Patient Reported Outcome Measures of Sleep Quality in Fibromyalgia: A COSMIN Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Considering Studies for This Review
2.1.1. Type of Studies
2.1.2. Type of Participants
2.1.3. Type of Outcome Measures
2.2. Search Strategy
2.3. Selection of Studies
2.4. Data Collection and Data Items
2.5. Risk of Bias and Quality of the Results Assessment
2.6. Data Analysis and Synthesis of Results
3. Results
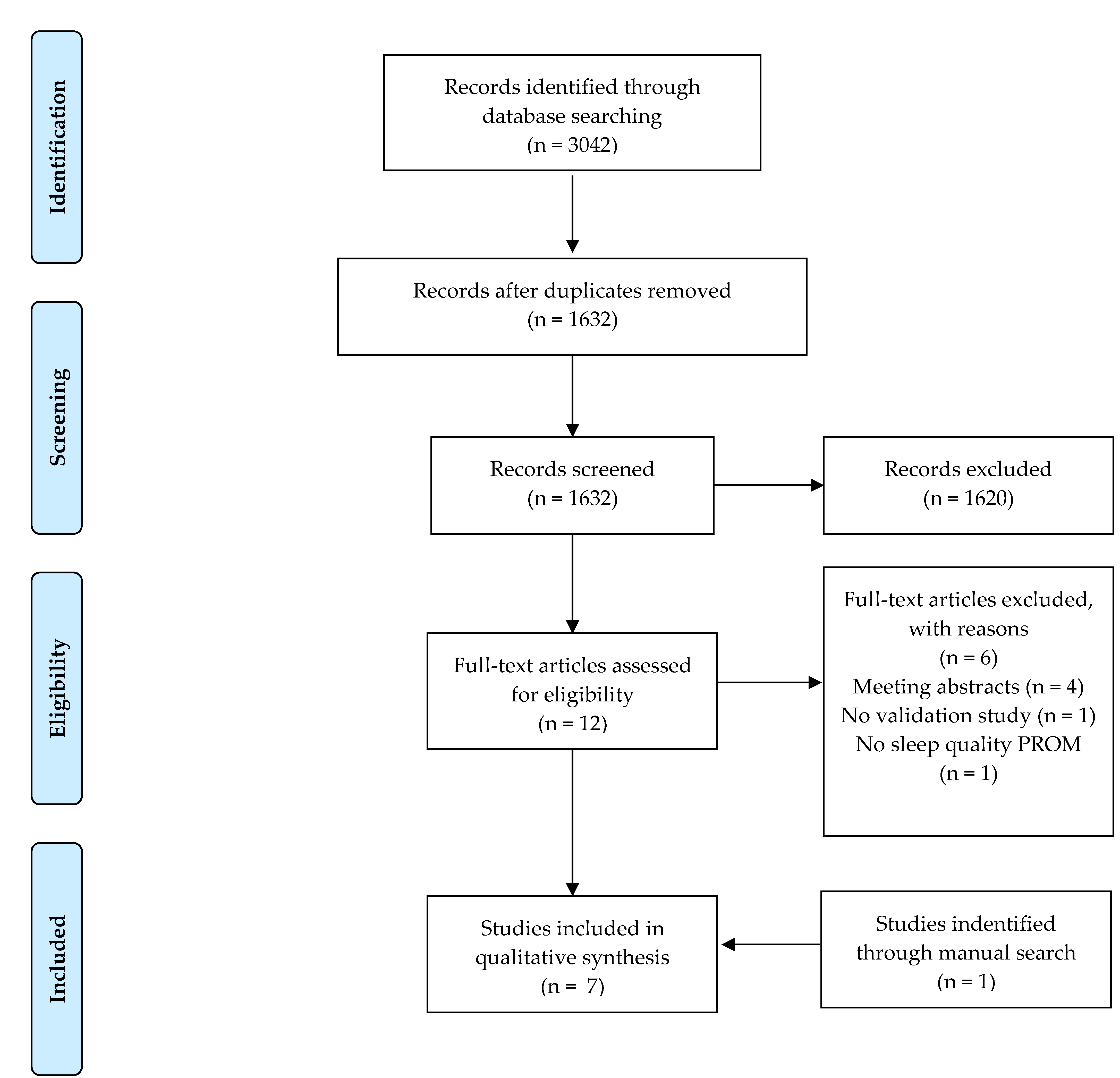
3.1. Study Selection
3.2. Risk of Bias
3.3. Characteristics of the Included PROMs and the Study Populations
3.3.1. Pittsburgh Sleep Quality Index (PSQI)
3.3.2. Jenkins Sleep Scale (JSS)
3.3.3. Sleep Quality-Numeric Rating Scale (SQ-NRS)
3.3.4. Medical Outcomes Study-Sleep Scale (MOS-SS)
3.3.5. Fibromyalgia Sleep Diary (FSD)
3.4. Results of Studies on the Measurement Properties in People Diagnosed with FM
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A1. Pubmed
Appendix A2. CINAHL Plus
| # | Query | Results |
|---|---|---|
| S1 | (MH “Sleep Hygiene”) OR (MH “Sleep”) | 17.544 |
| S2 | TI Sleep OR AB sleep | 54.713 |
| S3 | (MH “Fibromyalgia”) | 5.285 |
| S4 | TI (Fibromyalgia* OR “Muscular Rheumatism” OR Fibrositi*) OR AB (Fibromyalgia* OR “Muscular Rheumatism” OR Fibrositi*) | 5.299 |
| S5 | S1 OR S2 | 59.549 |
| S6 | S3 OR S4 | 6.382 |
| S7 | S5 AND S6 | 732 |
| S8 | (MH “Psychometrics”) or (TI psychometr* or AB psychometr*) or (TI clinimetr* or AB clinimetr*) or (TI clinometr* OR AB clinometr*) or (MH “Outcome Assessment”) or (TI outcome assessment or AB outcome assessment) or (TI outcome measure* or AB outcome measure*) or (MH “Health Status Indicators”) or (MH “Reproducibility of Results”) or (MH “Discriminant Analysis”) or ((TI reproducib* or AB reproducib*) or (TI reliab* or AB reliab*) or (TI unreliab* or AB unreliab*)) or ((TI valid* or AB valid*) or (TI coefficient or AB coefficient) or (TI homogeneity or AB homogeneity)) or (TI homogeneous or AB homogeneous) or (TI “coefficient of variation” or AB “coefficient of variation”) or (TI “internal consistency” or AB “internal consistency”) or (MH “Internal Consistency+”) or (MH “Reliability+”) or (MH “Measurement Error+”) or (MH “Content Validity+”) or “hypothesis testing” or “structural validity” or “cross-cultural validity” or (MH “Criterion-Related Validity+”) or “responsiveness” or “interpretability” or (TI reliab* or AB reliab*) and ((TI test or AB test) OR (TI retest or AB retest)) or (TI stability or AB stability) or (TI interrater or AB interrater) or (TI inter-rater or AB inter-rater) or (TI intrarater or AB intrarater) or (TI intra-rater or AB intrarater) or (TI intertester or AB intertester) or (TI inter-tester or AB inter-tester) or (TI intratester or AB intratester) or (TI intra-tester or AB intra-tester) or (TI interobserver or AB interobserver) or (TI inter-observer or AB inter-observer) or (TI intraobserver or AB intraobserver) or (TI intra-observer or AB intra-observer) or (TI intertechnician or AB intertechnician) or (TI inter-technician or AB inter-technician) or (TI intratechnician or AB intratechnician) or (TI intra-technician or AB intra-technician) or (TI interexaminer or AB interexaminer) or (TI inter-examiner or AB inter-examiner) or (TI intraexaminer or AB intraexaminer) OR (TI intra-examiner or AB intra-examiner) or (TI intra-examiner or AB intraexaminer) or (TI interassay or AB interassay) or (TI inter-assay or AB inter-assay) or (TI intraassay or AB intraassay) or (TI intra-assay or AB intra-assay) or (TI interindividual or AB interindividual) or (TI inter-individual or AB inter-individual) OR (TI intraindividual or AB intraindividual) or (TI intra-individual or AB intra-individual) or (TI interparticipant or AB interparticipant) or (TI inter-participant or AB inter-participant) or (TI intraparticipant or AB intraparticipant) or (TI intra-participant or AB intra-participant) or (TI kappa or AB kappa) or (TI kappa’s or AB kappa’s) or (TI kappas or AB kappas) or (TI repeatab* or AB repeatab*) or (TI responsive* or AB responsive*) or (TI interpretab* or AB interpretab*) | 568.245 |
| S9 | S7 AND S8 | 150 |
Appendix A3. Scopus
Appendix A4. Psychinfo
Appendix A5. ISI Web of Science
References
- Williams, D.A.; Arnold, L.M. Measures of Fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arthritis Care Res. 2011, 63, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Carmona, L.; Curbelo, R.J.; Isabel, C.G. Proms for Fibromyalgia. In Patient Reported Outcome Measures in Rheumatic Diseases; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–208. ISBN 978-3-319-3285-5. [Google Scholar]
- Mease, P.J.; Arnold, L.M.; Crofford, L.J.; Williams, D.A.; Russell, I.J.; Humphrey, L.; Abetz, L.; Martin, S.A. Identifying the Clinical Domains of Fibromyalgia: Contributions from Clinician and Patient Delphi Exercises. Arthritis Care Res. 2008, 59, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; Arnold, L.M.; Choy, E.H.; Clauw, D.J.; Crofford, L.J.; Glass, J.M.; Martin, S.A.; Morea, J.; Simon, L.; Strand, C.V.; et al. Fibromyalgia Syndrome Module at Omeract 9: Domain Construct. J. Rheumatol. 2009, 36, 2318–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, F.; Smythe, H.; Yunus, M.B.; Bennet, R.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- White, K.P.; Speechley, M.; Harth, M.; ØStbye, T. The London Fibromyalgia Epidemiology Study: Comparing the Demographic and Clinical Characteristics in 100 Random Community Cases of Fibromyalgia Versus Controls. J. Rheumatol. 1999, 26, 1577–1585. [Google Scholar]
- Theadom, A.; Cropley, M.; Humphrey, K.L. Exploring the Role of Sleep and Coping in Quality of Life in Fibromyalgia. J. Psychosom. Res. 2007, 62, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.-S.; DiBonaventura, M.D.; Chandran, A.B.; Cappelleri, J.C. The Association of Sleep Difficulties with Health-Related Quality of Life among Patients with Fibromyalgia. BMC Musculoskelet. Disord. 2012, 13, 199. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-L.; Chang, L.-Y.; Lee, H.-C.; Fang, S.-C.; Tsai, P.-S. Sleep Disturbances in Fibromyalgia: A Meta-Analysis of Case-Control Studies. J. Psychosom. Res. 2017, 96, 89–97. [Google Scholar] [CrossRef]
- Andrade, A.; Vilarino, G.T.; Sieczkowska, S.M.; Coimbra, D.R.; Bevilacqua, G.G.; Steffens, R.A.K. The Relationship between Sleep Quality and Fibromyalgia Symptoms. J. Health Psychol. 2018, 1–11. [Google Scholar] [CrossRef]
- Cheatle, M.D.; Foster, S.; Pinkett, A.; Lesneski, M.; Qu, D.; Dhingra, L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Anesthesiol. Clin. 2016, 34, 379–393. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. Cosmin Guideline for Systematic Reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. Press Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. Cosmin Risk of Bias Checklist for Systematic Reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Abedi, A.; Prinsen, C.A.C.; Shah, I.; Buser, Z.; Wang, J.C. Performance Properties of Health-Related Measurement Instruments in Whiplash: Systematic Review Protocol. Syst. Rev. 2019, 8, 199. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and Validity of the Spanish Version of the Pittsburgh Sleep Quality Index (Psqi) in Patients with Fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Crawford, B.K.; Piault, E.C.; Lai, C.; Sarzi-Puttini, P.; Crawford, B.K.; Piault, E.C.; Lai, C.; Sarzi-Puttini, P. Assessing Sleep in Fibromyalgia: Investigation of an Alternative Scoring Method for the Jenkins Sleep Scale Based on Data from Randomised Controlled Studies. Clin. Exp. Rheumatol. 2010, 28, 100–109. [Google Scholar]
- Martin, S.; Chandran, A.; Zografos, L.; Zlateva, G. Evaluation of the Impact of Fibromyalgia on Patients’ Sleep and the Content Validity of Two Sleep Scales. Health Qual. Life Outcomes 2009, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Cappelleri, J.C.; Bushmakin, A.G.; McDermott, A.M.; Dukes, E.; Sadosky, A.; Petrie, C.D.; Martin, S. Measurement Properties of the Medical Outcomes Study Sleep Scale in Patients with Fibromyalgia. Sleep Med. 2009, 10, 766–770. [Google Scholar] [CrossRef]
- Sadosky, A.; Dukes, E.; Evans, C. Reliability of a 1-Week Recall Period for the Medical Outcomes Study Sleep Scale (MOS-SS) in Patients with Fibromyalgia. Health Qual. Life Outcomes 2009, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Cappelleri, J.C.; Bushmakin, A.G.; McDermott, A.M.; Sadosky, A.B.; Petrie, C.D.; Martin, S. Psychometric Properties of a Single-Item Scale to Assess Sleep Quality among Individuals with Fibromyalgia. Health Qual. Life Outcomes 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, L.; Mannix, S.; Arnold, L.M.; Burbridge, C.; Howard, K.; McQuarrie, K.; Pitman, V.; Resnick, M.; Roth, T.; Symonds, T. Assessment of Sleep in Patients with Fibromyalgia: Qualitative Development of the Fibromyalgia Sleep Diary. Health Qual. Life Outcomes 2014, 12, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J.; Iii, C.F.R.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Jenkins, C.D.; Stanton, B.-A.; Niemcryk, S.J.; Rose, R.M. A Scale for the Estimation of Sleep Problems in Clinical Research. J. Clin. Epidemiol. 1988, 41, 313–321. [Google Scholar] [CrossRef]
- Hays, R.D.; Martin, S.A.; Sesti, A.M.; Spritzer, K.L. Psychometric Properties of the Medical Outcomes Study Sleep Measure. Sleep Med. 2005, 6, 41–44. [Google Scholar] [CrossRef]
- Stewart, A.; Ware, J. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach; Duke University Press: Durham, UK, 1992; ISBN 978-08-2231-212-3. [Google Scholar]
- Irving, G.; Neves, A.L.; Dambha-Miller, H.; Oishi, A.; Tagashira, H.; Verho, A.; Holden, J. International Variations in Primary Care Physician Consultation Time: A Systematic Review of 67 Countries. BMJ Open 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Baird, B.; Charles, A.; Honeyman, M.; Maguire, D.; Das, P. Understanding Pressures in General Practice; The King’s Fund: London, UK, 2016; ISBN 978-1-909029-61-3. [Google Scholar]
- Bigatti, S.M.; Hernandez, A.M.; Cronan, T.A.; Rand, K.L. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Rheum. 2008, 59, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Keskindag, B.; Karaaziz, M. The association between pain and sleep in fibromyalgia. Saudi Med. J. 2017, 38, 465–475. [Google Scholar] [CrossRef]
- Koca, T.T.; Karaca Acet, G.; Tanrikut, E.; Talu, B. Evaluation of sleep disorder and its effect on sexual dysfunction in patients with Fibromyalgia syndrome. Turk. J. Obstet. Gynecol. 2016, 13, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Turkoglu, G.; Selvi, Y. The relationship between chronotype, sleep disturbance, severity of fibromyalgia, and quality of life in patients with fibromyalgia. Chronobiol. Int. 2020, 37, 68–81. [Google Scholar] [CrossRef]
- Gomez-Hernandez, M.; Gallego-Izquierdo, T.; Martinez-Merinero, P.; Pecos-Martin, D.; Ferragut-Garcias, A.; Hita-Contreras, F.; Martinez-Amat, A.; Montanez-Aguilera, F.J.; Achalandabaso Ochoa, A. Benefits of adding stretching to a moderate-intensity aerobic exercise programme in women with fibromyalgia: A randomized controlled trial. Clin. Rehabil. 2020, 34, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Van Gordon, W.; Shonin, E.; Dunn, T.J.; Garcia-Campayo, J.; Griffiths, M.D. Meditation awareness training for the treatment of fibromyalgia syndrome: A randomized controlled trial. Br. J. Health Psychol. 2017, 22, 186–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lami, M.J.; Martínez, M.P.; Miró, E.; Sánchez, A.I.; Prados, G.; Cáliz, R.; Vlaeyen, J.W.S. Efficacy of Combined Cognitive-Behavioral Therapy for Insomnia and Pain in Patients with Fibromyalgia: A Randomized Controlled Trial. Cognit. Ther. Res. 2017, 42, 63–79. [Google Scholar] [CrossRef]
- Guinot, M.; Maindet, C.; Hodaj, H.; Hodaj, E.; Bachasson, D.; Baillieul, S.; Cracowski, J.L.; Launois, S. Effects of repetitive transcranial magnetic stimulation and multicomponent therapy in patients with fibromyalgia: A randomized controlled trial. Arthritis Care Res. (Hoboken) 2019. [Google Scholar] [CrossRef] [PubMed]
| Measurement Property | Rating | Criteria |
|---|---|---|
| Structural Validity | + | CTT CFA: CFI or TLI or comparable measure >0.95 OR RMSEA < 0.06 OR SRMR < 0.08 a IRT/Rasch No violation of unidimensionality b: CFI or TLI or comparable measure > 0.95 OR RMSEA < 0.06 OR SRMR < 0.08 AND no violation of local independence: residual correlations among the items after controlling for the dominant factor < 0.20 OR Q3s < 0.37 AND no violation of monotonicity: adequate looking graphs OR item scalability > 0.30 AND adequate model fit IRT: χ2 > 0.001 Rasch: infit and outfit mean squares ≥ 0.5 and ≤ 1.5 OR Z-standardized values > −2 and < 2 |
| ? | CTT: not all information for ‘+’ reported IRT/Rasch: model fit not reported | |
| − | Criteria for ‘+’ not met | |
| Internal Consistency | + | At least low evidence c for sufficient structural validity d AND Cronbach’s alpha(s) ≥ 0.70 for each unidimensional scale or subscale e |
| ? | Criteria for “At least low evidence c for sufficient structural validity d” not met | |
| − | At least low evidence c for sufficient structural validity d AND Cronbach’s alpha(s) < 0.70 for each unidimensional scale or subscale e | |
| Reliability | + | ICC or weighted Kappa ≥ 0.70 |
| ? | ICC or weighted Kappa not reported | |
| − | ICC or weighted Kappa < 0.70 | |
| Measurement Error | + | SDC or LoA < MIC d |
| ? | MIC not defined | |
| − | SDC or LoA > MIC d | |
| Hypotheses Testing for Construct Validity | + | The result is in accordance with the hypothesis f |
| ? | No hypothesis defined (by the review team) | |
| − | The result is not in accordance with the hypothesis f | |
| Cross-Cultural Validity/Measurement Invariance | + | No important differences found between group factors (such as age, gender, language) in multiple group factor analysis OR no important DIF for group factors (McFadden’s R2 < 0.02) |
| ? | No multiple group factor analysis OR DIF analysis performed | |
| − | Important differences between group factors OR DIF was found | |
| Criterion Validity | + | Correlation with gold standard ≥ 0.70 OR AUC ≥ 0.70 |
| ? | Not all information for ‘+’ reported | |
| − | Correlation with gold standard < 0.70 OR AUC < 0.70 | |
| Responsiveness | + | The result is in accordance with the hypothesis f OR AUC ≥ 0.70 |
| ? | No hypothesis defined (by the review team) | |
| − | The result is not in accordance with the hypothesis f OR AUC < 0.70 |
| PROM | Measurement Properties Assessed | Risk of Bias |
|---|---|---|
| PSQI | Internal Consistency | Low |
| Reliability | Low | |
| Structural validity | Low | |
| Hypothesis testing | Low | |
| JSS | Internal Consistency | Low |
| Reliability | Low | |
| Structural validity | Low | |
| Responsiveness | Low | |
| SQ-NRS | Content validity | Low |
| Reliability | Low | |
| Hypothesis testing | Low | |
| MOS-SS | Content validity | Low |
| Internal Consistency | Low | |
| Reliability | Low | |
| FSD | Content validity | Low |
| PROM * (Reference to First Article) | Construct(s) | Target Population | Mode of Administration (e.g., Self-Report, Interview-Based, Parent/Proxy Report etc.) | Recall Period | (Sub)scale(s) (Number of Items) | Response Options | Range of Scores/Scoring | Original Language | Available Translations |
|---|---|---|---|---|---|---|---|---|---|
| Pittsburgh Sleep Quality Index [24] | Sleep Quality | Patients diagnosed with major depressive disorder | Self-completed by the respondent | One month | Subscales: (1) subjective sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbances, (6) use of sleeping medication, and (7) daytime dysfunction. Items: 19 self-rated questions and 5 questions that are answered by the roommate or bedmate | The first four items are answered by providing some data related to the usual time of sleep, time to fall asleep, time awake at night, and hours of sleep per night. The other 12 items to be filled out by the patient plus the items to be filled in by the roommate or bed partner use the previous set of answers, and the respondent is asked to mark an X for the option that most corresponds to their experience: (1) Not during the past month, (2) Less than once a week, (3) Once or twice a week, or (4) Three or more time a week. In one of the items the possible answers are: (1) Very good, (2) Fairly good, (3) Fairly bad or (4) Very bad. Another item has the following answers: (1) No problem at all, (2) Only a very slight problem, (3) Somewhat of a problem, or (4) A very big problem. Finally, the last question has also four possible answers: (1) No bed partner or roommate, (2) Partner/roommate in other room, (3) Partner in the same room, but not same bed, or (4) Partner in the same bed. | The total score of the questionnaire is derived from the sum of the seven components of the questionnaire. Each of the items has, as explained above, four possible answers, so the scoring varies between 0 and 3. In this way, the maximum final score is 21 points and the minimum score is 0. a score lower than 5 points would indicate that the respondent is a “good sleeper” while ratings greater than 5 points would be indicative of poor sleep quality and moderate difficulties in three components or serious difficulties in at least two components of the seven that are evaluated. | English | Spanish with a sample of people diagnosed with FM [17] |
| Jenkins Sleep Scale [25] | Symptoms of insomnia | Patients 6 months after cardiac surgery Air traffic controllers | Self-administered | One month | The scale consists of four items: (1) Do you have trouble falling sleep? (2) Do you wake up several times per night? (3) Do you have trouble staying asleep? (Including waking far too early), and (4) Do you wake up after your usual amount of sleep feeling tired and worn out? | Each of the items is classified on a Likert scale of 6 points based on the frequency with which the respondent experiences each of the evaluated symptoms (0 = not at all, 1 = 1–3 days, 2 = 4–7 days, 3 = 8–14 days, 4 = 15–21 days, and 5 = 22–31 days). | According to the response options previously presented, the results of the JSS can vary from 0 to 20 in the total sum of the items. 0 points are indicative that there are no sleep problems and 20 points indicate significant sleep problems. | English | An alternative scoring method for the JSS was validated in Spanish with a sample of people diagnosed with FM [18]. |
| Sleep Quality Numeric Rating Scale [19] | Sleep Quality | People diagnosed with FM | Self-administered | Daily record of the quality of sleep | It is a tool with a single element. The patient is asked to choose the one that best describes their sleep quality during the last 24 h on a numerical scale. | A numerical scale of 11 points (0–10). | The scoring scale fluctuates in a range between 0 “best possible sleep” and 10 “worst possible sleep”. | English | - |
| Medical Outcomes Study-Sleep Scale [1,19,20,26] | Sleep quality and quantity | Healthy adults and adults diagnosed with neuropathic pain | Self-administered | One month | The MOS-SS is composed of 12 items that evaluate six sleep domains: initiation (time to fall asleep), quantity (hours of sleep each night), maintenance, respiratory problems, perceived adequacy, and drowsiness. | The first item: (1) 0–15 min, (2) 16–30 min, (3) 31–45 min, (4) 46–60 min, (5) More than 60 min. The second item is an open question allowing a response that ranges from 0–24 h. The remaining ten items use a set of 6-point answers based on the following values: (1) All of the time, (2) Most of the time, (3) A good bit of the time, (4) Some of the time, (5) A little of the time, (6) None of the time. | According to the authors, high scores indicate worse sleep problems. The exceptions are the items “sufficiency of sleep” and “quantity” where lower scores indicate worse sleep problems. | English | English with a sample of people diagnosed with FM [19,20,21] |
| Fibromyalgia Sleep Diary [23] | Sleep quality | People diagnosed with FM | Self-administered | Daily record of the quality of sleep | The FSD consist of eight items: (1) How difficult was it to fall asleep last night?, (2) How restless was your sleep last night?, (3) How difficult was it to get comfortable last night?, (4) How difficult was it to stay asleep last night?, (5) How deep was your sleep last night?, (6) How rested were you when you woke up for the day?, (7) How difficult was it to begin your day?, and (8) Did you have enough sleep last night? | A visual analogue scale of 11 points ranging from 0 to 10. | Not provided | English | - |
| Population | Disease Characteristics | Instrument Administration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PROM | Ref | N | Age Mean (SD, Range) yr | Gender % Female | Disease | Disease Duration Mean (SD) yr | Disease Severity | Setting | Country | Language | Response Rate |
| Pittsburgh Sleep Quality Index | 24 | Sample 1 = 34 Sample 2 = 45 Sample 3 = 17 Sample 4 = 52 | Sample 1: 50.9 (range: 21–80) Sample 2: 44.8 (range: 20–80) Sample 3: 42.2 (range: 19–57) Sample 4: 59.9 (range: 24–83) | Sample 1: 26.4% Sample 2: 64.4% Sample 3: 52.9% Sample 4: 23.07% | Sample 1: Major depressive disorder Sample 2: Disorder of Initiating and Maintaining Sleep Sample 3: Disorders of Excessive Somnolence Sample 4: Healthy subjects | - | - | Psychiatric Clinics | United States of America | English | 93.67% |
| 17 | 138 | 52.83 (±9.32) | 100% women | Fibromyalgia | 15.77 years (± 9.76) | Moderate: FIQ < 70 N = 68 FIQ score (51.02 ± 16.28) Severe: FIQ ≥ 70 N = 70 FIQ score (80.44 ± 6.20) | Community (FM association) | Spain | Spanish | Test: 100% Retest: 69.56% | |
| Jenkins Sleep Scale | 25 | Sample 1 = 300 Sample 2 = 467 | Sample 1: 37.1 (25–49) Sample 2: 54.9 (25–69) | Sample 1: 0% Sample 2: 20% | Sample 1: Air Traffic Controllers Sample 2: Cardiac valve surgery or coronary bypass | Sample 1: - Sample 2: - | Sample 1: - Sample 2; - | Sample 1: community Sample 2: Secondary health care | United States of America | English | Sample 1: 83.33% Sample 2: Test: 100% Retest: 91.22% |
| 18 | 195 | 46.5 (±11.35) | 94.4% | Fibromyalgia | ∼9 years | - | Clinical setting (unspecified) | United States of America | English | 97.95% | |
| Sleep Quality Numeric Rating Scale | 20 | Sample 1 = 748 Sample 2 = 745 | Sample 1: 48.8 (±10.9) Sample 2: 50.1 (±11.4) | Sample 1: 94.4% Sample 2: 94.5% | Fibromyalgia | Sample 1: ∼9 years Sample 2: ∼10 years | Mean pain score (0–10) Sample 1: 7.1 (±1.3) Sample 2: 6.7 (±1.3) | Clinical setting (unspecified) | United States of America | English | - |
| 19 | 20 | 50.3 (29–64) | 80% | Fibromyalgia | 8.9 (−1–18) | Pain level (0–10) (SD) 6 (1.6) | Community | United States of America | English | ||
| Medical Outcomes Study Sleep Scale | 27 | Sample 1 = 1011 Sample 2 = 173 | Sample 1: 46 (18–94 range) Sample 2: 72 (31–100 range) | Sample 1: 51% Sample 2: 53% | Sample 1: Healthy subjects Sample 2: Postherpetic neuralgia | Sample 1: -Sample 2: 33.8 months (35.9) | - | Clinical Setting (unspecified) | United States of America | English | Sample 1: - Sample 2: Test: 100% Re-test: 51.44% |
| 19 | 20 | 50.3 (29–64) | 80% | Fibromyalgia | 8.9 (−1–18) | Pain level (0–10) (SD) 6 (1.6) | Community | United States of America | English | ||
| 20 | Sample 1: 748 Sample 2: 745 | Sample 1: 48.8 (±10.9) Sample 2: 50.1 (±11.4) | Sample 1: 94.4% Sample 2: 94.5% | Fibromyalgia | Sample 1: ∼9 years Sample 2: ∼10 years | Mean pain score (0–10) Sample 1: 7.1 (±1.3) Sample 2: 6.7 (±1.3) | Clinical setting (unspecified) | United States of America | English | - | |
| 21 | 129 | 49.4 (±11.0) | 91.3% | Fibromyalgia | ≥2 years | Moderate-to-severe in 88.1% of the sample | Community | United States of America | English | 100% | |
| Fibromyalgia Sleep Diary | 24 | FM experts = 4 FM patients = 34 | FM patients: 47.8 (±11.9) | FM patients: 88.2% | Fibromyalgia | Not reported | Not reported | Community-based clinical sites | United States of America | English | 100% |
| PROM (Ref) | Country (Language) in Which the PROM Was Evaluated | Internal Consistency | Test–Retest Reliability | Hypotheses Testing | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | ||
| Pittsburgh Sleep Quality Index [17] | Spain (Spanish) | 138 | + | α = 0.805 | 96 | + | ρ = 0.806 for the PSQI total score (p < 0.001). Lowest value ρ = 0.356 “daytime dysfunction” Highest value ρ = 0.718 “use of sleeping medication” | 96 | + | FIQ (total score) ρ = 0.304 (p < 0.01) SF-36 Physical functioning ρ = −0.372 (p < 0.01) Role physical ρ = −0.217 (p < 0.05) Role emotional ρ = −0.254 (p < 0.01) Vitality ρ = −0.247 (p < 0.05) Mental Health ρ = −0.208 (p < 0.05) Social functioning ρ = −0.426 (p < 0.01) Bodily pain ρ = −0.351 (p < 0.01) General Health NS |
| Pooled or summary result (overall rating) | 138 | 0.805 | 96 | 0.806 | 96 | FIQ: ρ = 0.304 (p < 0.01) SF-36: General Health NS Social functioning ρ = −0.426 (p < 0.01) | ||||
| PROM (Ref) | Country (Language) in Which the PROM Was Evaluated | Internal Consistency | Criterion validity | Reliability | Responsiveness | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | n | Meth Qual | Results (Rating) | ||
| Jenkins Sleep Scale [18] | United States of America (English) | 195 | + | α = 0.70 | 195 | +/− | FIQ item 16 r = 0.68 FIQ item 17 r = 0.72 Pain VAS r = 54 Fatigue VAS r = 57 ESS r = 0.43 FOSQ total score r = −0.57 SF-36 Vitality score r = −0.66 | 195 | +/− | FIQ total score ICC 0.70 FIQ item 17 ICC 0.72 ESS ICC 0.69 Fatigue VAS ICC 0.66 Pain VAS ICC 0.61 | R: 38 NR: 115 | + | R: Pain VAS + FIQ total score SES = 1.62 NR Pain VAS + FIQ total score SES = −1.33 |
| Pooled or summary result (overall rating) | 195 | 0.70 | 0.43–0.72 | 195 | 0.61–0.72 | R: 38 NR: 115 | R: 1.62 NR: −1.33 | ||||||
| PROM (Ref) | Country (Language) in Which the PROM Was Evaluated | Criterion Validity | Test–Retest Reliability | Responsiveness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | ||
| Sleep Quality Numeric Rating Scale [20] | United States of America (English) | Sample 1 = 748 Sample 2 = 745 | + | PNRS Sample 1 r = 0.64, p < 0.001 Sample 2 r = 0.58, p < 0.001 MOS-SS Sample 1 Sleep disturbance r = 0.45, p < 0.001 Snoring r = 0.01, p = 0.884 Awaken Short of breath of with headache r = 0.21, p < 0.001 Quantity of sleep r = −0.31, p < 0.001 Sleep adequacy r = −0.21, p < 0.001 Somnolence r = 0.11, p = 0.004 Sample 2 Sleep disturbance r = 0.42, p < 0.001 Snoring r = 0.00, p = 0.993 Awaken Short of breath of with headache r = 0.14, p < 0.001 Quantity of sleep r = −0.34, p < 0.001 Sleep adequacy r = −0.32, p < 0.001 Somnolence r = 0.15, p < 0.001 | Sample 1 = 748 Sample 2 = 745 | + | Sample 1 ICC 0.90 Sample 2 ICC 0.91 | Pregabalin treatment Sample 1: 300 mg (n = 368) Sample 2: 450 mg (n = 373) Sample 3: 600 mg (n = 378) | + | Sample 1: SES = 0.46–0.52 Sample 2: SES = 0.59 Sample 3: SES = 0.73 |
| Pooled or summary result (overall rating) | 1493 | PNRS 0.58–0.64 MOS-SS 0.00–0.45 | 1493 | 0.90–0.91 | 0.46–0.73 | |||||
| PROM (Ref) | Country (Language) in Which the PROM Was Evaluated | Structural Validity | Internal Consistency | Test–Retest Reliability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | n | Meth Qual | Result (Rating) | ||
| Medical Outcomes Study Sleep Scale [20] | United States of America (English) | Sample 1 = 748 Sample 2 = 745 | − | CFA Bentler’s comparative fit index Baseline: 0.88 Week 5: 0.93 Week 9: 0.91 Week 13: 0.92 | Sample 1 = 748 Sample 2 = 745 | +/− | Sample 1: Week 1/week 13 Sleep disturbance subscale α = 0.78/α = 0.87 Somnolence subscale α = 0.72/α = 0.86 Sleep adequacy subscale α = 0.36/α = 0.74 Sample 2: Week 1/week 13 Sleep disturbance subscale α = 0.80/α = 0.87 Somnolence subscale α = 0.71/α = 0.75 Sleep adequacy subscale α = 0.61/α = 0.74 | |||
| Medical Outcomes Study Sleep Scale [21] | United States of America (English) | 140 | + | Week 1 = ICC 0.81Week 4 = ICC 0.89 | ||||||
| Pooled or summary result (overall rating) | 1493 | 0.88–0.93 | 1493 | 0.36–0.87 | 140 | 0.81–0.89 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent-Sanz, C.; Marco-Mitjavila, A.; Pastells-Peiró, R.; Valenzuela-Pascual, F.; Blanco-Blanco, J.; Gea-Sánchez, M. Patient Reported Outcome Measures of Sleep Quality in Fibromyalgia: A COSMIN Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2992. https://doi.org/10.3390/ijerph17092992
Climent-Sanz C, Marco-Mitjavila A, Pastells-Peiró R, Valenzuela-Pascual F, Blanco-Blanco J, Gea-Sánchez M. Patient Reported Outcome Measures of Sleep Quality in Fibromyalgia: A COSMIN Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(9):2992. https://doi.org/10.3390/ijerph17092992
Chicago/Turabian StyleCliment-Sanz, Carolina, Anna Marco-Mitjavila, Roland Pastells-Peiró, Fran Valenzuela-Pascual, Joan Blanco-Blanco, and Montserrat Gea-Sánchez. 2020. "Patient Reported Outcome Measures of Sleep Quality in Fibromyalgia: A COSMIN Systematic Review" International Journal of Environmental Research and Public Health 17, no. 9: 2992. https://doi.org/10.3390/ijerph17092992
APA StyleCliment-Sanz, C., Marco-Mitjavila, A., Pastells-Peiró, R., Valenzuela-Pascual, F., Blanco-Blanco, J., & Gea-Sánchez, M. (2020). Patient Reported Outcome Measures of Sleep Quality in Fibromyalgia: A COSMIN Systematic Review. International Journal of Environmental Research and Public Health, 17(9), 2992. https://doi.org/10.3390/ijerph17092992





