.; Houck, K.A.; Judson, R.S.; Richard, A.M.; Knudsen, T.B.; Dix, D.J.; Kavlock, R.J. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 2010, 118, 1714–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Ågerstrand, M.; Beronius, A.; Beausoleil, C.; Bergman, Å.; Bero, L.A.; Bornehag, C.-G.; Boyer, C.S.; Cooper, G.S.; Cotgreave, I.; et al. A proposed framework for the systematic review and integrated assessment (SYRINA) of endocrine disrupting chemicals. Environ. Health 2016, 15, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Coulter, D.P.; Mahapatra, C.T.; Sepulveda, M.S. Intersex in fishes and amphibians: Population implications, prevalence, mechanisms and molecular biomarkers. J. Appl. Toxicol. JAT 2015, 35, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Cacela, D.; Beltman, D.; Teh, S.J.; Okihiro, M.S.; Hinton, D.E.; Denslow, N.; Zelikoff, J.T. Biochemical and toxicopathic biomarkers assessed in smallmouth bass recovered from a polychlorinated biphenyl-contaminated river. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2003, 8, 371–393. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.D.; Foster, S.W.; Reeser, S.J. Intersex (testicular oocytes) in smallmouth bass from the Potomac River and selected nearby drainages. J. Aquat. Anim. Health 2007, 19, 242–253. [Google Scholar] [CrossRef]
- Hinck, J.E.; Blazer, V.S.; Schmitt, C.J.; Papoulias, D.M.; Tillitt, D.E. Widespread occurrence of intersex in black basses (Micropterus spp.) from U.S. rivers, 1995–2004. Aquat. Toxicol. 2009, 95, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.R.; Miller, D.L.; Ingram, T.R.; Tannehill, J.E. Intersex condition of shoal bass in the Flint River, Georgia. J. Aquat. Anim. Health 2011, 23, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kellock, K.A.; Trushel, B.E.; Ely, P.C.; Jennings, C.A.; Bringolf, R.B. Survey of intersex largemouth bass from impoundments in Georgia USA. Trans. Am. Fish. Soc. 2014, 143, 565–572. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Fisher, D.J. Intersex (testicular oocytes) in largemouth bass (Micropterus salmoides) on the Delmarva Peninsula, USA. Environ. Toxicol. Chem. SETAC 2014, 33, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Blazer, V.S.; Pinkney, A.E.; Guy, C.P.; Major, A.M.; Munney, K.; Mierzykowski, S.; Lingenfelser, S.; Secord, A.; Patnode, K.; et al. Evidence of estrogenic endocrine disruption in smallmouth and largemouth bass inhabiting Northeast U.S. national wildlife refuge waters: A reconnaissance study. Ecotoxicol. Environ. Saf. 2016, 124, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lee Pow, C.S.D.; Law, J.M.; Kwak, T.J.; Cope, W.G.; Rice, J.A.; Kullman, S.W.; Aday, D.D. Endocrine active contaminants in aquatic systems and intersex in common sport fishes. Environ. Toxicol. Chem. 2017, 36, 959–968. [Google Scholar] [CrossRef]
- Kadlec, S.M.; Johnson, R.D.; Mount, D.R.; Olker, J.H.; Borkholder, B.D.; Schoff, P.K. Testicular oocytes in smallmouth bass in northeastern Minnesota in relation to varying levels of human activity. Environ. Toxicol. Chem. SETAC 2017, 36, 3424–3435. [Google Scholar] [CrossRef]
- Abdel-moneim, A.; Deegan, D.; Gao, J.; De Perre, C.; Doucette, J.S.; Jenkinson, B.; Lee, L.; Sepúlveda, M.S. Gonadal intersex in smallmouth bass Micropterus dolomieu from northern Indiana with correlations to molecular biomarkers and anthropogenic chemicals. Environ. Pollut. 2017, 230, 1099–1107. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Pinkney, A.E.; Guy, C.P.; Major, A.M.; Munney, K.; Blazer, V.S.; Alvarez, D.A.; Walsh, H.L.; Sperry, A.; Braham, R.; et al. Temporal evaluation of estrogenic endocrine disruption markers in smallmouth bass (Micropterus dolomieu) reveals seasonal variability in intersex. Sci. Total Environ. 2019, 646, 245–256. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Henderson, H.; Mazik, P.M.; Jenkins, J.A.; Alvarez, D.A.; Young, J.A. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: Spatial and temporal comparisons of biological effects. Environ. Monit. Assess. 2012, 184, 4309–4334. [Google Scholar] [CrossRef] [Green Version]
- NJDEP-DWMS, Bureau of Freshwater and Biological Monitoring. Available online: https://www.state.nj.us/dep/wms/bfbm/surfacewaterhome.html (accessed on 15 August 2016).
- Williams, B.M.; Smalling, K.L.; Iwanowicz, L.R.; Blazer, V.S.; Boetsma, A.; Abatemarco, J.; Adams, C.; Atkinson, R.; Braham, R.; Bjorklund, B.; et al. Estrogen equivalents of surface water and smallmouth bass estrogenic biomarker data in New Jersey, 2016–2017. In Data Release; U.S. Geological Survey: Reston, VA, USA, 2019; p. 28. [Google Scholar]
- Ciparis, S.; Iwanowicz, L.R.; Voshell, J.R. Effects of watershed densities of animal feeding operations on nutrient concentrations and estrogenic activity in agricultural streams. Sci. Total Environ. 2012, 414, 268–276. [Google Scholar] [CrossRef]
- Sanseverino, J.; Gupta, R.K.; Layton, A.C.; Patterson, S.S.; Ripp, S.A.; Saidak, L.; Simpson, M.L.; Schultz, T.W.; Sayler, G.S. Use of Saccharomyces cerevisiae BLYES expressing bacterial bioluminescence for rapid, sensitive detection of estrogenic compounds. Appl. Environ. Microbiol. 2005, 71, 4455–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazer, V.S.; Walsh, H.L.; Braham, R.P.; Smith, C. Necropsy-based wild fish health assessment. JoVE 2018, 139, e57946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, L.G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts; American Histolabs. Inc.: Gaithersburg, MD, USA, 1992. [Google Scholar]
- Denslow, N.D.; Chow, M.C.; Kroll, K.J.; Green, L. Vitellogenin as a biomarker of exposure for estrogen or estrogen mimics. Ecotoxicology 1999, 8, 385–398. [Google Scholar] [CrossRef]
- Hahn, C.M.; Iwanowicz, L.R.; Cornman, R.S.; Mazik, P.M.; Blazer, V.S. Transcriptome discovery in non-model wild fish species for the development of quantitative transcript abundance assays. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 20, 27–40. [Google Scholar] [CrossRef]
- NJDEP Digital Downloads. Available online: https://www.nj.gov/dep/gis/listall.html (accessed on 12 September 2018).
- Martinovic-Weigelt, D.; Minarik, T.A.; Curran, E.M.; Marchuk, J.S.; Pazderka, M.J.; Smith, E.A.; Goldenstein, R.L.; Miresse, C.L.; Matlon, T.J.; Schultz, M.M.; et al. Environmental estrogens in an urban aquatic ecosystem: I. Spatial and temporal occurrence of estrogenic activity in effluent-dominated systems. Environ. Int. 2013, 61, 127–137. [Google Scholar] [CrossRef]
- Yao, B.; Li, R.; Yan, S.; Chan, S.A.; Song, W. Occurrence and estrogenic activity of steroid hormones in Chinese streams: A nationwide study based on a combination of chemical and biological tools. Environ. Int. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Berninger, J.P.; DeMarini, D.M.; Warren, S.H.; Simmons, J.E.; Wilson, V.S.; Conley, J.; Armstrong, M.D.; Iwanowicz, L.; Kolpin, D.W.; Kuivila, K.M.; et al. Predictive analysis using chemical-gene interaction networks consistent with observed endocrine activity and mutagenicity of U.S. streams. Environ. Sci. Technol. 2019, 53, 8611–8620. [Google Scholar] [CrossRef]
- Di Dea Bergamasco, A.M.; Eldridge, M.; Sanseverino, J.; Sodre, F.F.; Montagner, C.C.; Pescara, I.C.; Jardim, W.F.; De Aragao Umbuzeiro, G. Bioluminescent yeast estrogen assay (BLYES) as a sensitive tool to monitor surface and drinking water for estrogenicity. J. Environ. Monit. 2011, 13, 3288–3293. [Google Scholar] [CrossRef]
- Young, J.; Iwanowicz, L.; Sperry, A.; Blazer, V. A landscape-based reconnaissance survey of estrogenic activity in streams of the upper Potomac, upper James, and Shenandoah Rivers, USA. Environ. Monit. Assess. 2014, 186, 5531–5545. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; Neale, P.A.; Arnal, C.; Aneck-Hahn, N.H.; Balaguer, P.; Bruchet, A.; Escher, B.I.; Esperanza, M.; Grimaldi, M.; Leroy, G.; et al. Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Res. 2018, 139, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gao, Y.; Li, Q.; Li, G.; Guo, Q.; Yan, C. Estrogenic compounds and estrogenicity in surface water, sediments, and organisms from Yundang Lagoon in Xiamen, China. Arch. Environ. Contam. Toxicol. 2011, 61, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.R.; Giller, G.S.; Barber, L.B.; Fitzgerald, K.C.; Skelly, D.K. Suburbanization, estrogen contamination, and sex ratio in wild amphibian populations. Proc. Natl. Acad. Sci. USA 2015, 112, 11881–11886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prochazkova, T.; Sychrova, E.; Vecerkova, J.; Javurkova, B.; Otoupalikova, A.; Pernica, M.; Simek, Z.; Smutna, M.; Lepsova-Skacelova, O.; Hilscherova, K. Estrogenic activity and contributing compounds in stagnant water bodies with massive occurrence of phytoplankton. Water Res. 2018, 136, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Division of Fish & Wildlife. Available online: https://www.state.nj.us/dep/fgw/hacktown.htm (accessed on 17 March 2020).
- Marie, B.; Huet, H.; Marie, A.; Djediat, C.; Puiseux-Dao, S.; Catherine, A.; Trinchet, I.; Edery, M. Effects of a toxic cyanobacterial bloom (Planktothrix agardhii) on fish: Insights from histopathological and quantitative proteomic assessments following the oral exposure of medaka fish (Oryzias latipes). Aquat. Toxicol. 2012, 114–115, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, X.; Indran, I.R.; Zhang, S.-J.; Lv, Z.; Li, J.; Holmes, M.; Tang, Y.Z.; Yong, E.L. Phytoplankton blooms: An overlooked marine source of natural endocrine disrupting chemicals. Ecotoxicol. Environ. Saf. 2014, 107, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Vajda, A.M.; Barber, L.B.; Gray, J.L.; Lopez, E.M.; Woodling, J.D.; Norris, D.O. Reproductive disruption in fish downstream from an estrogenic wastewater effluent. Environ. Sci. Technol. 2008, 42, 3407–3414. [Google Scholar] [CrossRef]
- Hicks, K.A.; Fuzzen, M.L.; McCann, E.K.; Arlos, M.J.; Bragg, L.M.; Kleywegt, S.; Tetreault, G.R.; McMaster, M.E.; Servos, M.R. Reduction of intersex in a wild fish population in response to major municipal wastewater treatment plant upgrades. Environ. Sci. Technol. 2017, 51, 1811–1819. [Google Scholar] [CrossRef]
- Hara, A.; Hiramatsu, N.; Fujita, T. Vitellogenesis and choriogenesis in fishes. Fish. Sci. 2016, 82, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Blazer, V.S.; Iwanowicz, D.D.; Walsh, H.L.; Sperry, A.J.; Iwanowicz, L.R.; Alvarez, D.A.; Brightbill, R.A.; Smith, G.; Foreman, W.T.; Manning, R. Reproductive health indicators of fishes from Pennsylvania watersheds: Association with chemicals of emerging concern. Environ. Monit. Assess. 2014, 186, 6471–6491. [Google Scholar] [CrossRef] [Green Version]
- Bjerregaard, P.; Kinnberg, K.L.; Mose, M.P.; Holbech, H. Investigation of the potential endocrine effect of nitrate in zebrafish Danio rerio and brown trout Salmo trutta. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2018, 211, 32–40. [Google Scholar] [CrossRef]
- Kellock, K.A.; Moore, A.P.; Bringolf, R.B. Chronic nitrate exposure alters reproductive physiology in fathead minnows. Environ. Pollut. 2018, 232, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.; Cedergreen, N.; Hayes, T.; Hansen, M. Nitrate: An environmental endocrine disruptor? A review of evidence and research needs. Environ. Sci. Technol. 2018, 52, 3869–3887. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Edwards, T.M. Is nitrate an ecologically relevant endocrine disruptor in vertebrates? Integr. Comp. Biol. 2005, 45, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodziej, E.P.; Harter, T.; Sedlak, D.L. Dairy wastewater, aquaculture, and spawning fish as sources of steroid hormones in the aquatic environment. Environ. Sci. Technol. 2004, 38, 6377–6384. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Walsh, H.L.; Shaw, C.H.; Iwanowicz, L.R.; Braham, R.P.; Mazik, P.M. Indicators of exposure to estrogenic compounds at Great Lakes Areas of Concern: Species and site comparisons. Environ. Monit. Assess. 2018, 190, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraudie, P.; Gerbron, M.; Hill, E.; Minier, C. Roach (Rutilus rutilus) reproductive cycle: A study of biochemical and histological parameters in a low contaminated site. Fish Physiol. Biochem. 2010, 36, 767–777. [Google Scholar] [CrossRef]
- Tyler, C.R.; Lange, A.; Paull, G.C.; Katsu, Y.; Iguchi, T. The roach (Rutilus rutilus) as a sentinel for assessing endocrine disruption. Environ. Sci. Int. J. Environ. Physiol. Toxicol. 2007, 14, 235–253. [Google Scholar]
- Jobling, S.; Coey, S.; Whitmore, J.G.; Kime, D.E.; Van Look, K.J.; McAllister, B.G.; Beresford, N.; Henshaw, A.C.; Brighty, G.; Tyler, C.R.; et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002, 67, 515–524. [Google Scholar] [CrossRef]
- Harris, C.A.; Hamilton, P.B.; Runnalls, T.J.; Vinciotti, V.; Henshaw, A.; Hodgson, D.; Coe, T.S.; Jobling, S.; Tyler, C.R.; Sumpter, J.P. The consequences of feminization in breeding groups of wild fish. Environ. Health Perspect. 2011, 119, 306–311. [Google Scholar] [CrossRef] [Green Version]


| Site | Sex | Collection Dates | n | Age (years) | Length (mm) | Weight (g) | Condition Factor (Fulton’s K) |
|---|---|---|---|---|---|---|---|
| Round Valley Reservoir (RV) | M | 4/5 and 4/12 | 12 | 5.6 | 323.8 | 394.5 | 1.15 |
| F | 4/5/ and 4/12 | 8 | 5.1 | 312.6 | 330.1 | 1.08 | |
| Splitrock Reservoir (SPL) | M | 4/12 and 4/19 | 12 | 3.8 | 332.3 | 484.8 | 1.27 |
| F | 4/12 and 4/19 | 8 | 3.3 | 345.4 | 467.1 | 1.18 | |
| Echo Lake (ECH) | M | 4/17 | 12 | 5.2 | 360.7 | 628.8 | 1.26 |
| F | 4/17 | 8 | 5.0 | 339.0 | 566.1 | 1.29 | |
| Delaware River at Phillipsburg (PBURG) | M | 5/3 | 11 | 3.7 | 278.9 | 321.1 | 1.27 |
| F | 5/3 | 3 | 4.0 | 308.7 | 426.0 | 1.42 | |
| Swartswood Reservoir (SWA) | M | 4/18 | 13 | 4.9 | 358.5 | 630.2 | 1.23 |
| F | 4/18 | 7 | 5.6 | 360.9 | 695.1 | 1.30 | |
| Boonton Reservoir (BOON) | M | 4/19 | 17 | 6.4 | 443.7 | 1327.4 | 1.45 |
| F | 4/19 | 3 | 3.7 | 332.3 | 485.7 | 1.32 | |
| Yards Creek Reservoir (YCR) | M | 4/20 | 12 | 8.9 | 419.3 | 968.3 | 1.31 |
| F | 4/20 | 8 | 7.4 | 384.1 | 710.6 | 1.23 | |
| Delaware River at Lambertville (LVILLE) | M | 4/28 | 13 | 4.5 | 352.4 | 644.0 | 1.35 |
| F | 4/28 | 7 | 3.4 | 307.9 | 395.4 | 1.23 | |
| Canistear Reservoir (CAN) | M | 4/17 | 13 | 4.3 | 308.0 | 453.0 | 1.16 |
| F | 4/17 | 7 | 4.9 | 335.0 | 525.7 | 1.38 |
| Landscape Variable | All Sites (N = 101) | Rivers Only (N = 85) | Reservoirs Only (N = 16) |
|---|---|---|---|
| Percent Urban | 0.206 | 0.277 | −0.135 |
| Percent Agriculture | 0.0958 | 0.118 | 0.147 |
| Percent Cultivated Crops | 0.0761 | 0.0866 | 0.0678 |
| Percent Forest | −0.188 | −0.212 | −0.0765 |
| Percent Altered | 0.306 | 0.402 | −0.0176 |
| Watershed Area | 0.0557 | 0.128 | −0.0294 |
| Number of GW Discharge Permits | −0.0101 | 0.082 | −0.385 |
| Number of SW Discharge Permits | 0.154 | 0.249 | −0.130 |
| Number of Contaminated Sites | 0.157 | 0.253 | 0.059 |
| Number of Landfills | 0.0139 | 0.019 | 0.252 |
| Landscape Variable | Plasma Vitellogenin | Liver Vitellogenin |
|---|---|---|
| Percent Urban | −0.539 | 0.251 |
| Percent Agriculture | −0.219 | 0.0481 |
| Percent Cultivated Crops | −0.0075 | −0.0759 |
| Percent Forest | −0.229 | −0.389 |
| Percent Altered | −0.412 | 0.346 |
| Number of GW Discharge Permits | −0.330 | −0.0386 |
| Number of SW Discharge Permits | −0.329 | −0.138 |
| Number of Contaminated Sites | −0.404 | −0.109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanowicz, L.R.; Smalling, K.L.; Blazer, V.S.; Braham, R.P.; Sanders, L.R.; Boetsma, A.; Procopio, N.A.; Goodrow, S.; Buchanan, G.A.; Millemann, D.R.; et al. Reconnaissance of Surface Water Estrogenicity and the Prevalence of Intersex in Smallmouth Bass (Micropterus Dolomieu) Inhabiting New Jersey. Int. J. Environ. Res. Public Health 2020, 17, 2024. https://doi.org/10.3390/ijerph17062024
Iwanowicz LR, Smalling KL, Blazer VS, Braham RP, Sanders LR, Boetsma A, Procopio NA, Goodrow S, Buchanan GA, Millemann DR, et al. Reconnaissance of Surface Water Estrogenicity and the Prevalence of Intersex in Smallmouth Bass (Micropterus Dolomieu) Inhabiting New Jersey. International Journal of Environmental Research and Public Health. 2020; 17(6):2024. https://doi.org/10.3390/ijerph17062024
Chicago/Turabian StyleIwanowicz, Luke R., Kelly L. Smalling, Vicki S. Blazer, Ryan P. Braham, Lakyn R. Sanders, Anna Boetsma, Nicholas A. Procopio, Sandra Goodrow, Gary A. Buchanan, Daniel R. Millemann, and et al. 2020. "Reconnaissance of Surface Water Estrogenicity and the Prevalence of Intersex in Smallmouth Bass (Micropterus Dolomieu) Inhabiting New Jersey" International Journal of Environmental Research and Public Health 17, no. 6: 2024. https://doi.org/10.3390/ijerph17062024





