Perceived Health, Psychological Distress, and Subjective Well-Being among Older Adults with Parkinson’s Disease: A Cross-Lagged Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sampling and Data Collection
2.2. Measurement
2.3. Data Analysis
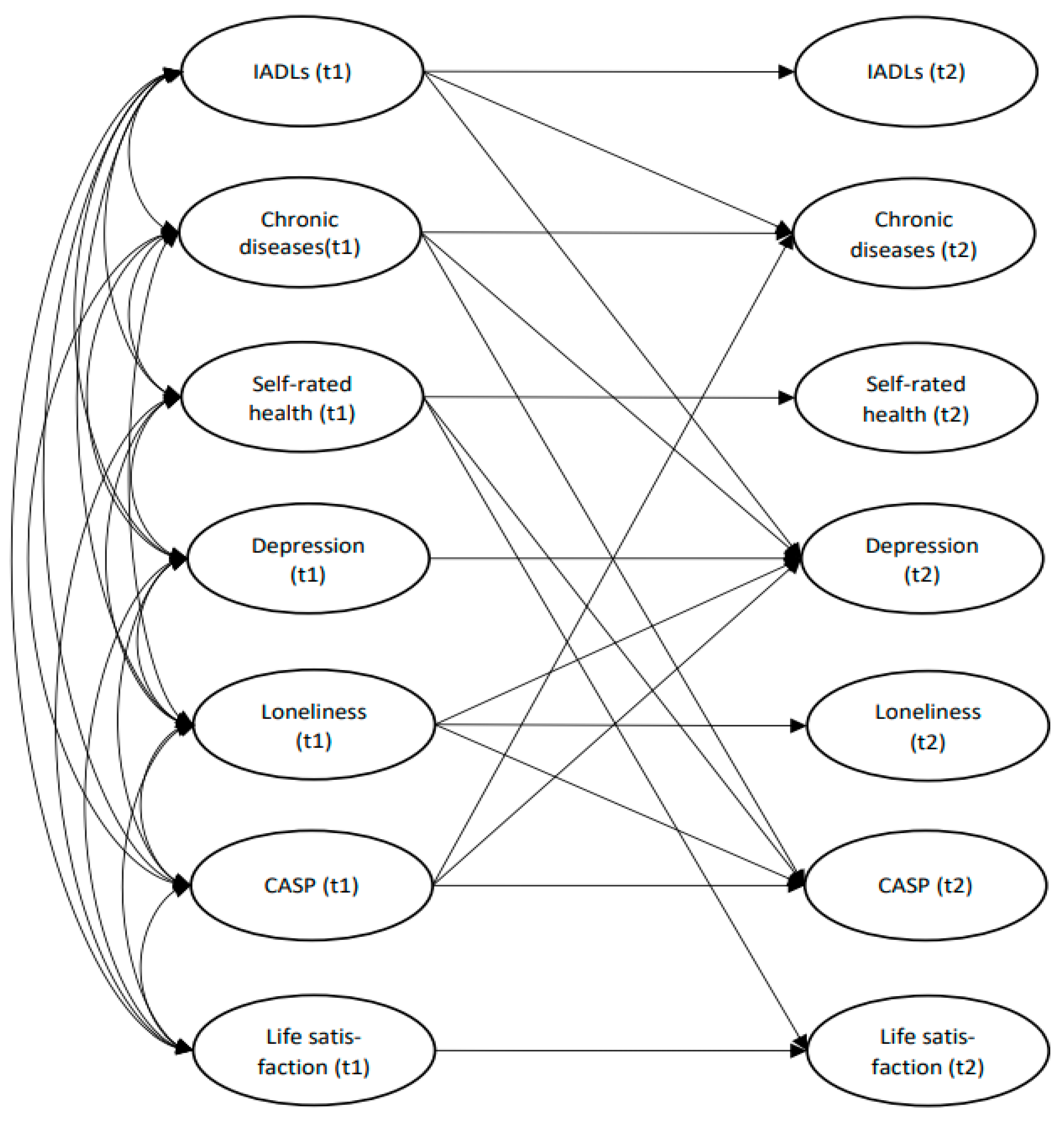
3. Results
3.1. Sample Frame
3.2. Correlations
3.3. Test of Time Effect Using t-Test
3.4. Auto-Regressive Effect and Cross-Lagged Associations
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ADLs (t1) | - | |||||||||||||||
| 2. ADLs (t2) | 0.115 | - | ||||||||||||||
| 3. IADLs (t1) | 0.217 ** | 0.117 | - | |||||||||||||
| 4. IADLs (t2) | 0.192 ** | 0.233 ** | 0.569 ** | - | ||||||||||||
| 5. CI (t1) | 0.028 | 0.090 | 0.138 ** | 0.059 | - | |||||||||||
| 6. CI (t2) | 0.155 * | 0.134 | 0.285 ** | 0.231 ** | 0.408 ** | - | ||||||||||
| 7. SRH (t1) | −0.323 ** | −0.223 ** | −0.385 ** | −0.238 ** | −0.174 ** | −0.175 * | - | |||||||||
| 8. SRH (t2) | −0.190 ** | −0.360 ** | −0.209 ** | −0.359 ** | −0.022 | −0.098 | 0.343 ** | - | ||||||||
| 9. DP (t1) | 0.165 ** | 0.046 | 0.498 ** | 0.289 ** | 0.137 ** | 0.107 | −0.361 ** | −0.078 | - | |||||||
| 10. DP (t2) | 0.085 | 0.094 | 0.375 ** | 0.433 ** | 0.053 | 0.176 * | −0.259 ** | −0.419 ** | 0.427 ** | - | ||||||
| 11. LN (t1) | 0.105 | 0.203 * | 0.270 ** | 0.198 * | 0.111 | 0.116 | −0.193 ** | −0.106 | 0.382 ** | 0.273 ** | - | |||||
| 12. LN (t2) | 0.027 | 0.145 | 0.289 ** | 0.311 ** | 0.089 | 0.122 | −0.102 | −0.152 | 0.310 ** | 0.428 ** | 0.420 ** | - | ||||
| 13. CASP (t1) | −0.202 ** | −0.146 | −0.550 ** | −0.427 ** | −0.131 * | −0.234 ** | 0.475 ** | 0.255 ** | −0.614 ** | −0.430 ** | −0.416 ** | −0.311 ** | - | |||
| 14. CASP (t2) | −0.195 * | −0.143 | −0.301 ** | −0.494 ** | 0.038 | −0.159 | 0.389 ** | 0.377 ** | −0.359 ** | −0.574 ** | −0.305 ** | −0.436 ** | 0.547 ** | - | ||
| 15. LS (t1) | −0.109 * | −0.009 | −0.314 ** | −0.220 ** | −0.119 * | −0.205 ** | 0.330 ** | 0.149 * | −0.415 ** | −0.308 ** | −0.405 ** | −0.297 ** | 0.566 ** | 0.368 ** | - | |
| 16. LS (t2) | −0.033 | −0.074 | −0.232 ** | −0.288 ** | −0.107 | −0.091 | 0.265 ** | 0.332 ** | −0.268 ** | −0.413 ** | −0.252 ** | −0.356 ** | 0.355 ** | 0.552 ** | 0.156 * | - |
Appendix B
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, T.; Smyth, K.A.; Wallendal, M.S.; Hyde, T.; Leo, G.; Geldmacher, D.S. Parkinson disease: Research update and clinical management. South Med. J. 2012, 105, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maserejian, N.; Vinikoor-Imler, L.; Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD): 198. Mov. Disord. 2020, 35. Available online: https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/ (accessed on 26 November 2021).
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the US. NPJ Parkinsons Dis. 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.J.; Gasson, N.; Kane, R.; Bucks, R.S.; Loftus, A.M. Activities of daily living, depression, and quality of life in Parkinson’s disease. PLoS ONE 2014, 9, e102294. [Google Scholar]
- Pirogovsky, E.; Schiehser, D.M.; Obtera, K.M.; Burke, M.M.; Lessig, S.L.; Song, D.D.; Litvan, I.; Filoteo, J.V. Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology 2014, 28, 229. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Meyer, M.; Noldin, E.; Mertzenich, M.; Daleccio, M.; Gannon, S.; Pickett, K. Instrumental Activities of Daily Living (IADLs) for individuals with Parkinson’s Disease: Results From the Canadian Occupational Performance Measure (COPM) as Compared to a Standardized Rating Scale. Am. J. Occup. Ther. 2020, 74, 7411500045p1. [Google Scholar] [CrossRef]
- Forsaa, E.B.; Larsen, J.P.; Wentzel-Larsen, T.; Herlofson, K.; Alves, G. Predictors and course of health-related quality of life in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 1420–1427. [Google Scholar] [CrossRef]
- Harsanyiova, J.; Buday, T.; Kralova Trancikova, A. Parkinson’s disease and the gut: Future perspectives for early diagnosis. Front. Neurosci. 2020, 14, 626. [Google Scholar]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Kurtis, M.M.; Chaudhuri, K.R.; NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 2011, 26, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Behari, M.; Srivastava, A.K.; Pandey, R.M. Quality of life in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2005, 11, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Buczak-Stec, E.W.; König, H.H.; Hajek, A. Impact of incident Parkinson’s disease on satisfaction with life. Front. Neurol. 2018, 9, 589. [Google Scholar] [CrossRef]
- Jonasson, S.B.; Rantakokko, M.; Franzén, E.; Iwarsson, S.; Nilsson, M.H. Prediction of life satisfaction in people with Parkinson’s disease. Parkinsons Dis. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Berardi, A.; Regoli, E.; Tofani, M.; Valente, D.; Fabbrini, G.; Fabbrini, A.; Ruggieri, M.; Panuccio, F.; Galeoto, G. Tools to assess the quality of life in patients with Parkinson’s disease: A systematic review. Expert Rev. Pharm. Outcomes Res. 2021, 21, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M. Parkinson’s disease and quality of life: Issues and challenges beyond motor symptoms. Neurol. Clin. 2004, 22, S141–S148. [Google Scholar] [CrossRef] [PubMed]
- Bolluk, B.; Özel-Kizil, E.T.; Akbostanci, M.C.; Atbasoglu, E.C. Social anxiety in patients with Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 390–394. [Google Scholar] [CrossRef]
- Kano, O.; Ikeda, K.; Cridebring, D.; Takazawa, T.; Yoshii, Y.; Iwasaki, Y. Neurobiology of depression and anxiety in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachana, N.A.; Egan, S.J.; Laidlaw, K.; Dissanayaka, N.; Byrne, G.J.; Brockman, S.; Marsh, R.; Starkstein, S. Clinical issues in the treatment of anxiety and depression in older adults with Parkinson’s disease. Mov. Disord. 2013, 28, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Quelhas, R.; Costa, M. Anxiety, depression, and quality of life in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 413–419. [Google Scholar] [CrossRef]
- Rascol, O.; Payoux, P.; Ory, F.; Ferreira, J.J.; Brefel-Courbon, C.; Montastruc, J.L. Limitations of current Parkinson’s disease therapy. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 53, S3–S15. [Google Scholar] [CrossRef]
- Sagna, A.; Gallo, J.J.; Pontone, G.M. Systematic review of factors associated with depression and anxiety disorders among older adults with Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Smit, M.; Kuiper, A.; Han, V.; Jiawan, V.C.; Douma, G.; Van Harten, B.; Oen, J.M.; Pouwels, M.E.; Dieks, H.J.; Bartels, A.L.; et al. Psychiatric co-morbidity is highly prevalent in idiopathic cervical dystonia and significantly influences health-related quality of life: Results of a controlled study. Parkinsonism Relat. Disord. 2016, 30, 7–12. [Google Scholar] [CrossRef]
- Moguel-Cobos, G.; Saldivar, C.; Goslar, P.W.; Shill, H.A. The relationship between social anxiety disorder and motor symptoms of Parkinson disease: A pilot study. Psychosomatics 2020, 61, 321–326. [Google Scholar] [CrossRef]
- Vann-Ward, T.; Morse, J.M.; Charmaz, K. Preserving self: Theorizing the social and psychological processes of living with Parkinson disease. Qual. Health Res. 2017, 27, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Uitti, R.J. Treatment of Parkinson’s disease: Focus on quality of life issues. Parkinsonism Relat. Disord. 2012, 18, S34–S36. [Google Scholar] [CrossRef]
- Vescovelli, F.; Sarti, D.; Ruini, C. Subjective and psychological well-being in Parkinson’s Disease: A systematic review. Acta Neurol. Scand. 2018, 138, 12–23. [Google Scholar] [CrossRef]
- Kummer, A.; Cardoso, F.; Teixeira, A.L. Suicidal ideation in Parkinson’s disease. CNS Spectr. 2009, 14, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Mursaleen, L.R.; Stamford, J.A. Drugs of abuse and Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 209–217. [Google Scholar]
- Rahman, S.; Griffin, H.J.; Quinn, N.P.; Jahanshahi, M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.K.; Cronin-Golomb, A. Impact of anxiety on quality of life in Parkinson’s disease. Parkinson’s Dis. 2012, 2012, 1–8. [Google Scholar]
- Leroi, I.; Ahearn, D.J.; Andrews, M.; McDonald, K.R.; Byrne, E.J.; Burns, A. Behavioural disorders, disability and quality of life in Parkinson’s disease. Age Ageing 2011, 40, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Shulman, L.M.; Taback, R.L.; Bean, J.; Weiner, W.J. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2001, 16, 507–510. [Google Scholar] [CrossRef]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data resource profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Malter, F.; Börsch-Supan, A. SHARE Compliance Profiles–Wave 4; MEA, Max Planck Institute for Social Law and Social Policy: Munich, Germany, 2013. [Google Scholar]
- Malter, F.; Schuller, K.; Börsch-Supan, A. SHARE Compliance Profiles–Wave 6; MEA, Max Planck Institute for Social Law and Social Policy: Munich, Germany, 2016. [Google Scholar]
- Ware, J.E., Jr.; Gandek, B. Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Beekman, A.T.; Deeg, D.J.; Fuhrer, R.; Kivela, S.L.; Lawlor, B.A.; Lobo, A.; Magnusson, H.; Meller, I.; Van Oyen, H.; et al. Depression symptoms in late life assessed using the EURO–D scale: Effect of age, gender and marital status in 14 European centres. Br. J. Psychiatry 1999, 174, 339–345. [Google Scholar] [CrossRef]
- Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Cacioppo, J.T. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Res. Aging 2004, 26, 655–672. [Google Scholar] [CrossRef]
- Hyde, M.; Wiggins, R.D.; Higgs, P.; Blane, D.B. A measure of quality of life in early old age: The theory, development and properties of a needs satisfaction model (CASP-19). Aging Ment. Health 2003, 7, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.; Lucas, R.E. Assessing the validity of single-item life satisfaction measures: Results from three large samples. Qual. Life Res. 2014, 23, 2809–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentler, P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. A multidisciplinary journal cutoff criteria for fit indexes in covariance structure analysis. Struct. Equ. Modeling 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Stutts, L.A.; Speight, K.L.; Yoo, S.; Little, I.D. Positive psychological predictors of psychological health in individuals with Parkinson’s Disease. J. Clin. Psychol. Med. Settings 2020, 27, 182–189. [Google Scholar] [CrossRef]
- Gison, A.; Dall’Armi, V.; Donati, V.; Rizza, F.; Giaquinto, S. Dispositional optimism, depression, disability and quality of life in Parkinson’s disease. Funct. Neurol. 2014, 29, 113. [Google Scholar] [PubMed]
- Lundervold, D.A.; Pahwa, R.; Lyons, K.E. Behavioral relaxation training for Parkinson’s disease related dyskinesia and comorbid social anxiety. Int. J. Behav. Consult. Ther. 2013, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Sheehy, T.L.; McDonough, M.H.; Zauber, S.E. Social comparisons, social support, and self-perceptions in group exercise for people with Parkinson’s disease. J. Appl. Sport Psychol. 2017, 29, 285–303. [Google Scholar] [CrossRef] [Green Version]
| Time 1 | Time 2 | t-Value | p-Value | Cohen’s d | |
|---|---|---|---|---|---|
| Measures | Mean (SD) | Mean (SD) | |||
| ADLs | 0.92 (0.26) | 0.91 (0.28) | 0.391 | 0.231 | - |
| IADLs | 1.51 (1.92) | 3.64 (2.54) | −11.040 | 0.000 | 0.95 |
| Chronic diseases | 3.43 (1.87) | 3.28 (2.01) | 0.946 | 0.345 | - |
| Self-rated health | 1.72 (0.76) | 1.62 (0.68) | 1.505 | 0.134 | - |
| Depression | 3.79 (2.55) | 3.92 (2.55) | −0.612 | 0.541 | - |
| Loneliness | 1.40 (0.68) | 1.50 (0.62) | 1.392 | 0.134 | - |
| CASP-quality of life scale | 34.08 (6.46) | 32.83 (6.03) | 2.487 | 0.014 | 0.21 |
| Life satisfaction | 7.06 (2.12) | 6.68 (2.11) | 2.060 | 0.031 | 0.18 |
| Path | β | S.E. | t-Value | R2 | |
|---|---|---|---|---|---|
| Time 1 | Time 2 | ||||
| IADLs | IADLs | 0.550 *** | 0.072 | 8.544 | 0.49 |
| CASP-quality of life | −0.217 *** | 0.071 | −3.318 | ||
| Chronic diseases | Chronic diseases | 0.365 *** | 0.064 | 5.748 | 0.23 |
| IADLs | 0.265 *** | 0.064 | 4.164 | ||
| Self-rated health | Self-rated health | 0.328 *** | 0.067 | 4.828 | 0.09 |
| Depression | Depression | 0.185 * | 0.088 | 2.234 | 0.31 |
| Loneliness | 0.161 * | 0.080 | 2.157 | ||
| CASP-quality of life | −0.244 ** | 0.093 | −2.747 | ||
| Chronic diseases | 0.184 ** | 0.065 | 3.072 | ||
| IADLs | 0.249 ** | 0.083 | 3.256 | ||
| Loneliness | Loneliness | 0.539 *** | 0.073 | 7.355 | 0.25 |
| CASP-quality of life | CASP-quality of life | 0.445 *** | 0.078 | 5.832 | 0.38 |
| Chronic diseases | −0.208 *** | 0.065 | −3.388 | ||
| Self-rated health | 0.196 ** | 0.073 | 2.831 | ||
| Loneliness | −0.228 ** | 0.077 | −3.069 | ||
| Life satisfaction | Life satisfaction | 0.401 *** | 0.075 | 5.418 | 0.20 |
| Self-rated health | 0.151 * | 0.075 | 2.050 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S. Perceived Health, Psychological Distress, and Subjective Well-Being among Older Adults with Parkinson’s Disease: A Cross-Lagged Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12566. https://doi.org/10.3390/ijerph182312566
Lee S. Perceived Health, Psychological Distress, and Subjective Well-Being among Older Adults with Parkinson’s Disease: A Cross-Lagged Analysis. International Journal of Environmental Research and Public Health. 2021; 18(23):12566. https://doi.org/10.3390/ijerph182312566
Chicago/Turabian StyleLee, Sunwoo. 2021. "Perceived Health, Psychological Distress, and Subjective Well-Being among Older Adults with Parkinson’s Disease: A Cross-Lagged Analysis" International Journal of Environmental Research and Public Health 18, no. 23: 12566. https://doi.org/10.3390/ijerph182312566
APA StyleLee, S. (2021). Perceived Health, Psychological Distress, and Subjective Well-Being among Older Adults with Parkinson’s Disease: A Cross-Lagged Analysis. International Journal of Environmental Research and Public Health, 18(23), 12566. https://doi.org/10.3390/ijerph182312566






