Decreased Methane Emissions Associated with Methanogenic and Methanotrophic Communities in a Pig Manure Windrow Composting System under Calcium Superphosphate Amendment
Abstract
1. Introduction
2. Materials and Methods
2.1. Windrow Composting Construction
2.2. Measurement of CH4 Fluxes
2.3. Physicochemical Parameters Determination
2.4. DNA Extraction and Real-Time Quantitative PCR (q-PCR) Assays of the Functional Genes
2.5. Illumina MiSeq Sequencing of mcrA and pmoA Genes
2.6. Analysis of Illumina MiSeq Sequencing Data
2.7. Statistical Analysis
3. Results
3.1. CH4 Fluxes
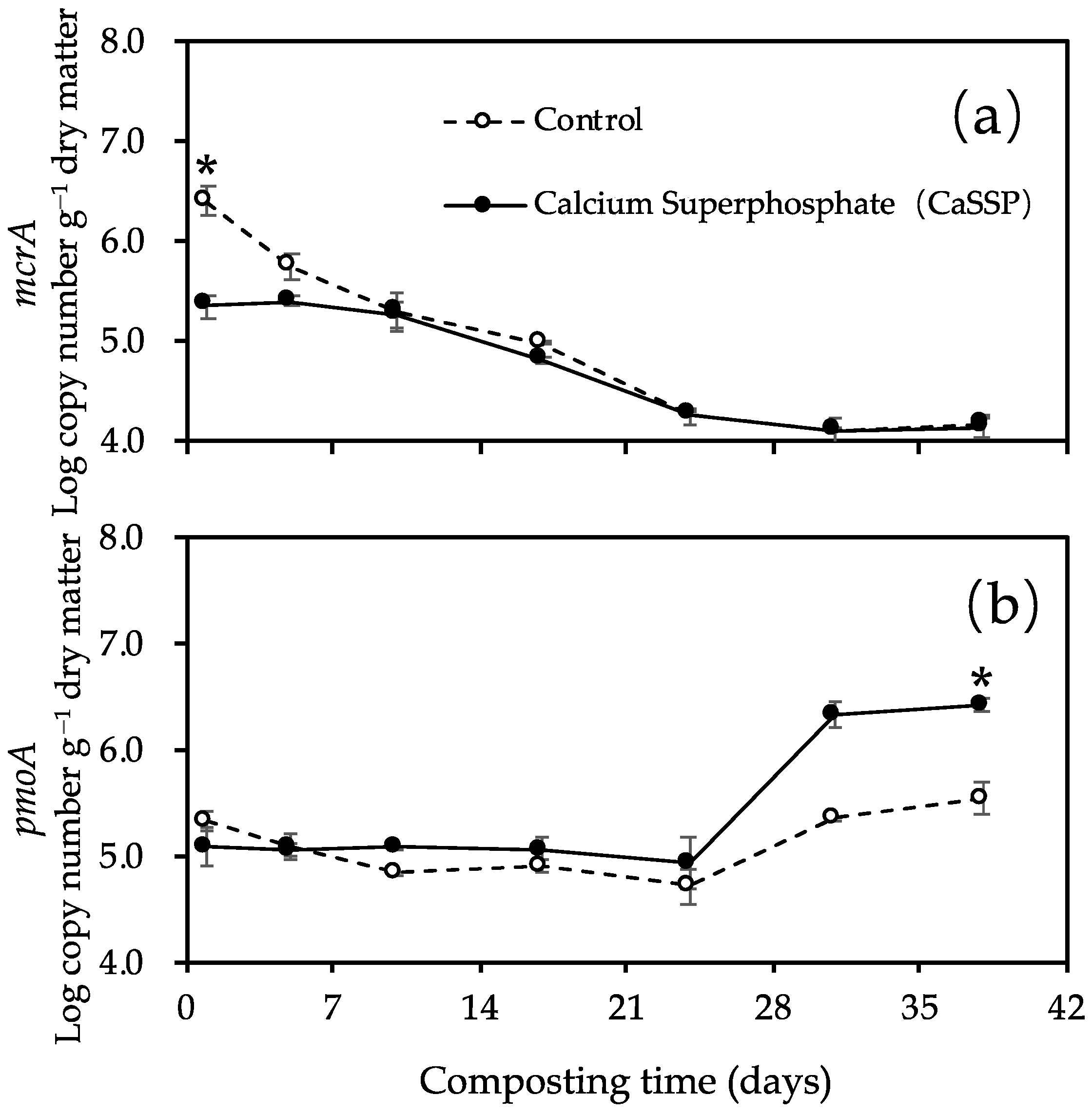
3.2. Methanogenic and Methanotrophic Abundances
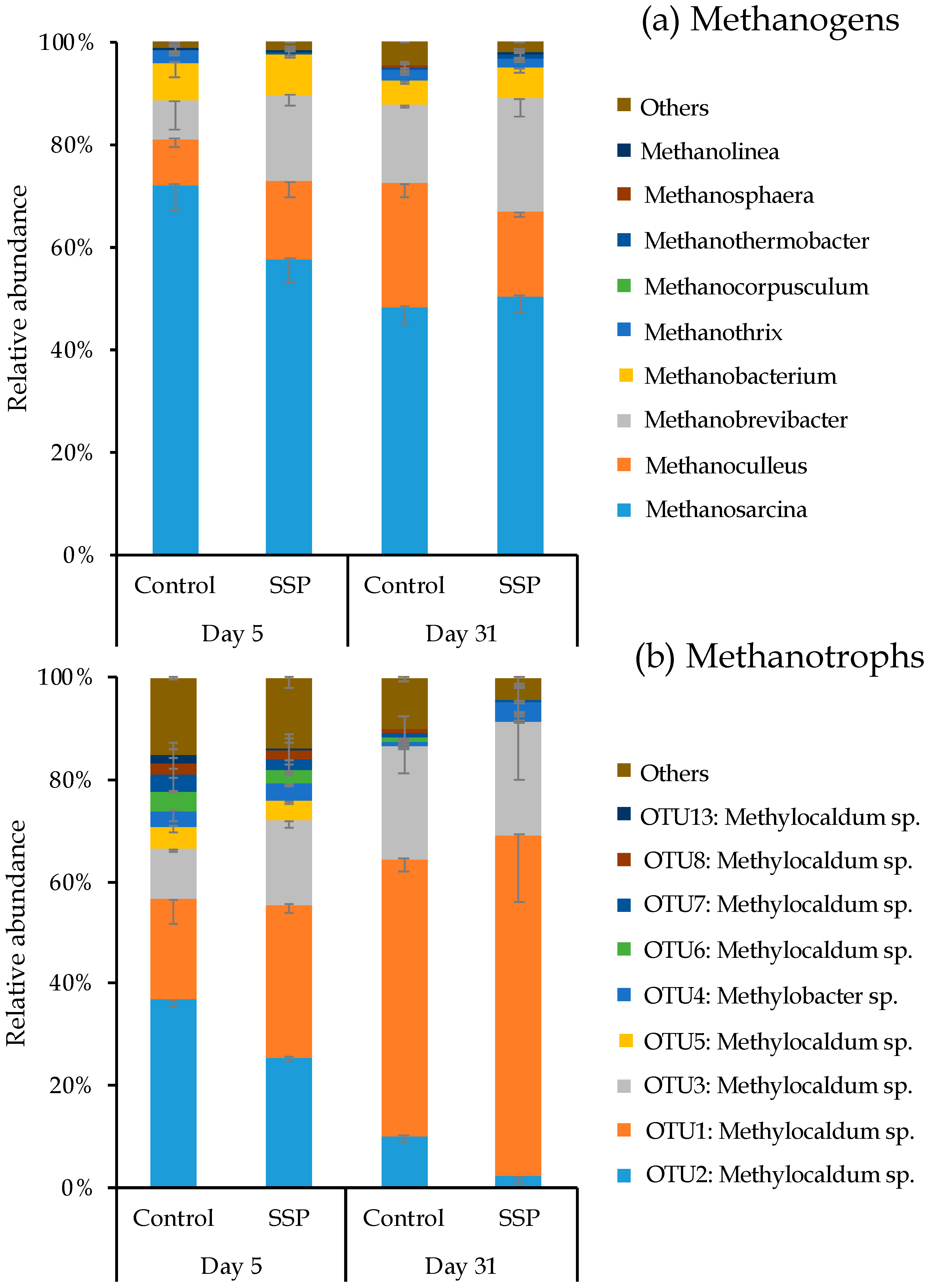
3.3. Response of Methanogenic and Methanotrophic Community to CaSSP Addition
4. Discussion
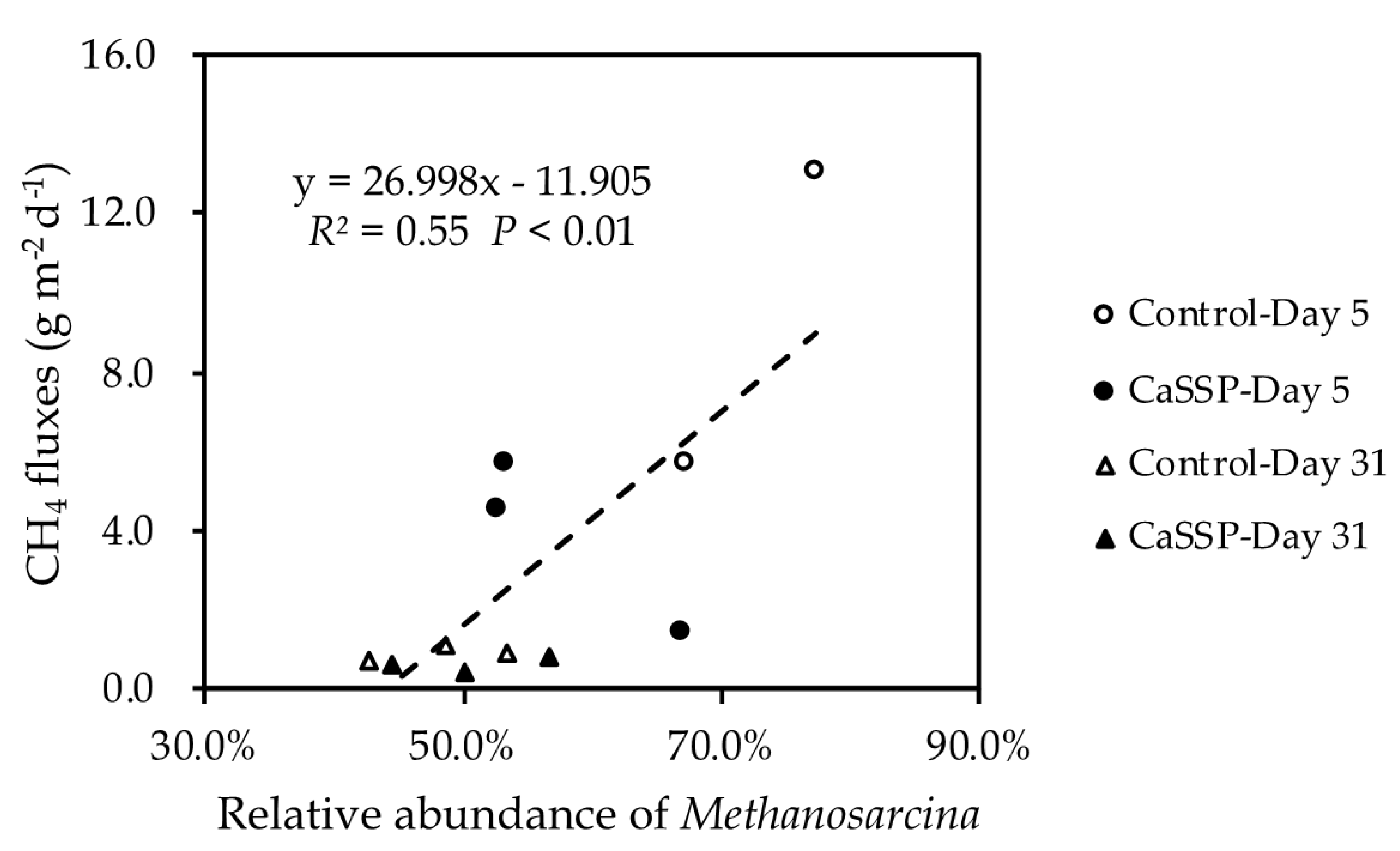
4.1. Methane Production by Methanogenic Community
4.2. Methane Oxidation by Methanotrophic Community
4.3. Physicochemical Parameters Involved in the CaSSP Affected CH4 Emissions during Composting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Oenema, O.; Tamminga, S. Nitrogen in global animal production and management options for improving nitrogen use efficiency. Sci. China Ser. C Life Sci. 2005, 48, 871–887. [Google Scholar] [CrossRef]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. The Third National Communication on Climate Change of the People’s Republic of China. (TNCCCC). 2018. Available online: http://english.mee.gov.cn/Resources/Reports/reports/201907/P020190702568751604320.pdf (accessed on 1 December 2018).
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Technical Specification for Sanitation Treatment of Livestock and Poultry Manure; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2018.
- Zheng, J.; Liu, J.; Han, S.; Wang, Y.; Wei, Y. N2O emission factors of full-scale animal manure windrow composting in cold and warm seasons. Bioresour. Technol. 2020, 316, 123905. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Impraim, R.; Coates, T.; Flesch, T.; Trouvé, R.; van Grinsven, H.; Cao, Y.; Hill, J.; Chen, D. Lignite effects on NH3, N2O, CO2 and CH4 emissions during composting of manure. J. Environ. Manag. 2020, 271. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, H.; Ma, J. Methane and Nitrous Oxide Emissions from Rice-Based Ecosystems; University of Science and Technology of China Press: Hefei, China, 2019. [Google Scholar]
- Sonoki, T.; Furukawa, T.; Jindo, K.; Suto, K.; Aoyama, M.; Sánchez-Monedero, M.Á. Influence of biochar addition on methane metabolism during thermophilic phase of composting. J. Basic Microbiol. 2013, 53, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- McDonald, I.R.; Radajewski, S.; Murrell, J.C. Stable isotope probing of nucleic acids in methanotrophs and methylotrophs: A review. Org. Geochem. 2005, 36, 779–787. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.H.; Freeman, C.; Fenner, N.; Kang, H. Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biol. Biochem. 2008, 40, 2874–2880. [Google Scholar] [CrossRef]
- Sharma, R.; Ryan, K.; Hao, X.; Larney, F.J.; McAllister, T.A.; Topp, E. Real-Time Quantification of mcr A, pmo A for Methanogen, Methanotroph Estimations during Composting. J. Environ. Qual. 2011, 40, 199–205. [Google Scholar] [CrossRef]
- Wanapat, M.; Cherdthong, A.; Phesatcha, K.; Kang, S. Dietary sources and their effects on animal production and environmental sustainability. Anim. Nutr. 2015, 1, 96–103. [Google Scholar] [CrossRef]
- Iguchi, H.; Umeda, R.; Taga, H.; Oyama, T.; Yurimoto, H.; Sakai, Y. Community composition and methane oxidation activity of methanotrophs associated with duckweeds in a fresh water lake. J. Biosci. Bioeng. 2019, 128, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xiong, Y.; Yuan, J.; Shaaban, M.; Zhou, W.; Hu, R. The influence of soil temperature, methanogens and methanotrophs on methane emissions from cold waterlogged paddy fields. J. Environ. Manag. 2020, 264, 110421. [Google Scholar] [CrossRef]
- Li, S.; Song, L.; Gao, X.; Jin, Y.; Liu, S.; Shen, Q.; Zou, J. Microbial abundances predict methane and nitrous oxide fluxes from a windrow composting system. Front. Microbiol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, G.; Shi, H.; Wang, Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag. 2015, 36, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Han, Y.; Zhu, H.; Deng, L.; Yang, W.; Meng, Q.; Sun, Y.; Egbeagu, U.U.; Sheng, S.; Wu, X.; et al. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 2020, 721, 137759. [Google Scholar] [CrossRef]
- Peng, S.; Li, H.; Xu, Q.; Lin, X.; Wang, Y. Addition of zeolite and superphosphate to windrow composting of chicken manure improves fertilizer efficiency and reduces greenhouse gas emission. Environ. Sci. Pollut. Res. 2019, 26, 36845–36856. [Google Scholar] [CrossRef]
- Pennock, D.; Yates, T.; Bedard-Haughn, A.; Phipps, K.; Farrell, R.; McDougal, R. Landscape controls on N2O and CH4 emissions from freshwater mineral soil wetlands of the Canadian Prairie Pothole region. Geoderma 2010, 155, 308–319. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Chen, S.; Li, D.; Tang, H.; Chadwick, D.; Li, S.; Li, W.; Li, G. Effects of phosphogypsum, superphosphate, and dicyandiamide on gaseous emission and compost quality during sewage sludge composting. Bioresour. Technol. 2018, 270, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Miao, Y.; Geng, Y.; Huang, M.; Zhang, Y.; Song, X.; Li, S.; Zou, J. Calcium superphosphate-mediated reshaping of denitrifying bacteria community contributed to n2 o mitigation in pig manure windrow composting. Int. J. Environ. Res. Public Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Hao, X.; Larney, F.J. Greenhouse gas emissions during co-composting of cattle feedlot manure with construction and demolition (C&D) waste. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Bertora, C.; Peyron, M.; Pelissetti, S.; Grignani, C.; Sacco, D. Assessment of methane and nitrous oxide fluxes from paddy field by means of static closed chambers maintaining plants within headspace. J. Vis. Exp. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Li, S.; Jin, Y.; Wu, S.; Chen, J.; Hu, T.; Wang, H.; Liu, S.; Zou, J. Linking methane emissions to methanogenic and methanotrophic communities under different fertilization strategies in rice paddies. Geoderma 2019, 347, 233–243. [Google Scholar] [CrossRef]
- Zou, J.; Liu, S.; Qin, Y.; Pan, G.; Zhu, D. Sewage irrigation increased methane and nitrous oxide emissions from rice paddies in southeast China. Agric. Ecosyst. Environ. 2009, 129, 516–522. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, S.; Guo, Y.; Liu, Q.; Zou, J. Methane and nitrous oxide emissions from organic and conventional rice cropping systems in Southeast China. Biol. Fertil. Soils 2010, 46, 825–834. [Google Scholar] [CrossRef]
- Shang, Q.; Yang, X.; Gao, C.; Wu, P.; Liu, J.; Xu, Y.; Shen, Q.; Zou, J.; Guo, S. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: A 3-year field measurement in long-term fertilizer experiments. Glob. Chang. Biol. 2011, 17, 2196–2210. [Google Scholar] [CrossRef]
- Steinberg, L.M.; Regan, J.M. Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef]
- Costello, A.M.; Lidstrom, M.E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl. Environ. Microbiol. 1999, 65, 5066–5074. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Ji, C.; Zhang, N.; Song, M.; Kong, D.; Liu, S.; Zhang, X.; Liu, X.; Zou, J.; et al. An additive effect of elevated atmospheric CO2 and rising temperature on methane emissions related to methanogenic community in rice paddies. Agric. Ecosyst. Environ. 2018, 257, 165–174. [Google Scholar] [CrossRef]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that participate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol. Lett. 1995, 132, 203–208. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Yang, S.; Liebner, S.; Alawi, M.; Ebenhöh, O.; Wagner, D. Taxonomic database and cut-off value for processing mcrA gene 454 pyrosequencing data by MOTHUR. J. Microbiol. Methods 2014, 103, 3–5. [Google Scholar] [CrossRef]
- Degelmann, D.M.; Borken, W.; Drake, H.L.; Kolb, S. Different atmospheric methane-oxidizing communities in European beach and Norway spruce soils. Appl. Environ. Microbiol. 2010, 76, 3228–3235. [Google Scholar] [CrossRef]
- Zhu-Barker, X.; Bailey, S.K.; Paw, U.K.T.; Burger, M.; Horwath, W.R. Greenhouse gas emissions from green waste composting windrow. Waste Manag. 2017, 59, 70–79. [Google Scholar] [CrossRef]
- Yang, Y.; Kumar Awasthi, M.; Du, W.; Ren, X.; Lei, T.; Lv, J. Compost supplementation with nitrogen loss and greenhouse gas emissions during pig manure composting. Bioresour. Technol. 2020, 297, 122435. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, W.; Yuan, J.; Li, G.; Luo, Y. Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting. Waste Manag. 2017, 68, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Huang, G.; Li, J.; Han, L. Particle-scale visualization of the evolution of methanogens and methanotrophs and its correlation with CH4 emissions during manure aerobic composting. Waste Manag. 2018, 78, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, X.; Fang, C.; He, X.; Han, L.; Huang, G. Exploring the mechanisms of decreased methane during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane. Waste Manag. 2018, 78, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Wei, S.; Wang, W.; Lin, X. Windrow composting mitigated CH4 emissions: Characterization of methanogenic and methanotrophic communities in manure management. FEMS Microbiol. Ecol. 2014, 90, 575–586. [Google Scholar] [CrossRef]
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef]
- Paul, K.; Nonoh, J.O.; Mikulski, L.; Brune, A. “Methanoplasmatales”, thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl. Environ. Microbiol. 2012, 78, 8245–8253. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Xu, C.; Dou, F.; Shimizu, K.Y.; Cao, W. Rational utilization of leguminous green manure to mitigate methane emissions by influencing methanogenic and methanotrophic communities. Geoderma 2020, 361, 114071. [Google Scholar] [CrossRef]
- Miller, T.L.; Currenti, E.; Wolin, M.J. Anaerobic bioconversion of cellulose by Ruminococcus albus, Methanobrevibacter smithii, and Methanosarcina barkeri. Appl. Microbiol. Biotechnol. 2000, 54, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, D.O.; Asher, R.A.; Bauchop, T. Fermentation of cellulose to methane and carbon dioxide by a rumen anaerobic fungus in a triculture with Methanobrevibacter sp. strain RA1 and Methanosarcina barkeri. Appl. Environ. Microbiol. 1982, 44, 128–134. [Google Scholar] [CrossRef]
- Bowman, J.P.; Sly, L.T.; Nichols, P.D.; Hayward, A.C. Erratum: Revised taxonomy of the methanotrophs: Description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int. J. Syst. Bacteriol. 1994, 44, 375. [Google Scholar] [CrossRef][Green Version]
- Amaral, J.A.; Knowles, R. Growth of methanotrophs in methane and oxygen counter gradients. FEMS Microbiol. Lett. 1995, 126, 215–220. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, S.T.; Ho, A.; Hong, C.O.; Lee, C.H.; Kim, P.J. Cattle manure composting: Shifts in the methanogenic community structure, chemical composition, and consequences on methane production potential in a rice paddy. Appl. Soil Ecol. 2018, 124, 344–350. [Google Scholar] [CrossRef]
- Linquist, B.A.; Adviento-Borbe, M.A.; Pittelkow, C.M.; van Kessel, C.; van Groenigen, K.J. Fertilizer management practices and greenhouse gas emissions from rice systems: A quantitative review and analysis. Field Crop. Res. 2012, 135, 10–21. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- Sossa, K.; Alarcón, M.; Aspé, E.; Urrutia, H. Effect of ammonia on the methanogenic activity of methylaminotrophic methane producing Archaea enriched biofilm. Anaerobe 2004, 10, 13–18. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, M.; Zheng, F.; Guo, S.; Song, X.; Liu, S.; Li, S.; Zou, J. Decreased Methane Emissions Associated with Methanogenic and Methanotrophic Communities in a Pig Manure Windrow Composting System under Calcium Superphosphate Amendment. Int. J. Environ. Res. Public Health 2021, 18, 6244. https://doi.org/10.3390/ijerph18126244
Zhang Y, Huang M, Zheng F, Guo S, Song X, Liu S, Li S, Zou J. Decreased Methane Emissions Associated with Methanogenic and Methanotrophic Communities in a Pig Manure Windrow Composting System under Calcium Superphosphate Amendment. International Journal of Environmental Research and Public Health. 2021; 18(12):6244. https://doi.org/10.3390/ijerph18126244
Chicago/Turabian StyleZhang, Yihe, Mengyuan Huang, Fengwei Zheng, Shumin Guo, Xiuchao Song, Shuwei Liu, Shuqing Li, and Jianwen Zou. 2021. "Decreased Methane Emissions Associated with Methanogenic and Methanotrophic Communities in a Pig Manure Windrow Composting System under Calcium Superphosphate Amendment" International Journal of Environmental Research and Public Health 18, no. 12: 6244. https://doi.org/10.3390/ijerph18126244
APA StyleZhang, Y., Huang, M., Zheng, F., Guo, S., Song, X., Liu, S., Li, S., & Zou, J. (2021). Decreased Methane Emissions Associated with Methanogenic and Methanotrophic Communities in a Pig Manure Windrow Composting System under Calcium Superphosphate Amendment. International Journal of Environmental Research and Public Health, 18(12), 6244. https://doi.org/10.3390/ijerph18126244




