Endothelin-1 and LOX-1 as Markers of Endothelial Dysfunction in Obstructive Sleep Apnea Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Control Subjects
2.2. Polysomnography
2.3. LOX-1 and ET-1
2.4. Statistical Analysis
2.5. Approval of Commission of Bioethics
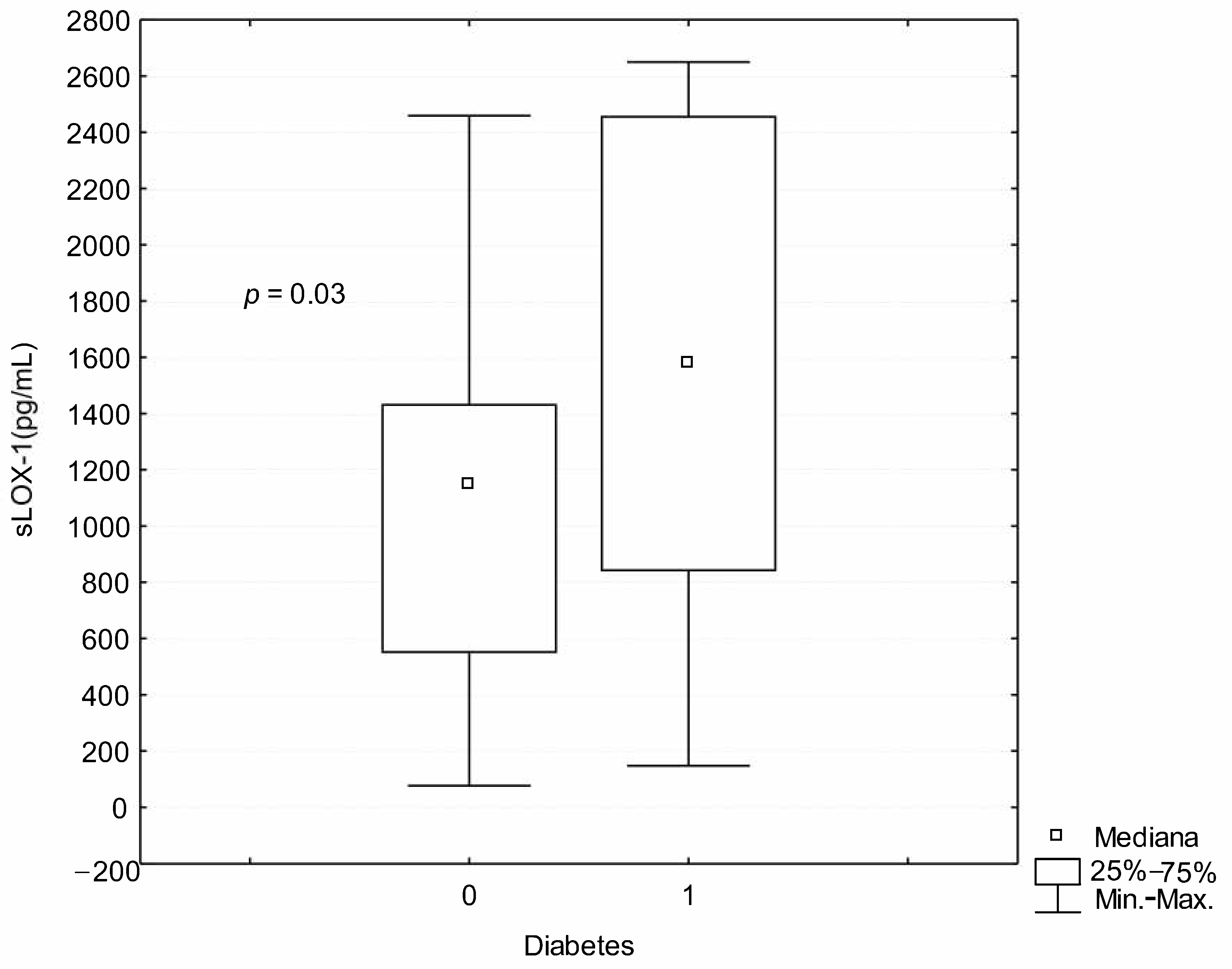
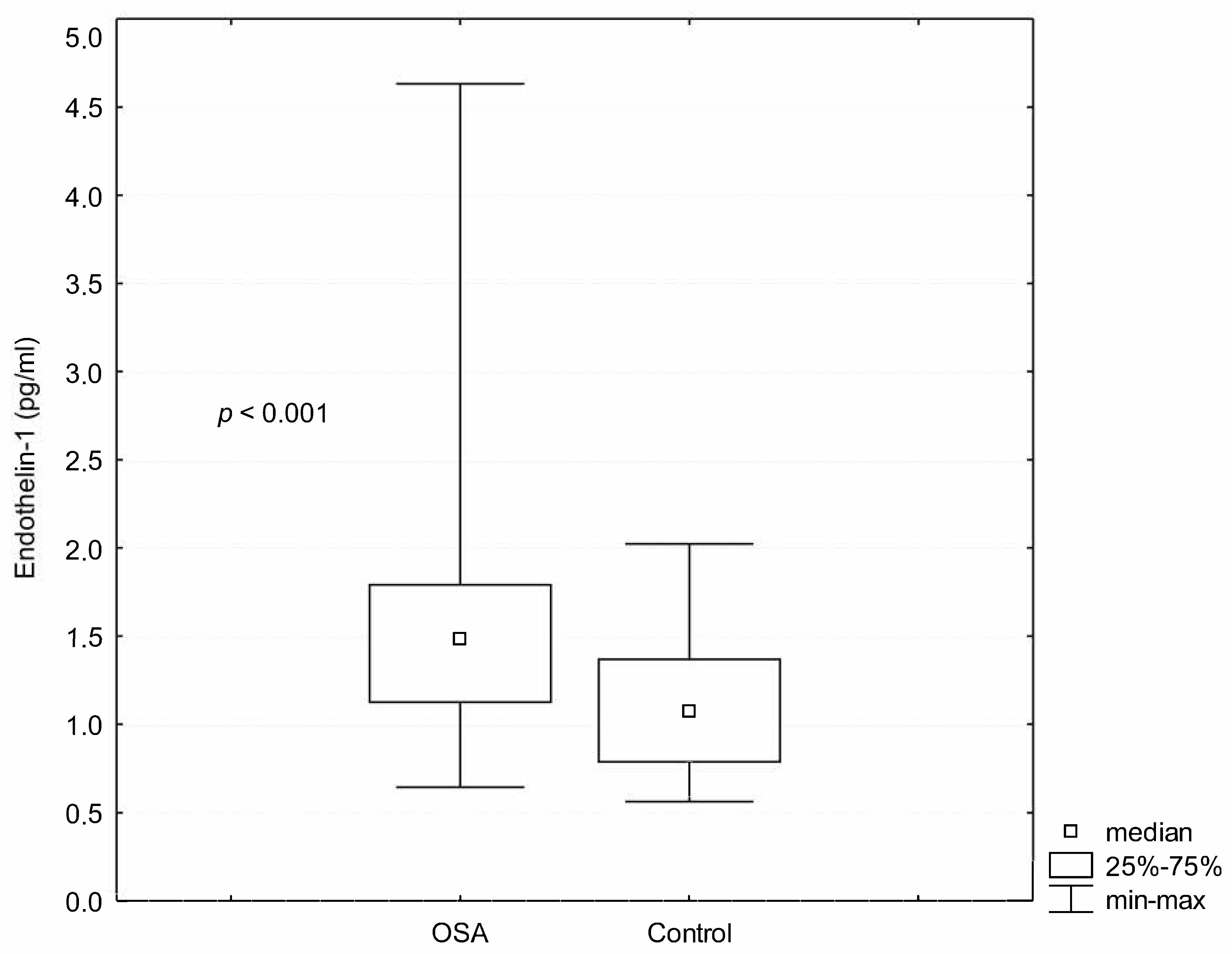
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Backer, W. Obstructive sleep apnea/hypopnea syndrome. Panminerva Med. 2013, 55, 191–195. [Google Scholar]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S. INCOSACT Initiative (International Collaboartion of Sleep Apnea Cardiovascular Trialists). Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef]
- Solarz, D.E.; Mullington, J.M.; Meier-Ewert, H.K. Sleep, Inflammation and cardiovascular disease. Front. Biosci. (Elite Ed.) 2012, 4, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea; A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular complications of sleep apnea: Role of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 985258. [Google Scholar] [CrossRef]
- Khalyfa, A.; Qiao, Z.; Gileles-Hillel, A.; Khalyfa, A.A.; Akbarpour, M.; Popko, B.; Gozal, D. Activation of the Integrated Stress Response and Metabolic Dysfunction in a Murine Model of Sleep Apnea. Am. J. Respir. Cell Mol. Biol. 2017, 57, 477–486. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. (Elite Ed.) 2012, 4, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarra, T.; Del Turco, S.; Berti, S.; Basta, G. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: Cardiovascular implications. J. Atheroscler. Thromb. 2010, 17, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirillo, A.; Catapano, A.L. Soluble lectin-like oxidized low density lipoprotein receptor-1 as a biochemical marker for atherosclerosis -related diseases. Dis. Markers 2013, 35, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Takanabe-Mori, R.; Ono, K.; Wada, H.; Takaya, T.; Ura, S.; Yamakage, H.; Satoh-Asahara, N.; Shimatsu, A.; Takahashi, Y.; Fujita, M.; et al. Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Plays an Important Role in Vascular Inflammation in Current Smokers. J. Atheroscler. Thromb. 2013, 20, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Civelek, S.; Kutnu, M.; Uzun, H.; Erdenen, F.; Altunoglu, E.; Andican, G.; Seven, A.; Sahin, A.O.; Burcak, G. Soluble Lectin-Like Oxidized LDL Receptor 1 as a Possible Mediator of Endothelial Dysfunction in Patients with Metabolic Syndrome. J. Clin. Lab. Anal. 2015, 29, 84–190. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Karathanasis, S.K.; Ding, Z.; Arulandu, A.; Varughese, K.I.; Mehta, J.L. LOX-1 in Atherosclerosis and Myocardial Ischemia: Biology, Genetics, and Modulation. J. Am. Coll. Cardiol. 2017, 69, 2759–2768. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [Green Version]
- Houde, M.; Desbiens, L.; D’Orleans-Juste, P. Endothelin-1: Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar] [CrossRef]
- Bousette, N.; Giaid, A.; Can, J. Endothelin-1 in atherosclerosis and other vasculopathies. Physiol. Pharmacol. 2003, 81, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.; Pugh, D.; Dhaun, N. Developments in the Role of Endothelin-1 in Atherosclerosis: A Potential Therapeutic Target? Am. J. Hypertens. 2019, 32, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, H.; Duerrschmidt, N.; Niemann, B.; Galle, J.; Sawamura, T.; Holtz, J. Induction of the oxLDL receptor LOX-1 by endothelin-1 in human endothelial cells. Biochem. Biophys. Res. Commun. 2001, 284, 961–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. American Academy of Sleep Medicine: Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Xia, Y.; Li, X.; Qian, Y.; Zou, J.; Fang, F.; Yi, H.; Wu, H.; Guan, J.; Yin, S. Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J. Clin. Sleep Med. 2020, 16, 475–482. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Shiu, S.W.M.; Wong, Y.; Leng, L.; Bucala, R. Soluble lectin-like oxidized low density lipoprotein receptor-1 in type 2 diabetes mellitus. J. Lipid Res. 2008, 49, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances endothelial LOX-1 expression: Role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes 2003, 52, 1843–1850. [Google Scholar] [CrossRef] [Green Version]
- Shiu, S.W.; Tan, K.C.; Wong, Y.; Leng, L.; Bucala, R. Glycoxidized LDL increases lectin-like oxidized low density lipoprotein receptor-1 in diabetes mellitus. Atherosclerosis 2009, 203, 522–527. [Google Scholar] [CrossRef]
- Hayashida, K.; Kume, N.; Murase, T. Serum Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Levels Are Elevated in Acute Coronary Syndrome: A Novel Marker for Early Diagnosis. ACC Curr. J. Rev. 2005, 14, 3. [Google Scholar] [CrossRef]
- Yavuzer, S.; Yavuzer, H.; Cengiz, M.; Erman, H.; Altıparmak, M.R.; Korkmazer, B.; Balci, H.; Simsek, G.; Yaldıran, A.L.; Karter, Y.; et al. Endothelial damage in white coat hypertension: Role of lectin-like oxidized low-density lipoprotein. J. Hum. Hypertens. 2015, 29, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Xu, Y.W.; Li, S.M.; Guo, J.J.; Sun, J.M.; Hong, J.C.; Chen, L.L. Baseline serum sLOX-1 concentrations are associated with 2-year major adverse cardiovascular and cerebrovascular events in patients after percutaneous coronary intervention. Dis. Markers 2019, 2019, 4925767. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Suehiro, A.; Nakanishi, M.; Sawamura, T.; Wakabayashi, I. Associations of atherosclerotic risk factors with oxidized low-density lipoprotein evaluated by LOX-1 ligand activity in healthy men. Clin. Chim. Acta 2011, 412, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Jia, K.; Huo, Z.; Huo, Q.; Liu, Z.; Li, Y.; Han, X.; Wang, R. Clinical analysis of lectin-like oxidized low-density lipoprotein receptor-1 in patients with in-restent restenosis after percutaneous coronary intervension. Medicine (Baltimore) 2018, 97, e0366. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, Y.; Zhang, Z.; Vallurupalli, S.; Mehta, J.L. Xanthine Oxidase induces Foam Cell Formation through LOX-1 and NLRP3 Activation. Cardiovasc. Drugs Ther. 2017, 31, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Min, M.; Sun, C.; Cheng, C.; Zhang, Y.; Liang, M.; Rizeq, F.K.; Sun, Y. A meta-analysis of the association between gout, serum uric acid levels, and obstructive sleep apnea. Sleep Breath 2019, 23, 1047–1057. [Google Scholar] [CrossRef]
- Kosacka, M.; Brzecka, A.; Piesiak, P.; Korzeniewska, A.; Jankowska, R. Soluble ligand CD40 and uric acid as markers of atheromatosis in patients with obstructive sleep apnea. Adv. Exp. Med. Biol. 2015, 839, 55–60. [Google Scholar] [CrossRef]
- Phillips, B.G.; Narkiewicz, K.; Pesek, C.A.; Haynes, W.G.; Dyken, M.E.; Somers, V.K. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J. Hypertens. 1999, 17, 61–66. [Google Scholar] [CrossRef]
- Karkoulias, K.; Lykouras, D.; Sampsonas, F.; Drakatos, P.; Canova, S.; Tsoukalas, G.; Spiropoulos, K. The role of Endothelin-1 in obstructive sleep apnea syndrome and pulmonary arterial hypertension: Pathogenesis and Endothelin-1 antagonists. Curr. Med. Chem. 2010, 17, 1059–1066. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Y.; Zou, F.; Xu, T.; Pan, P.; Hu, C.; Su, X. Serum adropin level is associated with endothelial dysfunction in patients with obstructive sleep apnea and hypopnea syndrome. Sleep Breath 2020, 1–7. [Google Scholar] [CrossRef]
- Grimpen, F.; Kanne, P.; Schulz, E.; Hagenah, G.; Hasenfuss, G.; Andreas, S. Endothelin-1 plasma levels are not elevated in patients with obstructive sleep apnoea. Eur. Respir. J. 2000, 15, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjørup, P.H.; Sadauskiene, L.; Wessels, J.; Nyvad, O.; Strunge, B.; Pedersen, E.B. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea relation to blood pressure and severity of disease. Am. J. Hypertens. 2007, 20, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Chen, Q.; Huang, J.; Chen, L.; Lin, T.; Lin, Q. Effect of continuous positive airway pressure on endothelin-1 in patients with obstructive sleep apnea: A meta -analysis. Eur. Arch. Otorhinolaryngol. 2019, 276, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.; Pathak, A.; Grassi, G.; van de Borne, P. Endothelin contributes to the blood pressure rise triggered by hypoxia in severe obstructive sleep apnea. J. Hypertens. 2017, 35, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sinning, D.; Landmesser, U. Low-density Lipoprotein-Cholesterol Lowering Strategies for Prevention of Atherosclerotic Cardiovascular Disease: Focus on siRNA Treatment Targeting PCSK9 (Inclisiran). Curr. Cardiol. Rep. 2020, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Kocyigit, I.; Lindholm, B. The endothelin system as target for therapeutic interventions in cardiovascular and renal disease. Clin. Chim. Acta 2020, 506, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, L.I.; Semenova, N.V.; Osipova, E.V.; Madaeva, I.M. Lipid status and oxidative stress in menopausal women with obstructive apnea syndrome. Ter. Arkh. 2019, 91, 48–53. [Google Scholar] [CrossRef]
- Xu, C.Y.; Li, D.J.; Wu, C.L.; Lou, H.J.; Jiang, H.W.; Ding, G.Q. Serum sLOX-1 Levels Are Correlated with the Presence and Severity of Obstructive Sleep Apnea. Genet. Test. Mol. Biomarkers 2015, 19, 272–276. [Google Scholar] [CrossRef] [Green Version]
| Parameters | OSA Patients | Control Group | p |
|---|---|---|---|
| Age (years) | 55.58 ± 11.03 | 52.80 ± 14.19 | 0.55 |
| BMI (kg/m2) | 32.63 ± 7.05 | 29.78 ± 7.15 | 0.07 |
| LOX-1 (pg/mL) | 1127.50 ± 642.01 | 983.62 ± 615.18 | 0.435 |
| ET-1 (pg/mL) | 1.58 ± 0.65 | 1.09 ± 0.38 | <0.001 |
| AHI (/hour) | 28.20 ± 17.90 | 2.15 ± 1.82 | <0.00001 |
| DI (/hour) | 23.75 ± 19.12 | 2.81 ± 2.32 | <0.00001 |
| Mean SaO2 during sleep (%) | 91.37 ± 8.19 | 94.05 ± 2.07 | 0.006 |
| Minimum saturation (%) | 79.46 ± 8.83 | 86.05 ± 4.90 | 0.0006 |
| Glucose (mg/dL) | 95.96 ± 14.98 | 89.50 ± 8.56 | 0.037 |
| CRP (mg/L) | 4.02 ± 3.20 | 3.13 ± 2.96 | 0.437 |
| Urid acid (mg/dL) | 5.90 ± 1.50 | 4.90 ± 1.05 | 0.006 |
| Total cholesterol (mg/dL) | 203.23 ± 38.66 | 218.00 ± 52.87 | 0.197 |
| LDL cholesterol (mg/dL) | 122.21 ± 32.58 | 136.73 ± 45.09 | 0.210 |
| HDL cholesterol (mg/dL) | 50.49 ± 12.91 | 51.15 ± 14.81 | 0.377 |
| Triglycerides (mg/dL) | 156.36 ± 87.58 | 150.21 ± 73.74 | 0.942 |
| Parameters | LOX-1 (pg/mL) | ET-1 (pg/mL) | ||
|---|---|---|---|---|
| R Spearman | p | R Spearman | p | |
| Age (years) | 0.08 | 0.45 | 0.079 | 0.499 |
| BMI(kg/m2) | 0.10 | 0.38 | −0.032 | 0.785 |
| AHI (/hour) | 0.04 | 0.71 | −0.036 | 0.757 |
| DI(/hour) | 0.03 | 0.78 | −0.102 | 0.386 |
| Mean SaO2 during sleep (%) | −0.20 | 0.08 | −0.097 | 0.411 |
| Minimum SaO2 during sleep (%) | 0.02 | 0.82 | 0.076 | 0.514 |
| Glucose (mg/dL) | 0.23 | 0.04 | 0.063 | 0.596 |
| CRP (mg/L) | −0.05 | 0.63 | 0.028 | 0.809 |
| Urid acid (mg/dL) | 0.17 | 0.15 | −0.001 | 0.989 |
| Total cholesterol (mg/dL) | 0.08 | 0.47 | −0.203 | 0.083 |
| LDL cholesterol (mg/dL) | −0.02 | 0.85 | −0.263 | 0.027 |
| HDL cholesterol (mg/dL) | 0.009 | 0.93 | 0.163 | 0.167 |
| Triglicerides (mg/dL) | 0.18 | 0.10 | −0.098 | 0.411 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosacka, M.; Brzecka, A. Endothelin-1 and LOX-1 as Markers of Endothelial Dysfunction in Obstructive Sleep Apnea Patients. Int. J. Environ. Res. Public Health 2021, 18, 1319. https://doi.org/10.3390/ijerph18031319
Kosacka M, Brzecka A. Endothelin-1 and LOX-1 as Markers of Endothelial Dysfunction in Obstructive Sleep Apnea Patients. International Journal of Environmental Research and Public Health. 2021; 18(3):1319. https://doi.org/10.3390/ijerph18031319
Chicago/Turabian StyleKosacka, Monika, and Anna Brzecka. 2021. "Endothelin-1 and LOX-1 as Markers of Endothelial Dysfunction in Obstructive Sleep Apnea Patients" International Journal of Environmental Research and Public Health 18, no. 3: 1319. https://doi.org/10.3390/ijerph18031319






