COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study
Abstract
:1. Introduction
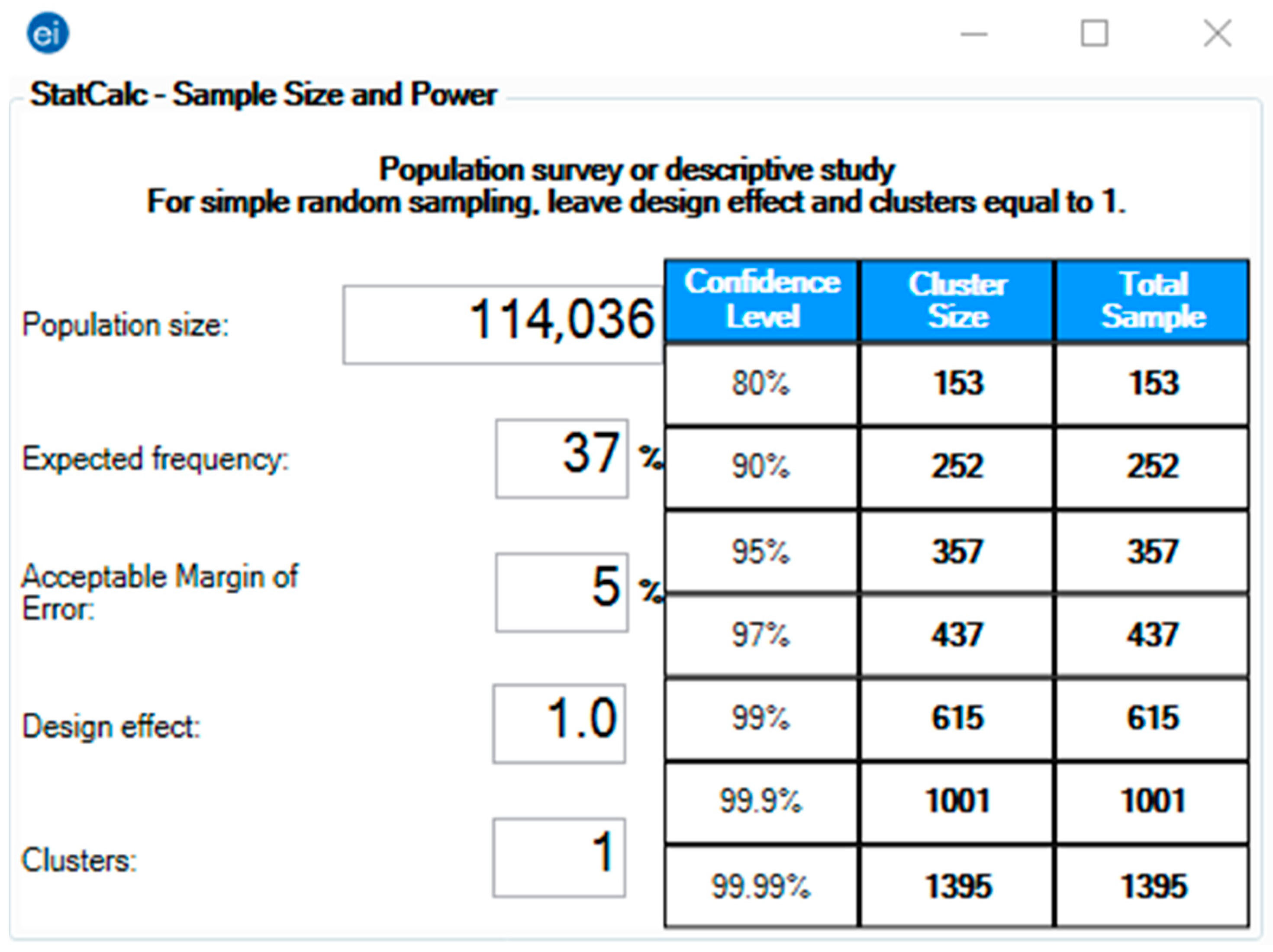
2. Materials and Methods
2.1. Design
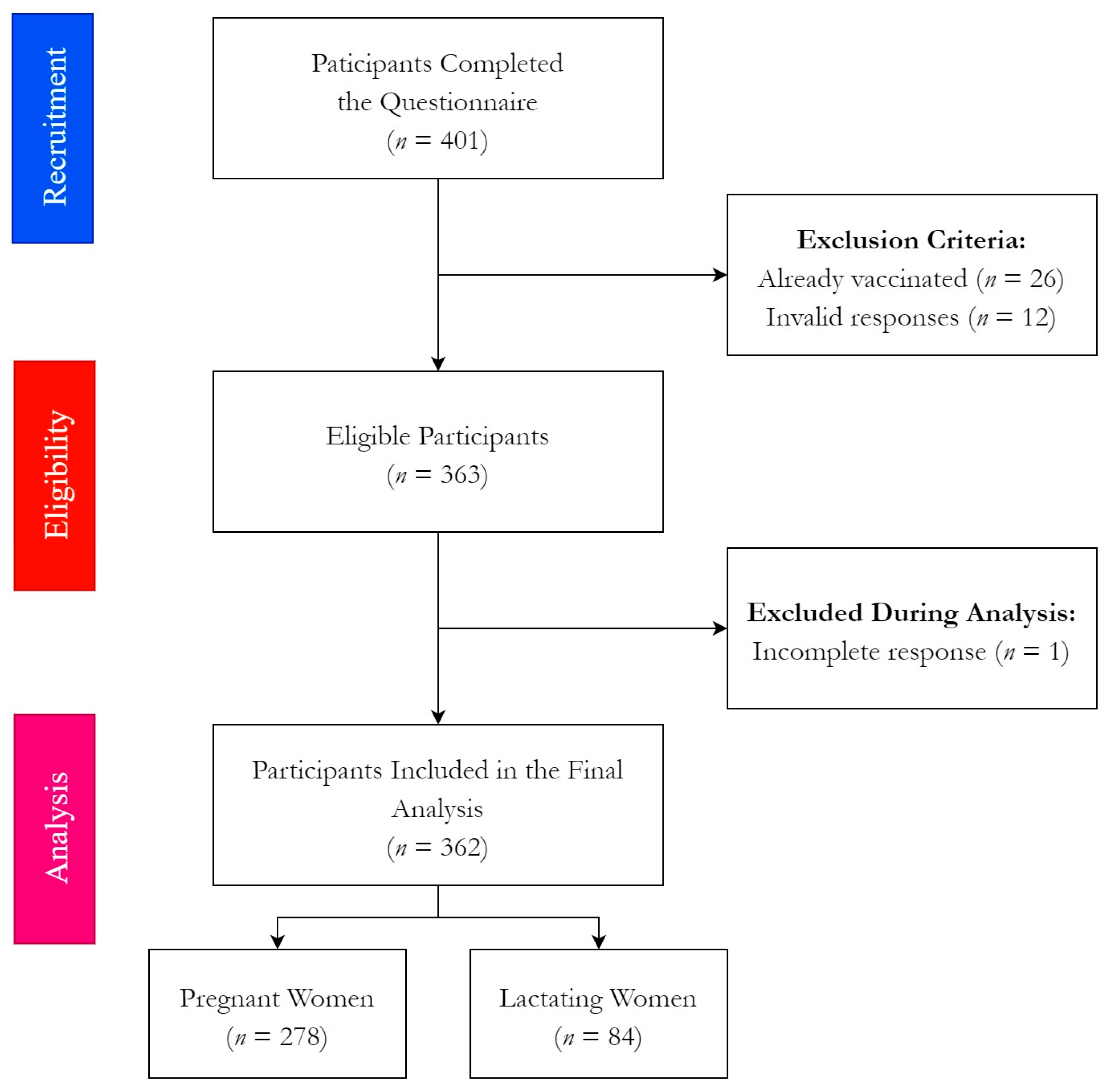
2.2. Participants
2.3. Instrument
2.4. Ethics
2.5. Analysis
3. Results
3.1. Demographic Characteristics
3.2. Clinical and Obstetric Characteristics
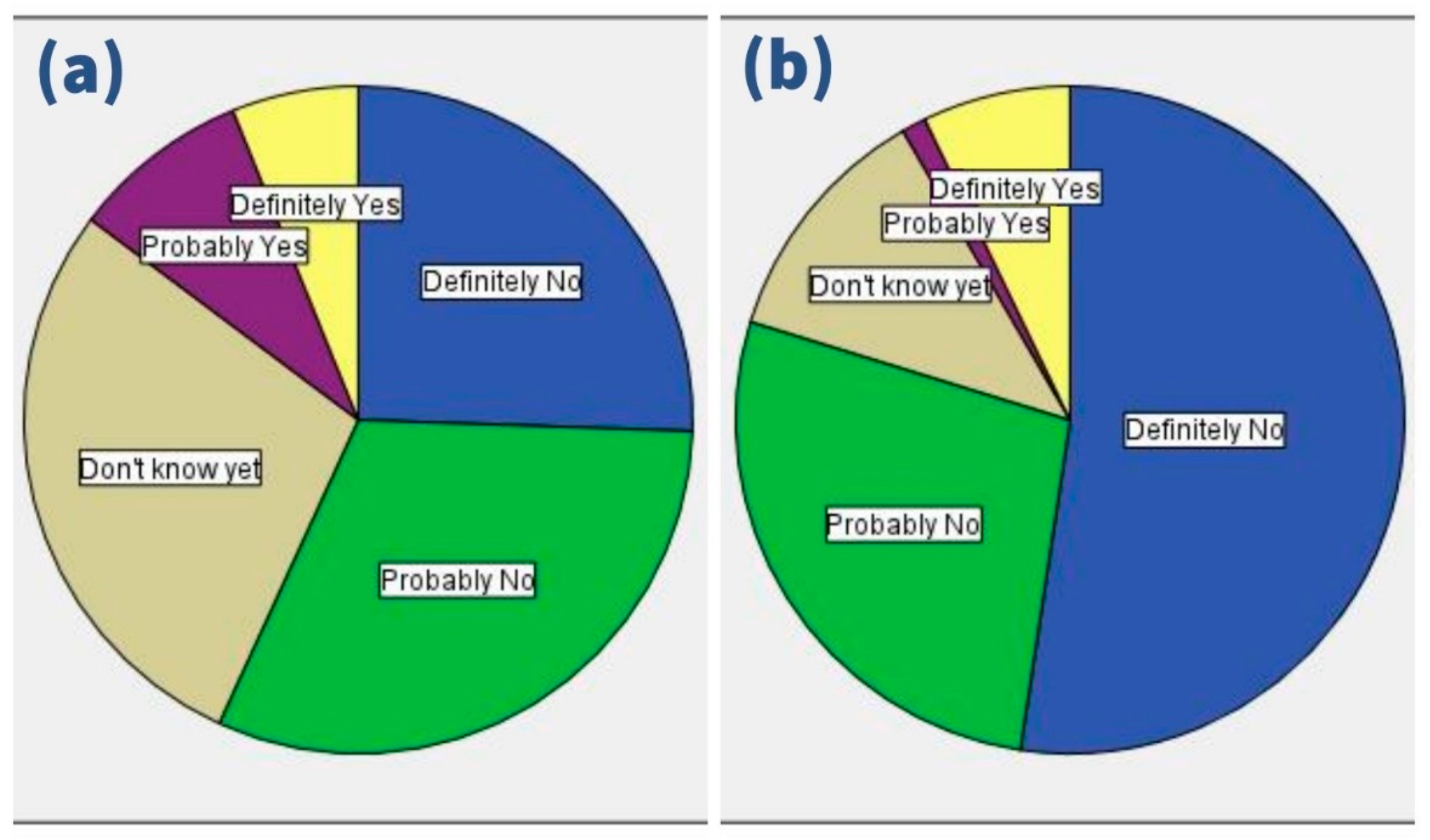
3.3. COVID-19 Vaccine-Related Attitudes
3.4. Psychosocial Predictors of Vaccine Hesitancy
3.5. Analysis of COVID-19 Vaccine Acceptance
3.6. Analysis of Responses to Physicians’ Recommendations
3.7. COVID-19 Vaccine Acceptance among Pregnant Women
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC) Pregnancy Complications. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications.html (accessed on 10 November 2021).
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F.; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Wilson, R.J.; Paterson, P.; Jarrett, C.; Larson, H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015, 33, 6420–6429. [Google Scholar] [CrossRef] [Green Version]
- Kukla, R. The ethics and cultural politics of reproductive risk warnings: A case study of California’s Proposition 65. Health Risk Soc. 2010, 12, 323–334. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Johnson, T.; Phillips, L.; Nelson, T.B. Sources of Vaccine Hesitancy: Pregnancy, Infertility, Minority Concerns, and General Skepticism. Open Forum Infect. Dis. 2021, ofab433. [Google Scholar] [CrossRef]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; et al. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef] [Green Version]
- Benis, A.; Seidmann, A.; Ashkenazi, S. Reasons for Taking the COVID-19 Vaccine by US Social Media Users. Vaccines 2021, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Glanz, J.M.; Wagner, N.M.; Narwaney, K.J.; Kraus, C.R.; Shoup, J.A.; Xu, S.; O’Leary, S.T.; Omer, S.B.; Gleason, K.S.; Daley, M.F. Web-based social media intervention to increase vaccine acceptance: A randomized controlled trial. Pediatrics 2017, 140, e20171117. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, S.T.; Narwaney, K.J.; Wagner, N.M.; Kraus, C.R.; Omer, S.B.; Glanz, J.M. Efficacy of a Web-Based Intervention to Increase Uptake of Maternal Vaccines: An RCT. Am. J. Prev. Med. 2019, 57, e125–e133. [Google Scholar] [CrossRef]
- Daley, M.F.; Glanz, J.M. Using Social Media to Increase Vaccine Acceptance. Acad. Pediatr. 2021, 21, S32–S33. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Abdulqader, H.; Morgado, M.; Domnori, S.; Koščík, M.; Mendes, J.J.; Klugar, M.; Kateeb, E. Global Prevalence and Drivers of Dental Students’ COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Trent, M.; Seale, H.; Chughtai, A.; Salmon, D.; MacIntyre, C. Trust in government, intention to vaccinate and COVID-19 vaccine hesitancy: A comparative survey of five large cities in the United States, United Kingdom, and Australia. Vaccine 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Schulz, W.S.; Chaudhuri, M.; Zhou, Y.; Dube, E.; Schuster, M.; MacDonald, N.E.; Wilson, R.; Eskola, J.; et al. Measuring vaccine hesitancy: The development of a survey tool. Vaccine 2015, 33, 4165–4175. [Google Scholar] [CrossRef] [Green Version]
- Ministerstvo Zdravotnictví ČR (MZČR) COVID-19: Přehled Vykázaných Očkování v ČR. Available online: https://onemocneni-aktualne.mzcr.cz/vakcinace-cr (accessed on 10 November 2021).
- Státní Ústav Pro Kontrolu Léčiv (SÚKL) Přehled: Nahlášená Podezření na Nežádoucí Účinky po Vakcínách Proti COVID-19. Available online: https://www.sukl.cz/tydenni-zpravy-o-prijatych-hlasenich-podezreni-na-nezadouci (accessed on 10 November 2021).
- Nour, S.; Plourde, G. Pharmacoepidemiology and Pharmacovigilance: Synergistic Tools to Better Investigate Drug Safety; Academic Press: Cambridge, MA, USA, 2018; pp. 1–224. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Antalová, N.; Krobot, M.; Zviadadze, N.; Serdiuk, I.; Koščík, M.; Klugar, M. Prevalence and Drivers of COVID-19 Vaccine Hesitancy among Czech University Students: National Cross-Sectional Study. Vaccines 2021, 9, 948. [Google Scholar] [CrossRef]
- Kosarkova, A.; Malinakova, K.; van Dijk, J.P.; Tavel, P. Vaccine Refusal in the Czech Republic Is Associated with Being Spiritual but not Religiously Affiliated. Vaccines 2021, 9, 1157. [Google Scholar] [CrossRef]
- Štěpánek, L.; Janošíková, M.; Nakládalová, M.; Štěpánek, L.; Boriková, A.; Vildová, H. Motivation to COVID-19 Vaccination and Reasons for Hesitancy in Employees of a Czech Tertiary Care Hospital: A Cross-Sectional Survey. Vaccines 2021, 9, 863. [Google Scholar] [CrossRef]
- Wilson, R.; Paterson, P.; Larson, H.J. Strategies to improve maternal vaccination acceptance. BMC Public Health 2019, 19, 342. [Google Scholar] [CrossRef]
- KoBoToolbox.org KoBoToolbox. Available online: https://support.kobotoolbox.org/welcome.html (accessed on 12 August 2020).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. UroToday Int. J. 2007, 335, 806–808. [Google Scholar] [CrossRef] [Green Version]
- Fakultní Nemocnice Brno (FN-Brno) Gynekologicko-Porodnická Klinika [Department of Gynecology and Obstetrics]. Available online: https://www.fnbrno.cz/gynekologicko-porodnicka-klinika/k1447 (accessed on 2 November 2021).
- Centers for Disease Control and Prevention, (CDC) Epi InfoTM for Windows. Available online: https://www.cdc.gov/epiinfo/pc.html (accessed on 25 December 2020).
- Centers for Disease Control and Prevention (CDC) Population Survey or Descriptive Study. Available online: https://www.cdc.gov/epiinfo/user-guide/statcalc/samplesize.html (accessed on 1 December 2021).
- Czech Statistical Office (CZSO) Aktuální Populační Vývoj v Kostce [Current Population Development in a Nutshell]. Available online: https://www.czso.cz/csu/czso/aktualni-populacni-vyvoj-v-kostce (accessed on 2 November 2021).
- Ayhan, S.G.; Oluklu, D.; Atalay, A.; Beser, D.M.; Tanacan, A.; Tekin, O.M.; Sahin, D. COVID-19 vaccine acceptance in pregnant women. Int. J. Gynecol. Obstet. 2021, 154, 291–296. [Google Scholar] [CrossRef]
- Tao, L.; Wang, R.; Han, N.; Liu, J.; Yuan, C.; Deng, L.; Han, C.; Sun, F.; Liu, M.; Liu, J. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: A multi-center cross-sectional study based on health belief model. Hum. Vaccines Immunother. 2021, 17, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA—J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proton Technologies AG General Data Protection Regulation (GDPR) Compliance Guidelines. Available online: https://gdpr.eu/ (accessed on 1 May 2020).
- SPSS Inc. IBM SPSS Statistics 28. Available online: https://www.ibm.com/support/pages/ibm-spss-statistics-28-documentation (accessed on 14 March 2021).
- NHMRC. Australian Guidelines for the Clinical Care of People with COVID-19. Available online: https://www.clinicalguidelines.gov.au/register/australian-guidelines-clinical-care-people-covid-19 (accessed on 17 September 2020).
- Mohan, S.; Reagu, S.; Lindow, S.; Alabdulla, M. COVID-19 vaccine hesitancy in perinatal women: A cross sectional survey. J. Perinat. Med. 2021, 49, 678–685. [Google Scholar] [CrossRef]
- Kalok, A.; Loh, S.Y.E.; Chew, K.T.; Abdul Aziz, N.H.; Shah, S.A.; Ahmad, S.; Mohamed Ismail, N.A.; Abdullah Mahdy, Z. Vaccine hesitancy towards childhood immunisation amongst urban pregnant mothers in Malaysia. Vaccine 2020, 38, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.M.; Minard, C.G.; Guffey, D.; Swaim, L.S.; Opel, D.J.; Boom, J.A. Prevalence of Vaccine Hesitancy among Expectant Mothers in Houston, Texas. Acad. Pediatr. 2018, 18, 154–160. [Google Scholar] [CrossRef]
- Maurici, M.; Dugo, V.; Zaratti, L.; Paulon, L.; Pellegrini, M.G.; Baiocco, E.; Rizzo, G.; Franco, E. Knowledge and attitude of pregnant women toward flu vaccination: A cross-sectional survey. J. Matern. Neonatal Med. 2015, 29, 3147–3150. [Google Scholar] [CrossRef]
- van Lier, A.; Steens, A.; Ferreira, J.A.; van der Maas, N.A.T.; de Melker, H.E. Acceptance of vaccination during pregnancy: Experience with 2009 influenza A (H1N1) in the Netherlands. Vaccine 2012, 30, 2892–2899. [Google Scholar] [CrossRef]
- Strassberg, E.R.; Power, M.; Schulkin, J.; Stark, L.M.; Mackeen, A.D.; Murtough, K.L.; Paglia, M.J. Patient attitudes toward influenza and tetanus, diphtheria and acellular pertussis vaccination in pregnancy. Vaccine 2018, 36, 4548–4554. [Google Scholar] [CrossRef]
- Thorpe, P.G.; Gilboa, S.M.; Hernandez-Diaz, S.; Lind, J.; Cragan, J.D.; Briggs, G.; Kweder, S.; Friedman, J.M.; Mitchell, A.A.; Honein, M.A. Medications in the first trimester of pregnancy: Most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol. Drug Saf. 2013, 22, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Mohammed Aljoher, A.; Abdullah Alsaeed, M.; Abdulltife AlKhlfan, M.; Wassil Almethen, A.; Abdullah Almukhaitah, M.; Zareen, H.; Ibrahim Ali, S.; Arabia, S. Pregnant Women Risk Perception of Medications and Natural Products Use During Pregnancy in Alahsa, Saudi Arabia. Egypt. J. Hosp. Med. 2018, 70, 13–20. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2021, in press. [Google Scholar] [CrossRef]
- Suzuki, S. Psychological status during the first trimester of pregnancy under the COVID-19 epidemic in Japan. J. Matern.-Fetal Neonatal Med. 2020, 1–2. [Google Scholar] [CrossRef]
- Otieno, N.A.; Otiato, F.; Nyawanda, B.; Adero, M.; Wairimu, W.N.; Ouma, D.; Atito, R.; Wilson, A.; Gonzalez-Casanova, I.; Malik, F.A.; et al. Drivers and barriers of vaccine acceptance among pregnant women in Kenya. Hum. Vaccines Immunother. 2020, 16, 2429–2437. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Varan, A.K.; Esteves-Jaramillo, A.; Siddiqui, M.; Sultana, S.; Ali, A.S.; Zaidi, A.K.M.; Omer, S.B. Influenza vaccine acceptance among pregnant women in urban slum areas, Karachi, Pakistan. Vaccine 2015, 33, 5103–5109. [Google Scholar] [CrossRef]
- Hoque, A.M.; Buckus, S.; Hoque, M.; Hoque, M.E.; Van Hal, G. COVID-19 Vaccine Acceptability among Pregnant Women at a Primary Health Care Facility in Durban, South Africa. Eur. J. Med. Health Sci. 2020, 2. [Google Scholar] [CrossRef]
- Hergenrather, K.C.; Zeglin, R.J.; McGuire-Kuletz, M.; Rhodes, S.D. Employment as a Social Determinant of Health: A Review of Longitudinal Studies Exploring the Relationship between Employment Status and Mental Health. Rehabil. Res. Policy Educ. 2015, 29, 261–290. [Google Scholar] [CrossRef]
- Hergenrather, K.C.; Zeglin, R.J.; McGuire-Kuletz, M.; Rhodes, S.D. Employment as a Social Determinant of Health: A Systematic Review of Longitudinal Studies Exploring the Relationship between Employment Status and Physical Health. Rehabil. Res. Policy Educ. 2015, 29, 2–26. [Google Scholar] [CrossRef]
- Khojasteh, F.; Arbabisarjou, A.; Boryri, T.; Safarzadeh, A.; Pourkahkhaei, M. The Relationship between Maternal Employment Status and Pregnancy Outcomes. Glob. J. Health Sci. 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, D.J.; Al-Tawfiq, J.; Babcock, H.; Bryant, K.; Drees, M.; Elshaboury, R.; Essick, K.; Fakih, M.; Henderson, D.; Javaid, W.; et al. Multisociety statement on coronavirus disease 2019 (COVID-19) vaccination as a condition of employment for healthcare personnel. Infect. Control Hosp. Epidemiol. 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Rosenstock, I.M. The Health Belief Model and Preventive Health Behavior. Health Educ. Behav. 1974, 2, 354–386. [Google Scholar] [CrossRef]
- Adams, A.; Hall, M.; Fulghum, J. Utilizing the Health Belief Model to Assess Vaccine Acceptance of Patients on Hemodialysis. Nephrol. Nurs. J. 2014, 41, 393–407. [Google Scholar] [PubMed]
- Glanz, K.; Rimer, B.K.; Viswanath, K. Theory, Research, and Practice in Health Behavior and Health Education. In Health Behavior and Health Education: Theory, Research, and Practice; Jossey-Bass: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rzymski, P.; Zeyland, J.; Poniedziałek, B.; Małecka, I.; Wysocki, J. The perception and attitudes toward COVID-19 vaccines: A cross-sectional study in poland. Vaccines 2021, 9, 382. [Google Scholar] [CrossRef]
- Salerno, L.; Craxì, L.; Amodio, E.; Lo Coco, G. Factors Affecting Hesitancy to mRNA and Viral Vector COVID-19 Vaccines among College Students in Italy. Vaccines 2021, 9, 927. [Google Scholar] [CrossRef]
- Ren, X.; Shen, F.; Gui, Y.; Wang, W.; Xing, B.; Huang, W. The attitudes of psychiatric patients towards COVID-19 vaccination in China: A cross-sectional study. BMC Psychiatry 2021, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Centers for Diseases Control and Prevention (CDC) V-Safe COVID-19 Vaccine Pregnancy Registry. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html (accessed on 9 November 2021).
- Adhikari, E.H.; Spong, C.Y. COVID-19 Vaccination in Pregnant and Lactating Women. JAMA 2021, 325, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.D.; Pippen, J.L.; Adesomo, A.A.; Rood, K.M.; Landon, M.B.; Costantine, M.M. Exclusion of Pregnant Women from Clinical Trials during the Coronavirus Disease 2019 Pandemic: A Review of International Registries. Am. J. Perinatol. 2020, 37, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.; Ayres-de-Campos, D.; Bancalari, E.; Benders, M.; Briana, D.; Di Renzo, G.C.; Fonseca, E.B.; Hod, M.; Poon, L.; Cortes, M.S.; et al. Equity in coronavirus disease 2019 vaccine development and deployment. Am. J. Obstet. Gynecol. 2021, 224, 423–427. [Google Scholar] [CrossRef]
- Ren, Z.; Bremer, A.A.; Pawlyk, A.C. Drug development research in pregnant and lactating women. Am. J. Obstet. Gynecol. 2021, 225, 33–42. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Kaeser, L.; Cernich, A.N. Involving Pregnant Individuals in Clinical Research on COVID-19 Vaccines. JAMA 2021, 325, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Gal Levin, E.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.R.; Racherla, S.; Tirumala, R.; Madathala, R.R.; Gajula, V. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: A cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021, 225, 458–460. [Google Scholar] [CrossRef]
- Kachikis, A.; Englund, J.A.; Singleton, M.; Covelli, I.; Drake, A.L.; Eckert, L.O. Short-term Reactions among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout. JAMA Netw. Open 2021, 4, e2121310. [Google Scholar] [CrossRef]
- Riad, A.; Schünemann, H.; Attia, S.; Peričić, T.P.; Žuljević, M.F.; Jürisson, M.; Kalda, R.; Lang, K.; Morankar, S.; Yesuf, E.A.; et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Environ. Res. Public Health 2021, 18, 7859. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Mekhemar, M.; Conrad, J.; Klugarová, J.; Koščík, M.; Klugar, M.; Attia, S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines 2021, 9, 673. [Google Scholar] [CrossRef]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef] [PubMed]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.-P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef]
- Riad, A.; Hocková, B.; Kantorová, L.; Slávik, R.; Spurná, L.; Stebel, A.; Havriľak, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals 2021, 14, 873. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Klugarová, J.; Antalová, N.; Kantorová, L.; Koščík, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccines among Young Adults (18–30 Years Old): An Independent Post-Marketing Study. Pharmaceuticals 2021, 14, 1049. [Google Scholar] [CrossRef]
- Dziedzic, A.; Riad, A.; Attia, S.; Klugar, M.; Tanasiewicz, M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J. Clin. Med. 2021, 10, 5338. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e10. [Google Scholar] [CrossRef]
- Edlow, A.G.; Li, J.Z.; Collier, A.R.Y.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M.; et al. Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies during the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, A.J.; Hofferth, S.L. A gestational vulnerability window for smoking exposure and the increased risk of preterm birth: How timing and intensity of maternal smoking matter. Reprod. Health 2019, 16, 43. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Berry, S.D.; Johnson, K.S.; Myles, L.; Herndon, L.; Montoya, A.; Fashaw, S.; Gifford, D. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J. Am. Geriatr. Soc. 2021, 69, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Karafillakis, E.; Francis, M.R.; Paterson, P.; Larson, H.J. Trust, emotions and risks: Pregnant women’s perceptions, confidence and decision-making practices around maternal vaccination in France. Vaccine 2021, 39, 4117–4125. [Google Scholar] [CrossRef] [PubMed]
- Kateeb, E.; Danadneh, M.; Pokorná, A.; Klugarová, J.; Abdulqader, H.; Klugar, M.; Riad, A. Predictors of Willingness to Receive COVID-19 Vaccine: Cross-Sectional Study of Palestinian Dental Students. Vaccines 2021, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.; Weitzer, J.; Laubichler, M.D.; Birmann, B.M.; Bertau, M.; Zenk, L.; Caniglia, G.; Jäger, C.C.; Steiner, G. Correlates of COVID-19 vaccine hesitancy in Austria: Trust and the government. J. Public Health 2021, fdab122. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Dinga, J.N.; Sinda, L.K.; Titanji, V.P.K. Assessment of vaccine hesitancy to a COVID-19 vaccine in cameroonian adults and its global implication. Vaccines 2021, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Holzmann-Littig, C.; Braunisch, M.C.; Kranke, P.; Popp, M.; Seeber, C.; Fichtner, F.; Littig, B.; Carbajo-Lozoya, J.; Allwang, C.; Frank, T.; et al. COVID-19 Vaccination Acceptance and Hesitancy among Healthcare Workers in Germany. Vaccines 2021, 9, 777. [Google Scholar] [CrossRef]
- Guillon, M.; Kergall, P. Factors associated with COVID-19 vaccination intentions and attitudes in France. Public Health 2021, 198, 200–207. [Google Scholar] [CrossRef]
- Riad, A.; Huang, Y.; Abdulqader, H.; Morgado, M.; Domnori, S.; Koščík, M.; Mendes, J.J.; Klugar, M.; Kateeb, E.; Score, I. Universal Predictors of Dental Students’ Attitudes towards COVID-19 Vaccination: Machine Learning-Based Approach. Vaccines 2021, 9, 1158. [Google Scholar] [CrossRef]
- Beck, U.; Lash, S.; Wynne, B. Risk Society: Towards a New Modernity; Sage: Newbury Park, CA, USA, 1992; Volume 17, ISBN 0803983468. [Google Scholar]
- Fairhead, J.; Leach, M. Vaccine Anxieties: Global Science, Child Health and Society; Taylor & Francis: Milton, UK, 2012; ISBN 1136549226. [Google Scholar]
- Poltorak, M.; Leach, M.; Fairhead, J.; Cassell, J. ‘MMR talk’ and vaccination choices: An ethnographic study in Brighton. Soc. Sci. Med. 2005, 61, 709–719. [Google Scholar] [CrossRef]
- Nganga, S.W.; Otieno, N.A.; Adero, M.; Ouma, D.; Chaves, S.S.; Verani, J.R.; Widdowson, M.A.; Wilson, A.; Bergenfeld, I.; Andrews, C.; et al. Patient and provider perspectives on how trust influences maternal vaccine acceptance among pregnant women in Kenya. BMC Health Serv. Res. 2019, 19, 747. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C.R.; Bottrell, K.; Paterson, P.; Schulz, W.S.; Vandrevala, T.; Larson, H.J.; Jones, C.E. Influenza and pertussis vaccination in pregnancy: Portrayal in online media articles and perceptions of pregnant women and healthcare professionals. Vaccine 2018, 36, 7625–7631. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Calvert, A.; Metz, J.; Kilich, E.; MacLeod, R.; Beadon, K.; Heath, P.T.; Khalil, A.; Finn, A.; Snape, M.D.; et al. Determinants of influenza and pertussis vaccination uptake in pregnancy a multicenter questionnaire study of pregnant women and healthcare professionals. Pediatr. Infect. Dis. J. 2019, 38, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Harrison, M.J.; Kushner, K.E.; Benzies, K.; Rempel, G.; Kimak, C. Women’s Satisfaction with Their Involvement in Health Care Decisions during a High-Risk Pregnancy. Birth 2003, 30, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Fabry, P.; Gagneur, A.; Pasquier, J.C. Determinants of A (H1N1) vaccination: Cross-sectional study in a population of pregnant women in Quebec. Vaccine 2011, 29, 1824–1829. [Google Scholar] [CrossRef]
- Ymba, A.; Perrey, C. Acceptability of tetanus toxoid vaccine by pregnant women in two health centres in Abidjan (Ivory Coast). Vaccine 2003, 21, 3497–3500. [Google Scholar] [CrossRef]
- Afridi, N.K.; Hatcher, J.; Mahmud, S.; Nanan, D. Toxoid vaccination status among females of reproductive age in Peshawar. JCPSP 2005, 15, 391–395. [Google Scholar]
- Ozer, A.; Arikan, D.C.; Kirecci, E.; Ekerbicer, H.C. Status of Pandemic Influenza Vaccination and Factors Affecting It in Pregnant Women in Kahramanmaras, an Eastern Mediterranean City of Turkey. PLoS ONE 2010, 5, e14177. [Google Scholar] [CrossRef]
- Meharry, P.M.; Colson, E.R.; Grizas, A.P.; Stiller, R.; Vázquez, M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern. Child Health J. 2013, 17, 156–164. [Google Scholar] [CrossRef]
- Helen Skirrow, A.; Barnett, S.; Bell, S.; Riaposova, L.; Mounier-Jack, S.; Kampmann, B.; Holder, B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: A multi-methods study in the UK. medRxiv 2021. [Google Scholar] [CrossRef]
- Park, C.-Y. News Media Exposure and Self-Perceived Knowledge: The Illusion of Knowing. Int. J. Public Opin. Res. 2001, 13, 419–425. [Google Scholar] [CrossRef]
- Capobianco, G.; Saderi, L.; Aliberti, S.; Mondoni, M.; Piana, A.; Dessole, F.; Dessole, M.; Cherchi, P.L.; Dessole, S.; Sotgiu, G. COVID-19 in pregnant women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Kasraeian, M.; Zare, M.; Vafaei, H.; Asadi, N.; Faraji, A.; Bazrafshan, K.; Roozmeh, S. COVID-19 pneumonia and pregnancy; a systematic review and meta-analysis. J. Matern. Neonatal Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Pormohammad, A.; Sheikh Neshin, S.A.; Ghorbani, S.; Bose, D.; Alimohammadi, S.; Basirjafari, S.; Mohammadi, M.; Rasmussen-Ivey, C.; Razizadeh, M.H.; et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, e2208. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.; Patel, C.; Abuelgasim, E.; Harky, A. COVID-19 (SARS-CoV-2) Infection in Pregnancy: A Systematic Review. Gynecol. Obstet. Investig. 2020, 85, 295–306. [Google Scholar] [CrossRef] [PubMed]


| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Status | Pregnant * | 278 | 76.8% |
| Lactating | 84 | 23.2% | |
| Trimester * | 1st trimester (0–13 weeks) | 12 | 4.3% |
| 2nd trimester (14–28 weeks) | 28 | 10.1% | |
| 3rd trimester (>28 weeks) | 238 | 85.6% | |
| Age Group | 19–31 years-old | 183 | 51.5% |
| 32–44 years-old | 172 | 48.5% | |
| Education Level | Basic (Elementary) Education | 17 | 4.7% |
| Secondary (Vocational) Education | 139 | 38.5% | |
| Bachelor’s Degree | 52 | 14.4% | |
| Master’s Degree or Higher | 153 | 42.4% | |
| Employment Status | Employed | 329 | 91.1% |
| Unemployed | 32 | 8.9% | |
| Healthcare Profession | Yes | 68 | 20.7% |
| No | 260 | 79.3% | |
| Region | South Moravian | 292 | 80.9% |
| Vysočina | 10 | 2.8% | |
| Olomouc | 9 | 2.5% | |
| Pardubice | 9 | 2.5% | |
| Moravian-Silesian | 8 | 2.2% | |
| Central Bohemian | 7 | 1.9% | |
| Zlín | 7 | 1.9% | |
| Other | 19 | 5.3% | |
| Household Composition | <4 years-old | 178 | 49.9% |
| 4–17 years-old | 82 | 23% | |
| 18–65 years-old | 349 | 97.2% | |
| >65 years-old | 28 | 7.8% |
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Previous Pregnancies | One | 117 | 32.6% |
| Two | 59 | 16.4% | |
| Three | 33 | 9.2% | |
| Four | 6 | 1.7% | |
| None | 144 | 40.1% | |
| Previous Live Births | One | 119 | 57.2% |
| Two | 49 | 23.6% | |
| Three | 8 | 3.8% | |
| Four | 1 | 0.5% | |
| None | 31 | 14.9% | |
| Previous Pregnancies Complications | Yes | 72 | 34.3% |
| No | 138 | 65.7% | |
| Previous High-Risk Pregnancy | Yes | 69 | 19.1% |
| No | 293 | 80.9% | |
| Chronic Disease | Allergy | 77 | 21.3% |
| Anaemia | 14 | 3.9% | |
| Asthma | 26 | 7.2% | |
| Cancer | 0 | 0% | |
| Chronic Hypertension | 3 | 0.8% | |
| Depression | 3 | 0.8% | |
| Diabetes Mellitus | 7 | 1.9% | |
| Gastrointestinal Disease | 9 | 2.5% | |
| Obesity | 12 | 3.3% | |
| Skin-related Disorder | 23 | 6.4% | |
| Thyroid Disease | 46 | 12.7% | |
| Total | 164 | 45.3% | |
| Vaccine During Pregnancy | Yes | 10 | 2.8% |
| No | 352 | 97.2% |
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| COVID-19 Infection | Yes | 78 | 21.5% |
| No | 284 | 78.5% | |
| Infection During Pregnancy | Yes | 29 | 37.2% |
| No | 49 | 62.8% | |
| Clinical Course | Mild | 29 | 37.2% |
| Moderate | 49 | 62.8% |
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Intention | Immediate Acceptance | 13 | 3.6% |
| Delayed Acceptance | 241 | 66.6% | |
| Rejection | 108 | 29.8% | |
| Position from Physician’s Recommendation | Acceptance | 48 | 13.3% |
| Hesitancy | 89 | 24.6% | |
| Rejection | 225 | 62.2% |
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Preferred Vaccine Type | mRNA-based Vaccine | 212 | 58.6% |
| Viral Vector-based Vaccine | 24 | 6.6% | |
| Inactivated Virus Vaccine | 12 | 3.3% | |
| None | 114 | 31.5% | |
| First Priority | Safe for the child | 209 | 61.5% |
| Safe for the mother | 63 | 18.5% | |
| Effective for the child | 44 | 12.9% | |
| Effective for the mother | 24 | 7.1% | |
| Second Priority | Safe for the child | 61 | 18.5% |
| Safe for the mother | 155 | 47% | |
| Effective for the child | 61 | 18.5% | |
| Effective for the mother | 53 | 16.1% | |
| Third Priority | Safe for the child | 36 | 10.9% |
| Safe for the mother | 68 | 20.6% | |
| Effective for the child | 116 | 35.2% | |
| Effective for the mother | 110 | 33.3% | |
| Fourth Priority | Safe for the child | 27 | 8.2% |
| Safe for the mother | 50 | 15.1% | |
| Effective for the child | 137 | 41.4% | |
| Effective for the mother | 117 | 35.3% |
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| [Media] Do reports you hear/read in the media/on social media make you re-consider the choice to take the COVID-19 vaccine? | No = 0 | 235 | 65.3% |
| Not Sure = 1 | 95 | 26.4% | |
| Yes = 2 | 30 | 8.3% | |
| [Government] Do you trust that your government is making decisions in your best interest concerning what vaccines are provided (e.g., your government purchases the highest quality vaccines available)? | No = 0 | 212 | 58.7% |
| Not Sure = 1 | 118 | 32.7% | |
| Yes = 2 | 31 | 8.6% | |
| [Industry] Do you trust pharmaceutical companies to provide credible data on COVID-19 vaccine safety and effectiveness? | No = 0 | 156 | 43.2% |
| Not Sure = 1 | 151 | 41.8% | |
| Yes = 2 | 54 | 15% | |
| [Health Professional] Do you trust your health care provider to tell you about the risks and benefits of vaccines honestly? | No = 0 | 97 | 26.9% |
| Not Sure = 1 | 174 | 48.3% | |
| Yes = 2 | 89 | 24.7% | |
| [Partner] My decision whether to get vaccinated or not is driven by my husband/partner? | No = 0 | 300 | 83.1% |
| Not Sure = 1 | 25 | 6.9% | |
| Yes = 2 | 36 | 10% | |
| [Risk-benefit Ratio] Do you think that the benefits of COVID-19 vaccines outweigh their reported side effects/adverse reactions? | No = 0 | 79 | 21.9% |
| Not Sure = 1 | 182 | 50.6% | |
| Yes = 2 | 99 | 27.5% | |
| [Perceived Knowledge] Do you feel you have enough information about COVID-19 vaccines and their safety? | No = 0 | 127 | 35.6% |
| Not Sure = 1 | 114 | 31.9% | |
| Yes = 2 | 116 | 32.5% | |
| [Safety] Do you think that there is enough evidence about COVID-19 vaccine safety during pregnancy? | No = 0 | 294 | 82.4% |
| Not Sure = 1 | 61 | 17.1% | |
| Yes = 2 | 2 | 0.6% |
| Variable | Outcome | Lactating Women (n = 84) | Pregnant Women (n = 278) | ||||
|---|---|---|---|---|---|---|---|
| Acceptance (n = 41) | Rejection (n = 43) | Sig. | Acceptance (n = 213) | Rejection (n = 65) | Sig. | ||
| Trimester | 0–13 weeks | N/A | N/A | N/A | 5 (41.7%) | 7 (58.3%) | 0.008 |
| 14–28 weeks | N/A | N/A | N/A | 16 (57.1%) | 12 (42.9%) | 0.010 | |
| >28 weeks | N/A | N/A | N/A | 192 (80.7%) | 46 (19.3%) | <0.001 | |
| Age | µ ± σ | 31.0 ± 4.7 | 32.8 ± 4.8 | 0.095 | 31.6 ± 4.3 | 30.4 ± 5.0 | 0.046 |
| Education Level | Basic | 2 (66.7%) | 1 (33.3%) | 0.611 | 6 (42.9%) | 8 (57.1%) | 0.005 |
| Secondary | 14 (45.2%) | 17 (54.8%) | 0.609 | 81 (75%) | 27 (25%) | 0.611 | |
| Bachelor’s | 8 (61.5%) | 5 (38.5%) | 0.318 | 27 (69.2%) | 12 (30.8%) | 0.240 | |
| Masters’/Higher | 17 (45.9%) | 20 (54.1%) | 0.641 | 99 (85.3%) | 17 (14.7%) | 0.004 | |
| Employment Status | Yes | 36 (46.8%) | 41 (53.2%) | 0.259 | 200 (79.4%) | 52 (20.6%) | 0.002 |
| No | 5 (71.4%) | 2 (28.6%) | 13 (52%) | 12 (48%) | |||
| Healthcare Professional | Yes | 13 (72.2%) | 5 (27.8%) | 0.016 | 37 (74%) | 13 (26%) | 0.295 |
| No | 23 (39.7%) | 35 (60.3%) | 163 (80.7%) | 39 (19.3%) | |||
| Household | <4 years-old | 39 (50.6%) | 38 (49.4%) | 1.000 | 74 (73.3%) | 27 (26.7%) | 0.250 |
| 4–17 years-old | 11 (39.3%) | 17 (60.7%) | 0.186 | 33 (61.1%) | 21 (38.9%) | 0.003 | |
| 18–65 years-old | 39 (48.8%) | 41 (51.2%) | 0.494 | 206 (76.6%) | 63 (23.4%) | 1.000 | |
| >65 years-old | 5 (71.4%) | 2 (28.6%) | 0.432 | 14 (66.7%) | 7 (33.3%) | 0.282 | |
| Previous Pregnancies | Yes | 25 (44.6%) | 31 (55.4%) | 0.212 | 122 (76.7%) | 37 (23.3%) | 0.970 |
| No | 16 (59.3%) | 11 (40.7%) | 90 (76.9%) | 27 (23.1%) | |||
| Previous Live Births | Yes | 19 (41.3%) | 27 (58.7%) | 0.162 | 97 (74%) | 34 (26%) | 0.043 |
| No | 4 (80%) | 2 (20%) | 24 (92.3%) | 2 (7.7%) | |||
| Pregnancies Complications | Yes | 9 (50%) | 9 (50%) | 0.621 | 46 (85.2%) | 8 (14.8%) | 0.080 |
| No | 15 (42.9%) | 20 (57.1%) | 75 (72.8%) | 28 (27.2%) | |||
| High-Risk Pregnancies | Yes | 10 (55.6%) | 8 (44.4%) | 0.518 | 40 (78.4%) | 11 (21.6%) | 0.735 |
| No | 31 (47%) | 35 (53%) | 173 (76.2%) | 54 (23.8%) | |||
| Chronic Illness | Yes | 17 (44.7%) | 21 (55.3%) | 0.497 | 97 (77%) | 29 (23%) | 0.896 |
| No | 24 (52.2%) | 22 (47.8%) | 116 (76.3%) | 36 (23.7%) | |||
| COVID-19 | Yes | 7 (50%) | 7 (50%) | 0.922 | 50 (78.1%) | 14 (21.9%) | 0.746 |
| Infection | No | 34 (48.6%) | 36 (51.4%) | 163 (76.2%) | 51 (23.8%) | ||
| During | Yes | 3 (75%) | 1 (25%) | 0.559 | 22 (88%) | 3 (12%) | 0.126 |
| Pregnancy | No | 4 (40%) | 6 (60%) | 28 (71.8%) | 11 (28.2%) | ||
| Clinical | Mild | 3 (60%) | 2 (40%) | 1.000 | 16 (66.7%) | 8 (33.3%) | 0.086 |
| Course | Moderate | 4 (44.4%) | 5 (55.6%) | 34 (85%) | 6 (15%) | ||
| Psychosocial Predictors | Media | 0.7 ± 0.9 | 0.2 ± 0.5 | 0.001 | 0.5 ± 0.6 | 0.2 ± 0.4 | <0.001 |
| Government | 0.6 ± 0.8 | 0.1 ± 0.5 | 0.002 | 0.7 ± 0.6 | 0.2 ± 0.5 | <0.001 | |
| Industry | 0.9 ± 0.8 | 0.1 ± 0.4 | <0.001 | 1.0 ± 0.6 | 0.2 ± 0.4 | <0.001 | |
| Health Professional | 1.2 ± 0.8 | 0.4 ± 0.6 | <0.001 | 1.1 ± 0.6 | 0.6 ± 0.7 | <0.001 | |
| Partner | 0.3 ± 0.7 | 0.1 ± 0.4 | 0.040 | 0.3 ± 0.7 | 0.1 ± 0.4 | 0028 | |
| Risk/Benefit Ratio | 1.3 ± 0.7 | 0.6 ± 0.8 | <0.001 | 1.2 ± 0.6 | 0.7 ± 0.6 | <0.001 | |
| Knowledge | 1.0 ± 0.9 | 1.1 ± 0.9 | 0.562 | 0.9 ± 0.8 | 1.1 ± 0.9 | 0.153 | |
| Safety | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.608 | 0.3 ± 0.5 | 0.1 ± 0.2 | <0.001 | |
| Variable | Outcome | Lactating Women (n = 84) | Pregnant Women (n = 278) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rejection (n = 67) | Hesitancy (n = 10) | Acceptance (n = 7) | Sig. | Rejection (n = 158) | Hesitancy (n = 79) | Acceptance (n = 41) | Sig. | ||
| Trimester | 0–13 weeks | N/A | N/A | N/A | N/A | 10 (83.3%) | 1 (8.3%) | 1 (8.3%) | 0.219 |
| 14–28 weeks | N/A | N/A | N/A | N/A | 22 (78.6%) | 4 (14.3%) | 2 (7.1%) | 0.060 | |
| >28 weeks | N/A | N/A | N/A | N/A | 126 (52.9%) | 74 (31.1%) | 38 (16%) | 0.006 | |
| Age | µ ± σ | 32.2 ± 4.9 | 30.3 ± 5.1 | 31.3 ± 2.9 | 0.490 | 30.9 ± 4.6 | 32.0 ± 4.4 | 32.1 ± 4.0 | 0.100 |
| Education Level | Basic | 1 (33.3%) | 2 (66.7%) | 0 (0%) | 0.054 | 10 (71.4%) | 1 (7.1%) | 3 (21.4%) | 0.126 |
| Secondary | 26 (83.9%) | 4 (12.9%) | 1 (3.2%) | 0.506 | 70 (64.8%) | 28 (25.9%) | 10 (9.3%) | 0.052 | |
| Bachelor’s | 9 (69.2%) | 1 (7.7%) | 3 (23.1%) | 0.113 | 26 (66.7%) | 6 (15.4%) | 7 (17.9%) | 0.150 | |
| Masters’/Higher | 31 (83.8%) | 3 (8.1%) | 3 (8.1%) | 0.615 | 51 (44%) | 44 (37.9%) | 21 (18.1%) | 0.001 | |
| Employment Status | Yes | 63 (81.8%) | 7 (9.1%) | 7 (9.1%) | 0.055 | 141 (56%) | 74 (29.4%) | 37 (14.7%) | 0.616 |
| No | 4 (57.1%) | 3 (42.9%) | 0 (0%) | 16 (64%) | 5 (20%) | 4 (16%) | |||
| Healthcare Professional | Yes | 13 (72.2%) | 2 (11.1%) | 3 (16.7%) | 0.459 | 33 (66%) | 12 (24%) | 5 (10%) | 0.263 |
| No | 49 (84.5%) | 5 (8.6%) | 4 (6.9%) | 108 (53.5%) | 62 (30.7%) | 32 (15.8%) | |||
| Household | <4 years-old | 61 (79.2%) | 9 (11.7%) | 7 (9.1%) | 0.697 | 62 (61.4%) | 26 (25.7%) | 13 (12.9%) | 0.437 |
| 4–17 years-old | 25 (89.3%) | 3 (10.7%) | 0 (0%) | 0.107 | 36 (66.7%) | 10 (18.5%) | 8 (14.8%) | 0.164 | |
| 18–65 years-old | 63 (78.8%) | 10 (12.5%) | 7 (8.8%) | 1.000 | 154 (57.2%) | 75 (27.9%) | 40 (14.9%) | 0.579 | |
| >65 years-old | 4 (57.1%) | 2 (28.6%) | 1 (14.3%) | 0.198 | 13 (61.9%) | 5 (23.8%) | 3 (14.3%) | 0.949 | |
| Previous Pregnancies | Yes | 47 (83.9%) | 6 (10.7%) | 3 (5.4%) | 0.242 | 91 (57.2%) | 46 (28.9%) | 22 (13.8%) | 0.848 |
| No | 19 (70.4%) | 4 (14.8%) | 4 (14.8%) | 66 (56.4%) | 32 (27.4%) | 19 (16.2%) | |||
| Previous Live Births | Yes | 40 (87%) | 5 (10.9%) | 1 (2.2%) | 0.009 | 78 (59.5%) | 36 (27.5%) | 17 (13%) | 0.398 |
| No | 2 (40%) | 1 (20%) | 2 (40%) | 12 (46.2%) | 10 (38.5%) | 4 (15.4%) | |||
| Pregnancy Complications | Yes | 13 (72.2%) | 2 (11.1%) | 3 (16.7%) | 0.048 | 28 (51.9%) | 18 (33.3%) | 8 (14.8%) | 0.602 |
| No | 31 (88.6%) | 4 (11.4%) | 0 (0%) | 62 (60.2%) | 28 (27.2%) | 13 (12.6%) | |||
| High-Risk Pregnancies | Yes | 13 (72.2%) | 2 (11.1%) | 3 (16.7%) | 0.379 | 28 (54.9%) | 11 (21.6%) | 12 (23.5%) | 0.114 |
| No | 54 (81.8%) | 8 (12.1%) | 4 (6.1%) | 130 (57.3%) | 68 (30%) | 29 (12.8%) | |||
| Chronic Illness | Yes | 31 (81.6%) | 1 (2.6%) | 6 (15.8%) | 0.007 | 70 (55.6%) | 36 (28.6%) | 20 (15.9%) | 0.875 |
| No | 36 (78.3%) | 9 (19.6%) | 1 (2.2%) | 88 (57.9%) | 43 (28.3%) | 21 (13.8%) | |||
| COVID-19 | Yes | 12 (85.7%) | 1 (7.1%) | 1 (7.1%) | 1.000 | 33 (51.6%) | 20 (31.3%) | 11 (17.2%) | 0.597 |
| Infection | No | 55 (78.6%) | 9 (12.9%) | 6 (8.6%) | 125 (58.4%) | 59 (27.6%) | 30 (14%) | ||
| During | Yes | 3 (75%) | 0 (0%) | 1 (25%) | 0.505 | 12 (48%) | 6 (24%) | 7 (28%) | 0.166 |
| Pregnancy | No | 9 (90%) | 1 (10%) | 0 (0%) | 21 (53.8%) | 14 (35.9%) | 4 (10.3%) | ||
| Clinical | Mild | 5 (100%) | 0 (0%) | 0 (0%) | 1.000 | 17 (70.8%) | 4 (16.7%) | 3 (12.5%) | 0.057 |
| Course | Moderate | 7 (77.8%) | 1 (11.1%) | 1 (11.1%) | 16 (40%) | 16 (40%) | 8 (20%) | ||
| Psychosocial Predictors | Media | 0.4 ± 0.7 | 0.7 ± 0.9 | 0.6 ± 1.0 | 0.454 | 0.3 ± 0.6 | 0.6 ± 0.6 | 0.4 ± 0.6 | 0.002 |
| Government | 0.3 ± 0.6 | 0.4 ± 0.7 | 1.3 ± 1.0 | 0.003 | 0.4 ± 0.6 | 0.7 ± 0.6 | 0.9 ± 0.6 | <0.001 | |
| Industry | 0.3 ± 0.7 | 0.8 ± 0.8 | 1.4 ± 0.8 | <0.001 | 0.5 ± 0.7 | 1.1 ± 0.6 | 1.2 ± 0.5 | <0.001 | |
| H. Professional | 0.6 ± 0.8 | 1.0 ± 0.7 | 2.0 ± 0.0 | <0.001 | 0.9 ± 0.7 | 1.3 ± 0.6 | 1.3 ± 0.5 | <0.001 | |
| Partner | 0.1 ± 0.5 | 0.8 ± 0.9 | 0.3 ± 0.8 | <0.001 | 0.2 ± 0.6 | 0.3 ± 0.6 | 0.5 ± 0.8 | 0.020 | |
| Risk/Benefit Ratio | 0.8 ± 0.8 | 1.2 ± 0.8 | 2.0 ± 0.0 | 0.001 | 1.0 ± 0.7 | 1.2 ± 0.6 | 1.3 ± 0.5 | 0.003 | |
| Knowledge | 1.0 ± 0.9 | 0.8 ± 0.9 | 1.6 ± 0.8 | 0.218 | 1.0 ± 0.8 | 0.8 ± 08 | 1.0 ± 0.7 | 0.085 | |
| Safety | 0.0 ± 0.2 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.068 | 0.1 ± 0.3 | 0.3 ± 0.5 | 0.6 ± 0.6 | <0.001 | |
| Predictor | B (SE) | Wald | AOR (CI 95%) | Sig. |
|---|---|---|---|---|
| Second Trimester (vs. First Trimester) | 0.163 (0.945) | 0.030 | 1.177 (0.185–7.504) | 0.863 |
| Third Trimester (vs. First Trimester) | 1.872 (0.859) | 4.746 | 6.501 (1.207–35.030) | 0.029 |
| Secondary Education (vs. Basic Education) | 1.299 (0.791) | 2.697 | 3.665 (0.778–17.274) | 0.101 |
| Bachelor’s (vs. Basic Education) | 1.026 (0.936) | 1.201 | 2.790 (0.445–17.475) | 0.273 |
| Master’s or Higher (vs. Basic Education) | 1.790 (0.857) | 4.360 | 5.992 (1.116–32.164) | 0.037 |
| Employed (vs. Unemployed) | 0.893 (0.665) | 1.804 | 2.442 (0.664–8.987) | 0.179 |
| No Previous Live Births (vs. Previous Live Births) | 1.107 (0.843) | 1.724 | 3.025 (0.580–15.795) | 0.189 |
| Predictor | B (SE) | Wald | AOR (CI 95%) | Sig. |
|---|---|---|---|---|
| Media: Yes (vs. No) | 1.916 (1.183) | 2.621 | 6.793 (0.668–69.087) | 0.105 |
| Government: Yes (vs. No) | 0.888 (0.904) | 0.966 | 2.431 (0.414–14.289) | 0.326 |
| Industry: Yes (vs. No) | 2.747 (1.115) | 6.070 | 15.590 (1.754–138.599) | 0.014 |
| Health Professional: Yes (vs. No) | 1.471 (0.626) | 5.527 | 4.355 (1.277–14.847) | 0.019 |
| Partner: Yes (vs. No) | 1.692 (1.153) | 2.156 | 5.433 (0.567–52.009) | 0.142 |
| Risk/Benefit Ratio: Yes (vs. No) | 2.742 (0.878) | 9.745 | 15.518 (2.774–86.795) | 0.002 |
| Knowledge: Yes (vs. No) | 0.093 (0.511) | 0.033 | 0.911 (0.335–2.480) | 0.855 |
| Safety: Yes (vs. No) | 19.746 (40,192.969) | 0.000 | 376 × 106 (0.000–∞) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riad, A.; Jouzová, A.; Üstün, B.; Lagová, E.; Hruban, L.; Janků, P.; Pokorná, A.; Klugarová, J.; Koščík, M.; Klugar, M. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 13373. https://doi.org/10.3390/ijerph182413373
Riad A, Jouzová A, Üstün B, Lagová E, Hruban L, Janků P, Pokorná A, Klugarová J, Koščík M, Klugar M. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2021; 18(24):13373. https://doi.org/10.3390/ijerph182413373
Chicago/Turabian StyleRiad, Abanoub, Anna Jouzová, Batuhan Üstün, Eliška Lagová, Lukáš Hruban, Petr Janků, Andrea Pokorná, Jitka Klugarová, Michal Koščík, and Miloslav Klugar. 2021. "COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study" International Journal of Environmental Research and Public Health 18, no. 24: 13373. https://doi.org/10.3390/ijerph182413373
APA StyleRiad, A., Jouzová, A., Üstün, B., Lagová, E., Hruban, L., Janků, P., Pokorná, A., Klugarová, J., Koščík, M., & Klugar, M. (2021). COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. International Journal of Environmental Research and Public Health, 18(24), 13373. https://doi.org/10.3390/ijerph182413373








