The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Participants
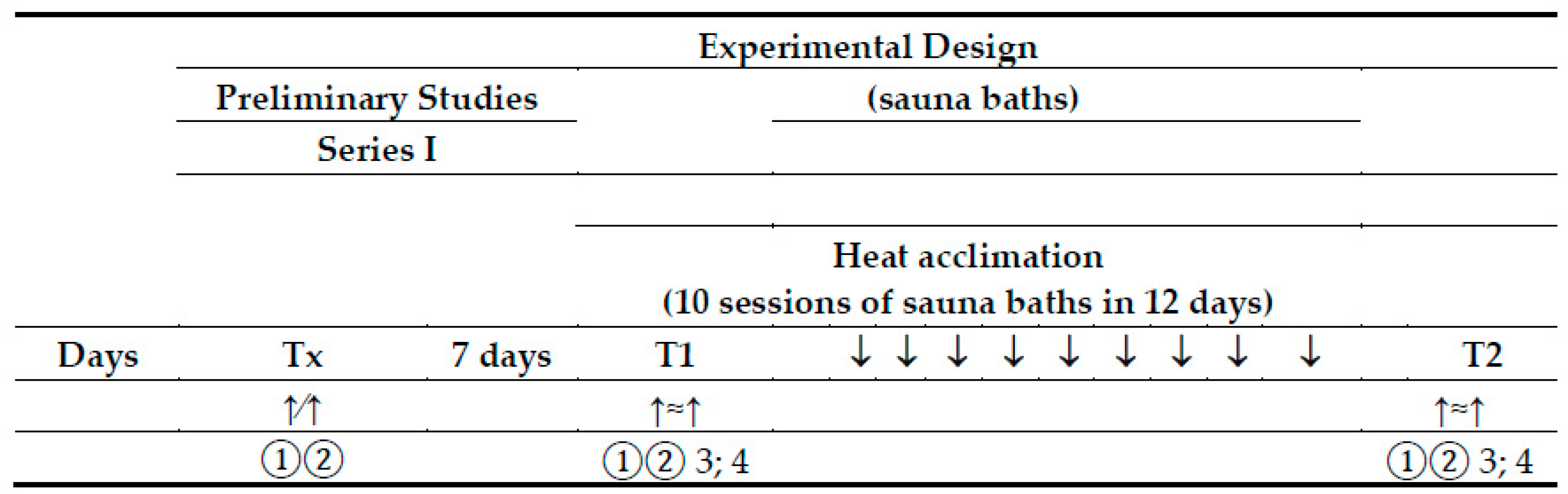
2.3. Experimental Design
2.3.1. Preliminary Session
2.3.2. Heat Acclimation—Finnish Sauna Treatments
2.3.3. Standard Submaximal Exercise Test
2.3.4. Training
2.4. Measures
2.4.1. Internal and Skin Temperatures and Blood Pressure
2.4.2. Calculations
2.4.3. Blood Sample Collection and Analyses
2.4.4. Calculation of Plasma Volume
2.5. Data Analysis and Statistics
3. Results
3.1. Participants
3.2. The Effect of Heat Acclimation on Physiological and Hematological Variables at Rest
3.3. The Effect of Heat Acclimation on Physiological Responses to Exercise
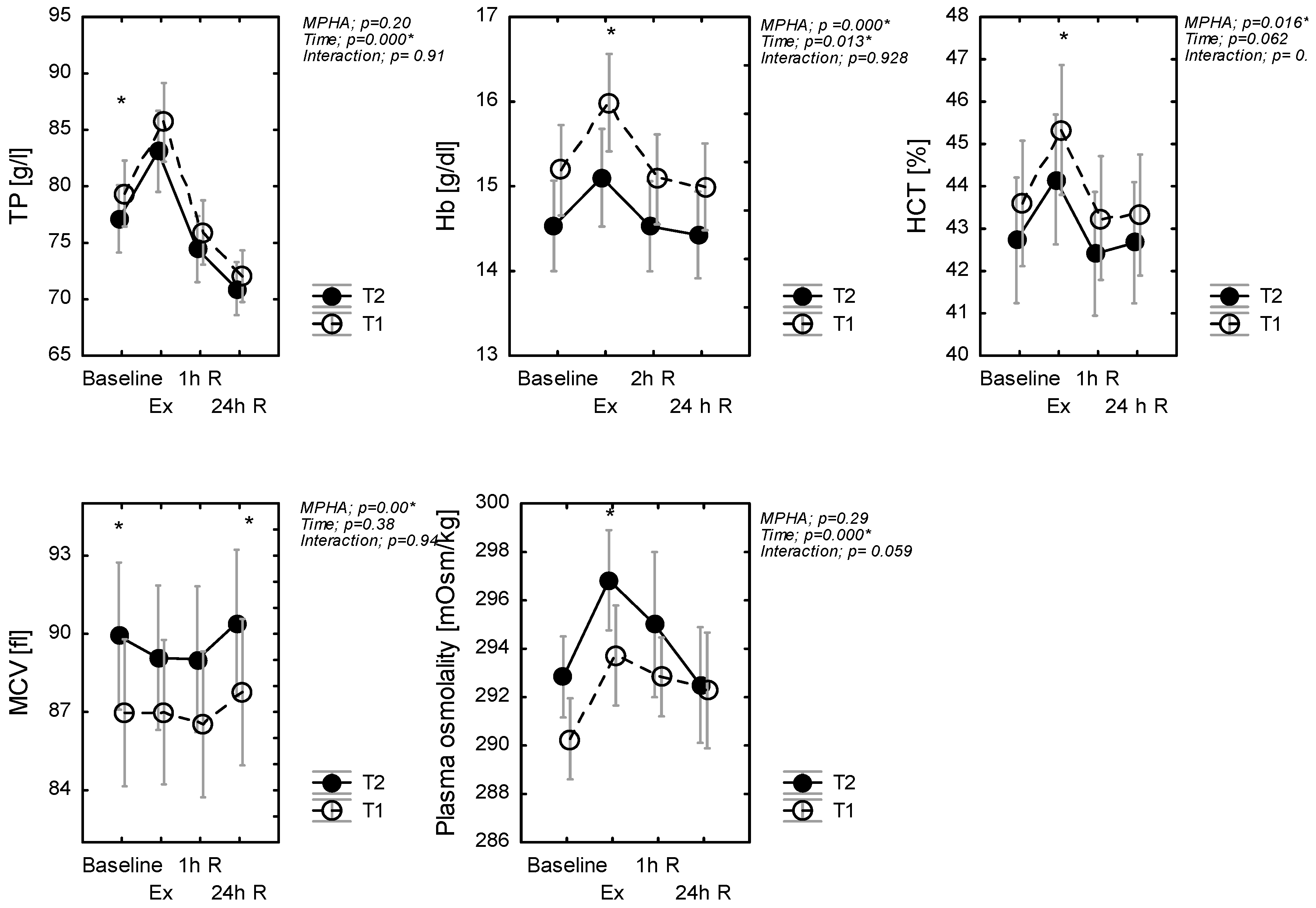
3.4. The Effect of Heat Acclimation on Changes in Hematological Biomarkers Following Exercise
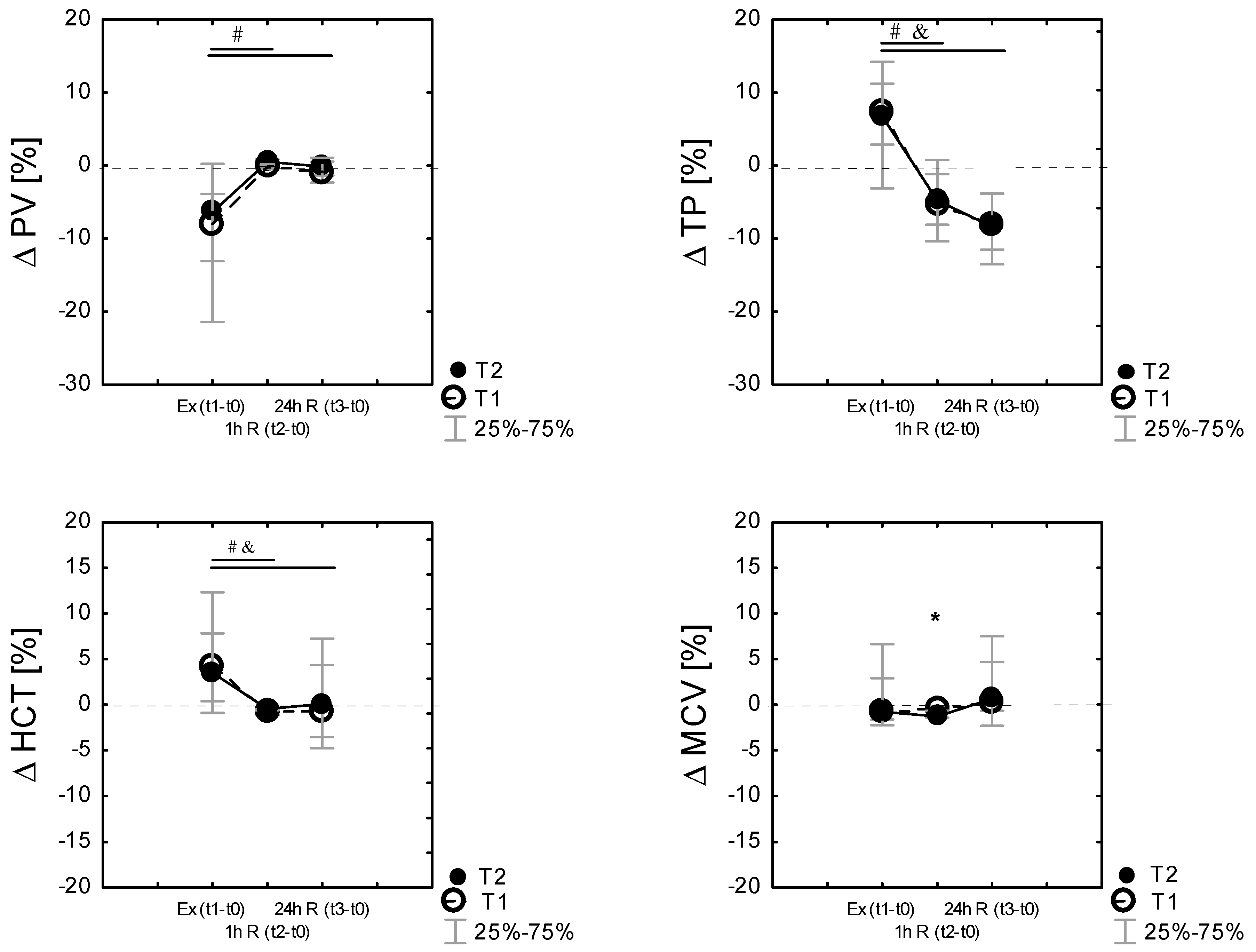
3.5. Changes in Plasma Volume Following Exercise
4. Discussion
4.1. Thermal and Physiological Changes after a Series of Ten Sauna Baths
4.2. Passive Mild Heat Acclimation and the Human Plasma Volume Changes
4.3. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, N.A.S.; Cotter, J.D. Heat adaptation: Guidelines for the optimisation of human performance. Int. Sportmed J. 2006, 7, 1–37. [Google Scholar]
- Garrett, A.T.; Creasy, R.; Rehrer, N.J.; Patterson, M.J.; Cotter, J.D. Effectiveness of short-term heat acclimation for highly trained athletes. Eur. J. Appl. Physiol. 2012, 112, 1827–1837. [Google Scholar] [CrossRef]
- Gibson, O.R.; Mee, J.A.; Tuttle, J.A.; Taylor, L.; Watt, P.W.; Maxwell, N.S. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J. Therm. Biol. 2015, 49–50, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Tyler, C.J.; Reeve, T.; Hodges, G.J.; Cheung, S.S. The Effects of Heat Adaptation on Physiology, Perception and Exercise Performance in the Heat: A Meta-Analysis. Sport Med. 2016, 46, 1699–1724. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, S.; Halliwill, J.R.; Sawka, M.N.; Minson, C.T. Heat acclimation improves exercise performance. J. Appl. Physiol. 2010, 109, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racinais, S.; Cocking, S.; Périard, J.D. Sports and environmental temperature: From warming-up to heating-up. Temperature 2017, 4, 227–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.J.S.; Crockford, M.J.; Moore, J.P.; Walsh, N.P. Heat acclimation responses of an ultra-endurance running group preparing for hot desert-based competition. Eur. J. Sport Sci. 2014, 14, 37–41. [Google Scholar] [CrossRef]
- Périard, J.D.; Racinais, S.; Sawka, M.N. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand. J. Med. Sci. Sport 2015, 25, 20–38. [Google Scholar] [CrossRef]
- Akerman, A.P.; Lucas, S.J.E.; Katare, R.; Cotter, J.D. Heat and dehydration additively enhance cardiovascular outcomes following orthostatically-stressful calisthenics exercise. Front. Physiol. 2017, 8, 1–178. [Google Scholar] [CrossRef] [Green Version]
- Garrett, A.T.; Rehrer, N.J.; Patterson, M.J. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sport Med. 2011, 41, 757–771. [Google Scholar] [CrossRef]
- Corbett, J.; Rendell, R.A.; Massey, H.C.; Costello, J.T.; Tipton, M.J. Inter-individual variation in the adaptive response to heat acclimation. J. Therm. Biol. 2018, 74, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.; Hales, J.R.; Strange, S.; Christensen, N.J.; Warberg, J.; Saltin, B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J. Physiol. 1993, 460, 467–485. [Google Scholar] [CrossRef]
- Garrett, A.T.; Goosens, N.G.; Rehrer, N.J.; Patterson, M.J.; Cotter, J.D. Induction and decay of short-term heat acclimation. Eur. J. Appl. Physiol. 2009, 107, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Daanen, H.A.M.; Racinais, S.; Périard, J.D. Heat Acclimation Decay and Re-Induction: A Systematic Review and Meta-Analysis. Sport Med. 2018, 48, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Leppaluoto, J.; Huttunen, P.; Hirvonen, J.; Väänänen, A.; Tuominen, M.; Vuori, J. Endocrine effects of repeated sauna bathing. Acta Physiol. Scand. 1986, 128, 467–470. [Google Scholar] [CrossRef]
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar] [CrossRef] [PubMed]
- Nadel, E.R.; Pandolf, K.B.; Roberts, M.F.; Stolwijk, J.A. Mechanisms of thermal acclimation to exercise and heat. J. Appl. Physiol. 1974, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Shvartz, E.; Shapiro, Y.; Magazanik, A.; Meroz, A.; Birnfeld, H.; Mechtinger, A.; Shibolet, S. Heat acclimation, physical fitness, and response to exercise in temperate and hot environments. J. Appl. Physiol. Resp. Environ. Exerc. Physiol. 1977, 43, 678–683. [Google Scholar] [CrossRef]
- Sawka, M.N.; Young, A.J.; Cadarette, B.S.; Levine, L.; Pandolf, K.B. Influence of heat stress and acclimation on maximal aerobic power. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 53, 294–298. [Google Scholar] [CrossRef]
- Périard, J.D.; Travers, G.J.S.; Racinais, S.; Sawka, M.N. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. Basic Clin. 2015, 196, 52–62. [Google Scholar] [CrossRef] [Green Version]
- James, L.J.; Moss, J.; Henry, J.; Papadopoulou, C.; Mears, S.A. Hypohydration impairs endurance performance: A blinded study. Physiol. Rep. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Waldron, M.; Jeffries, O.; Tallent, J.; Patterson, S.; Nevola, V. The time course of adaptations in thermoneutral maximal oxygen consumption following heat acclimation. Eur. J. Appl. Physiol. 2019, 119, 2391–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapara, M.A.; Dudnik, E.N.; Samartseva, V.G.; Kryzhanovskaya, S.Y.; Susta, D.; Glazachev, O.S. Passive Whole-Body Hyperthermia Increases Aerobic Capacity and Cardio-Respiratory Efficiency in Amateur Athletes. Health (Irvine. Calif) 2020, 12, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, C.L.; Sekiguchi, Y.; Fry, L.A.; Casa, D.J. Performance Changes Following Heat Acclimation and the Factors That Influence These Changes: Meta-Analysis and Meta-Regression. Front. Physiol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Senay, L.C.; Mitchell, D.; Wyndham, C.H. Acclimatization in a hot humid environment: Body fluid adjustments. J. Appl. Physiol. 1976, 40, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Greenleaf, J.E.; Bernauer, E.M. Role of thermal and exercise factors in the mechanism of hypervolemia. J. Appl. Physiol. 1980, 48, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.H.; Edwards, R.J.; Graveney, M.J.; Cochrane, L.A.; Davies, J.A. Blood volume and plasma protein responses to heat acclimatization in humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 50, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Convertino, V.A.; Eichner, E.R.; Schnieder, S.M.; Young, A.J. Blood volume: Importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med. Sci. Sports Exerc. 2000, 32, 332–348. [Google Scholar] [CrossRef]
- Patterson, M.J.; Stocks, J.M.; Taylor, N.A.S. Sustained and generalized extracellular fluid expansion following heat acclimation. J. Physiol. 2004, 559, 327–334. [Google Scholar] [CrossRef]
- Pilch, W.; Lech, G.; Pałka, T.; Błach, W.; Tota, Ł.; Cisoń, T. The knowledge about using Finnish sauna in post-event recovery among judokas. J. Combat. Sport Martial Arts 2014, 5, 43–47. [Google Scholar] [CrossRef]
- Racinais, S.; Alonso, J.M.; Coutts, A.J.; Flouris, A.D.; Girard, O.; González-Alonso, J.; Hausswirth, C.; Jay, O.; Lee, J.K.W.; Mitchell, N.; et al. Consensus recommendations on training and competing in the heat. Br. J. Sports Med. 2015, 49, 1164–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heathcote, S.L.; Hassmén, P.; Zhou, S.; Stevens, C.J. Passive Heating: Reviewing Practical Heat Acclimation Strategies for Endurance Athletes. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Greenleaf, J.E.; Kaciuba-Uscilko, H. Acclimatization to Heat in Humans; Polish Academy of Sciences: Warsaw, Poland, 1989; pp. 1–41. [Google Scholar]
- Moran, D.S.; Montain, S.J.; Pandolf, K.B. Evaluation of different levels of hydration using a new physiological strain index. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A. Blood volume: Its adaptation to endurance training. Med. Sci. Sports Exerc. 1991, 23, 1338–1348. [Google Scholar] [CrossRef]
- Gibson, O.R.; James, C.A.; Mee, J.A.; Willmott, A.G.B.; Turner, G.; Hayes, M.; Maxwell, N.S. Heat alleviation strategies for athletic performance: A review and practitioner guidelines. Temperature 2020, 7, 3–36. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.H.; Deakin, G.B.; Edwards, A.M.; Miller, C.M.; Pyne, D.B. Adaptation to Hot Environmental Conditions: An Exploration of the Performance Basis, Procedures and Future Directions to Optimise Opportunities for Elite Athletes. Sport Med. 2015, 45, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.S. Human heat adaptation. Compr. Physiol. 2014, 4, 325–365. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Clegg, M.E.; Walsh, M.L.; White, M.D. Adaptation of exercise ventilation during an actively-induced hyperthermia following passive heat acclimation. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallubinsky, H.; Schellen, L.; Kingma, B.R.M.; Dautzenberg, B.; van Baak, M.A.; van Marken Lichtenbelt, W.D. Thermophysiological adaptations to passive mild heat acclimation. Temperature 2017, 4, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Scoon, G.S.M.; Hopkins, W.G.; Mayhew, S.; Cotter, J.D. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J. Sci. Med. Sport 2007, 10, 259–262. [Google Scholar] [CrossRef]
- Tyka, A.; Pałka, T.; Tyka, A.K.; Szyguła, Z.; Cisoń, T. Repeated Sauna Bathing Effects on Males’ Capacity to Prolonged Exercise-Heat Performance. Med. Sport. 2008, 12, 150–154. [Google Scholar] [CrossRef]
- Stanley, J.; Halliday, A.; D’Auria, S.; Buchheit, M.; Leicht, A.S. Effect of sauna-based heat acclimation on plasma volume and heart rate variability. Eur. J. Appl. Physiol. 2015, 115, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Skurvydas, A. Heat acclimation does not reduce the impact of hyperthermia on central fatigue. Eur. J. Appl. Physiol. 2010, 109, 771–778. [Google Scholar] [CrossRef]
- Zurawlew, M.J.; Mee, J.A.; Walsh, N.P. Post-exercise hot water immersion elicits heat acclimation adaptations that are retained for at least two weeks. Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zurawlew, M.J.; Walsh, N.P.; Fortes, M.B.; Potter, C. Post-exercise hot water immersion induces heat acclimation and improves endurance exercise performance in the heat. Scand. J. Med. Sci. Sports 2016, 26, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Kissling, L.S.; Akerman, A.P.; Cotter, J.D. Heat-induced hypervolemia: Does the mode of acclimation matter and what are the implications for performance at Tokyo 2020? Temperature 2019, 7, 129–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Laukkanen, T.; Kunutsor, S.K.; Khan, H.; Willeit, P.; Zaccardi, F.; Laukkanen, J.A. Sauna exposure leads to improved arterial compliance: Findings from a non-randomised experimental study. Eur. J. Prev. Cardiol. 2018, 25, 130–138. [Google Scholar] [CrossRef]
- Hussain, J.N.; Greaves, R.F.; Cohen, M.M. A hot topic for health: Results of the Global Sauna Survey. Complement. Ther. Med. 2019, 44, 223–234. [Google Scholar] [CrossRef]
- O’Toole, M.L.; Paolone, A.M.; Ramsey, R.E.; Irion, G. The effects of heat acclimation on plasma volume and plasma protein of females. Int. J. Sports Med. 1983, 4, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Pokora, I.; Szyguła, Z.; Pałka, T.; Pilch, P.; Cisoń, T.; Malik, L.; Wiecha, S. Effect of a Single Finnish Sauna Session on White Blood Cell Profile and Cortisol Levels in Athletes and Non-Athletes. J. Hum. Kinet. 2013, 39, 127–135. [Google Scholar] [CrossRef]
- Cheung, S.S.; McLellan, T.M. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J. Appl. Physiol. 1998, 84, 1731–1739. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Rosenberg, I.; Armstrong, L.; Manz, F.; Canton, A.D.; Barclay, D.; Ritz, P.; Sawka, M.; Shirreffs, S.; Ferry, M. Hydration Assessment Techniques. Nutr. Rev. 2005, 63, S40–S54. [Google Scholar] [CrossRef] [PubMed]
- Michnik, A.; Duch, K.; Pokora, I.; Sadowska, E. Differences in cryostimulation and sauna effects on post-exercise changes in blood serum of athletes. Complementary Ther. Med. 2020, 51, 1–6. [Google Scholar] [CrossRef]
- Pryor, J.L.; Johnson, E.C.; Roberts, W.O.; Pryor, R.R. Application of evidence-based recommendations for heat acclimation: Individual and team sport perspectives. Temperature 2019, 6, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Racinais, S. Different Effects of Heat Exposure upon Exercise Performance in the Morning and Afternoon. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. 3), 80–89. [Google Scholar] [CrossRef]
- Todd, G.; Gordon, C.J.; Groeller, H.; Taylor, N.A.S. Does intramuscular thermal feedback modulate eccrine sweating in exercising humans? Acta Physiol. 2014, 212, 86–96. [Google Scholar] [CrossRef]
- Cotter, J.D.; Patterson, M.J.; Taylor, N.A.S. The topography of eccrine sweating in humans during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.A.J.; Hardy, J.D. Temperature regulation in man—A theoretical study. Pflügers Arch. 1966, 291, 129–162. [Google Scholar] [CrossRef] [PubMed]
- Burton, A. The application of the theory of heat flow to the study of energy metabolism. J. Nutr. 1934, 7, 497–533. [Google Scholar] [CrossRef]
- Pokora, I.; Zebrowska, A. Application of a physiological strain index in evaluating responses to exercise stress—A comparison between endurance and high intensity intermittent trained athletes. J. Hum. Kinet. 2016, 50, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Abdelhamid, A.; Ali, A.; Bunn, D.K.; Jennings, A.; John, W.G.; Kerry, S.; Lindner, G.; Pfortmueller, C.A.; Sjöstrand, F.; et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: Adding value to pathology laboratory reports. BMJ Open 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, M.B.; Davis, R.K.; Roseubaum, J.D.; Rossmeisil, E.C. Water diuresis produced during recumbency by the intravenous infusion. J. Clin. Invest. 1951, 30, 862–868. [Google Scholar] [CrossRef]
- Alis, R.; Sanchis-Gomar, F.; Primo-Carrau, C.; Lozano-Calve, S.; Dipalo, M.; Aloe, R.; Blesa, J.R.; Romagnoli, M.; Lippi, G. Hemoconcentration induced by exercise: Revisiting the Dill and Costill equation. Scand. J. Med. Sci. Sport 2015, 25, e630–e637. [Google Scholar] [CrossRef]
- Rocker, L.; Kirsch, K.; Heyduck, B.; Altenkirch, H. Influence of Prolonged Physical Exercise on Plasma Volume, Plasma Proteins, Electrolytes, and Fluid-Regulating Hormones. Int. J. Sports Med. 1989, 10, 270–274. [Google Scholar] [CrossRef]
- Koch, G.; Rocker, L. Plasma volume and intravascular protein masses in trained boys and fit young men. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1977, 43, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buono, M.J.; Heaney, J.H.; Canine, K.M. Acclimation to humid heat lowers resting core temperature. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, 1295–1299. [Google Scholar] [CrossRef]
- Castle, P.; Mackenzie, R.W.; Maxwell, N.; Webborn, A.D.J.; Watt, P.W. Heat acclimation improves intermittent sprinting in the heat but additional pre-cooling offers no further ergogenic effect. J. Sports Sci. 2011, 29, 1125–1134. [Google Scholar] [CrossRef]
- Stephenson, B.T.; Tolfrey, K.; Goosey-Tolfrey, V.L. Mixed Active and Passive, Heart Rate-Controlled Heat Acclimation Is Effective for Paralympic and Able-Bodied Triathletes. Front. Physiol. 2019, 10, 1214. [Google Scholar] [CrossRef]
- Racinais, S.; Mohr, M.; Buchheit, M.; Voss, S.; Gaoua, N.; Granthan, J.; Nybo, L. Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. Br. J. Sports Med. 2012, 46, 810–815. [Google Scholar] [CrossRef]
- Ravanelli, N.; Cramer, M.; Imbeault, P.; Jay, O. The optimal exercise intensity for the unbiased comparison of thermoregulatory responses between groups unmatched for body size during uncompensable heat stress. Physiol. Rep. 2017, 5, e13099. [Google Scholar] [CrossRef]
- Moss, J.N.; Bayne, F.M.; Castelli, F.; Naughton, M.R.; Reeve, T.C.; Trangmar, S.J.; Mackenzie, R.W.A.; Tyler, C.J. Short-term isothermic heat acclimation elicits beneficial adaptations but medium-term elicits a more complete adaptation. Eur. J. App. Physiol. 2020, 120, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Pandolf, K.; Burse, R.; Goldman, R. Role of physical fitness in heat acclimatisation, decay and reinduction. Ergonomics 1977, 20, 399–408. [Google Scholar] [CrossRef]
- Casadio, J.R.; Kilding, A.E.; Cotter, J.D.; Laursen, P.B. From Lab to Real World: Heat Acclimation Considerations for Elite Athletes. Sports Med. 2017, 47, 1467–1476. [Google Scholar] [CrossRef]
- Garrett, A.T.; Goosens, N.G.; Rehrer, N.J.; Patterson, M.J.; Harrison, J.; Sammut, I.; Cotter, J.D. Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am. J. Hum. Biol. 2014, 26, 311–320. [Google Scholar] [CrossRef]
- Neal, R.A.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effect of permissive dehydration on induction and decay of heat acclimation, and temperate exercise performance. Front. Physiol. 2016, 7, 564. [Google Scholar] [CrossRef] [Green Version]
- Jay, O.; Bain, A.R.; Deren, T.M.; Sacheli, M.; Cramer, M.N. Large differences in peak oxygen uptake do not independently alter changes in core temperature and sweating during exercise. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, 832–841. [Google Scholar] [CrossRef]
- Moran, D.S.; Shitzer, A.; Pandolf, K.B. A physiological strain index to evaluate heat stress. Am. J. Physiol. Integr. Comp. Physiol. 1998, 44, R129–R134. [Google Scholar] [CrossRef] [Green Version]
- Tikuisis, P.; Mclellan, T.M.; Selkirk, G. Perceptual versus physiological heat strain During Exercise-Heat Stress. Med. Sci. Sport Exerc. 2002, 34, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Okazaki, K.; Kamijo, Y.I.; Ikegawa, S.; Masuki, S.; Miyagawa, K.; Nose, H. Protein and carbohydrate supplementation during 5-day aerobic training enhanced plasma volume expansion and thermoregulatory adaptation in young men. J. Appl. Physiol. 2010, 109, 1247–1255. [Google Scholar] [CrossRef]
- Rendell, R.A.; Prout, J.; Costello, J.T.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effects of 10 days of separate heat and hypoxic exposure on heat acclimation and temperate exercise performance. Am. J. Physiol. Integrative Comp. Physiol. 2017, 313, R191–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senay, L.C. Plasma volumes and constituents of heat exposed men before and after acclimatization. J. Appl. Physiol. 1975, 38, 570–575. [Google Scholar] [CrossRef]
- Senay, L.C.; Kok, R. Effects of training and heat acclimatization on blood plasma contents of exercising men. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 43, 591–599. [Google Scholar] [CrossRef]
- Mourtakos, S.; Philippou, A.; Papageorgiou, A.; Lembessis, P.; Zaharinova, S.; Hasanova, Y.; Koynova, R.; Bersimis, F.; Tenchov, B.; Geladas, N.; et al. The effect of prolonged intense physical exercise of special forces volunteers on their plasma protein denaturation profile examined by differential scanning calorimetry. J. Therm. Biol. 2021, 96, 102860. [Google Scholar] [CrossRef] [PubMed]
- Duch, K.; Michnik, A.; Pokora, I.; Sadowska-Krępa, E. Modification of blood serum DSC profiles by sauna treatments in cross-country skiers during the exercise cycle. J. Therm. Anal. Calorim. 2020, 142, 1927–1932. [Google Scholar] [CrossRef]
- Fellman, N. Hormonal and Plasma volume alterations following endurance exercise. A Brief Review. Sports Med. 1992, 13, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fortney, S.M.; Nadel, E.R.; Wenger, C.B.; Bove, J.R. Effect of acute alterations of blood volume on circulatory performance in humans. J. Appl. Physiol. 1981, 50, 292–298. [Google Scholar] [CrossRef]
- Coyle, E.; Hopper, M.; Coggan, A. Maximal oxygen uptake relative to plasma volume expansion. Int. J. Sports Med. 1990, 11, 116–119. [Google Scholar] [CrossRef]
| Indicators | ± SD | Min | Max |
|---|---|---|---|
| Age [years] | 21.2 ± 2.99 | 18 | 27 |
| Body height [cm] | 178.8 ± 4.12 | 167 | 183 |
| Body mass [kg] | 70.6 ± 6.0 | 59 | 80.6 |
| BSA [m2] | 1.86 ± 0.13 | 1.71 | 2.18 |
| BMI [kg m−2] | 22.17 ± 1.83 | 18.4 | 24.9 |
| FM [kg] | 5.94 ± 2.56 | 1.3 | 11 |
| FFM [kg] | 50.47 ± 13.17 | 36.2 | 71.5 |
| TBW [kg] | 49.6 ± 6.1 | 43.0 | 60.7 |
| Training status [years] | 9.5 ± 10.5 | 7 | 12 |
| Indicators | ± SD | Min | Max |
|---|---|---|---|
| VO2max [mL·kg−1·min−1] | 64.5 ± 6.33 | 53 | 73 |
| HRmax [bs·min−1] | 191 ± 8.72 | 171 | 206 |
| Powermax [W] | 395.7 ± 9.1 | 310 | 497 |
| Powermax [W·kg−1] | 5.68 ± 0.5 | 5.3 | 6.2 |
| RERmax | 1.05 ± 0.6 | 0.95 | 1.21 |
| METmax | 17.9 ± 1.9 | 14.9 | 21.8 |
| Ve max [L·min−1] | 156 ± 25.1 | 120.4 | 198 |
| LA max [mmol·L−1] | 9.83 ± 1.84 | 6.7 | 13.5 |
| HRAT [bs·min−1] | 173.3 ± 7.7 | 160 | 183 |
| VAT [km·h−1] | 13.6 ± 0.9 | 12 | 14 |
| G% AT [%] | 1.25 ± 1.6 | 1 | 5 |
| Variables | T1 (n = 14) X ± SD | T2 (n = 14) X ± SD | p | ES | Effect of MPHA; p; η2p Effect of Exercise; p; η2p Interaction; p; η2p |
|---|---|---|---|---|---|
| VO2 rest [mL·kg−1·min−1] | 0.74 ± 0.04 | 0.75 ± 0.05 | 0.28 | 0.22 | MPHA; p = 0.08 Exercise; p = 0.001; 0.95 Interaction; p = 0.57 |
| VO2 last [mL·kg−1·min−1] | 3.67 ± 0.1 | 3.68 ± 0.12 | 0.41 | 0.09 | |
| HR rest [bs·min−1] | 66.5 ± 11.2 | 58.5 ± 3.78 *** | 0.001 | 1.08 | MPHA; p = 0.006; 0.44 Exercise; p = 0.001; 0.95 Interaction; p = 0.57 |
| HR last [bs·min−1] | 167.9 ± 2.6 | 162.3 ± 3.4 *** | 0.001 | 1.23 | |
| Tty rest [°C] | 36.5 ± 0.6 | 36.3 ± 0.4 | 0.31 | 0.40 | MPHA; p = 0.11 Exercise; p = 0.000; 0.58 Interaction; p = 0.57 |
| Tty last [°C] | 37.6 ± 0.6 | 37.3 ± 0.4 | 0.11 | 0.63 | |
| TsCh rest [°C] | 33.8 ± 1.4 | 33.2 ± 1.3 | 0.09 | 0.44 | MPHA; p = 0.17 Exercise; p = 0.84 Interaction; p = 0.72 |
| TsCh last [°C] | 33.8 ± 1.4 | 33.2 ± 2.1 | 0.3 | 0.34 | |
| TsF rest [°C] | 32.9 ± 1.0 | 32.2 ± 1.4 | 0.09 | 0.62 | MPHA; p = 0.79 Exercise; p = 0.001; 0.53 Interaction; p = 0.68 |
| TsF last [°C] | 33.3 ± 1.5 | 33.8 ± 1.3 | 0.08 | 0.35 | |
| TsTh rest [°C] | 31.2 ± 1.3 | 31.3 ± 0.8 | 0.38 | 0.15 | MPHA; p = 0.59 Exercise; p = 0.08 Interaction; p = 0.21 |
| TsTh last [°C] | 32.8 ± 1.4 | 32.4 ± 1.6 | 0.40 | 0.26 | |
| b rest [°C] | 35.6 ± 0.7 | 35.5 ± 0.4 | 0.53 | 0.31 | MPHA; p = 0.054; Exercise; p = 0.000; 0.65 Interaction; p = 0.84 |
| b last [°C] | 36.7 ± 0.5 | 36.4 ± 0.7 | 0.17 | 0.63 | |
| SK rest [°C] | 32.8 ± 1.2 | 32.4 ± 0.8 | 0.25 | 0.36 | MPHA; p = 0.45 Exercise; p = 0.001; 0.3 Interaction; p = 0.83 |
| SK last [°C] | 33.4 ± 1.3 | 33.2 ± 1.4 | 0.68 | 0.66 | |
| SBP rest [mmHg] | 138.3 ± 19.3 | 125.5 ± 6.2 * | 0.04 | 0.96 | MPHA; p = 0.03; 0.25 Exercise; p = 0.45 Interaction; p = 0.92 |
| SBP last [mmHg] | 136.8 ± 11.6 | 140.4 ± 14.2 | 0.65 | 0.28 | |
| DBP rest [mmHg] | 71.7 ± 2.9 | 70.8 ± 10.7 | 0.73 | 0.14 | MPHA; p = 0.34; Exercise; p = 0.51 Interaction; p = 0.74 |
| DBP last [mmHg] | 70.7 ± 5.3 | 71.5 ± 8.3 | 0.16 | 0.11 | |
| Power [W] last | 237.0 ± 36.5 | 237.5 ± 35.3 | 0.94 | 0.01 | |
| Power [W·kg−1] last | 3.39 ± 0.04 | 3.41 ± 0.07 | 0.19 | 0.36 |
| Variables | T1 (n = 14) X ± SD | T2 (n = 14) X ± SD | p | ES |
|---|---|---|---|---|
| Δ Tb [°C] | 0.89 ± 0.51 | 0.62 ± 0.64 | 0.243 | |
| Δ TSK [°C] | 0.57 ± 1.04 | 0.78 ± 1.03 | 0.7 | |
| Δ BM [kg] | −1.06 ± 0.6 | −1.13 ± 0.81 | 0.19 | |
| Δ Tty [°C] | 1.12 ± 0.8 | 1.0 ± 0.6 | 0.06 | |
| HRR [bs·min−1] | 99.4 ± 6.19 | 104.1 ± 8.56 | 0.35 | |
| fT (PSI) | 0.24 ± 0.11 | 0.22 ± 0.14 | 0.22 | |
| f HR (PSI) | 0.76 ± 0.12 | 0.77 ± 0.11 | 0.78 | |
| PSI | 6.03 ± 1.11 | 5.27 ± 0.62 | 0.052 | 0.87 |
| T1 | T2 | |||||
|---|---|---|---|---|---|---|
| Variables | Δ PV% | Δ TP% | Δ HCT [%] | Δ PV% | Δ TP% | Δ HCT [%] |
| Δ PV [%] | −0.44 | −0.71 * | ||||
| Δ TP [%] | −0.44 | 0.71 * | ||||
| Δ HCT [%] | −0.97 * | 0.23 | −0.98 * | 0.73 * | ||
| Δ OSM [%] | 0.11 | 0.39 | 0.03 | −0.05 | ||
| Δ MCV [%] | −0.83 * | 0.2 | 0.96 * | −0.89 * | 0.69 * | 0.93 * |
| Δ MCV [%] 1 h R (t2–t0) | 0.35 | 0.13 | 0.03 | −0.04 | ||
| Δ MCV [%] 24 h R (t3–t0) | 0.85 * | -0.25 | 0.88 * | 0.52 * | 0.07 | 0.88 * |
| T1 | T2 | |
|---|---|---|
| Variables | PSI | PSI |
| Δ PV [%] | −0.37 | −0.49 |
| Δ TP [%] | −0.01 | 0.76 * |
| Δ HCT [%] | 0.33 | 0.41 |
| Δ OSM [%] | 0.09 | 0.19 |
| Δ MCV [%] | 0.08 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokora, I.; Sadowska-Krępa, E.; Wolowski, Ł.; Wyderka, P.; Michnik, A.; Drzazga, Z. The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers. Int. J. Environ. Res. Public Health 2021, 18, 6906. https://doi.org/10.3390/ijerph18136906
Pokora I, Sadowska-Krępa E, Wolowski Ł, Wyderka P, Michnik A, Drzazga Z. The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers. International Journal of Environmental Research and Public Health. 2021; 18(13):6906. https://doi.org/10.3390/ijerph18136906
Chicago/Turabian StylePokora, Ilona, Ewa Sadowska-Krępa, Łukasz Wolowski, Piotr Wyderka, Anna Michnik, and Zofia Drzazga. 2021. "The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers" International Journal of Environmental Research and Public Health 18, no. 13: 6906. https://doi.org/10.3390/ijerph18136906
APA StylePokora, I., Sadowska-Krępa, E., Wolowski, Ł., Wyderka, P., Michnik, A., & Drzazga, Z. (2021). The Effect of Medium-Term Sauna-Based Heat Acclimation (MPHA) on Thermophysiological and Plasma Volume Responses to Exercise Performed under Temperate Conditions in Elite Cross-Country Skiers. International Journal of Environmental Research and Public Health, 18(13), 6906. https://doi.org/10.3390/ijerph18136906







