Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
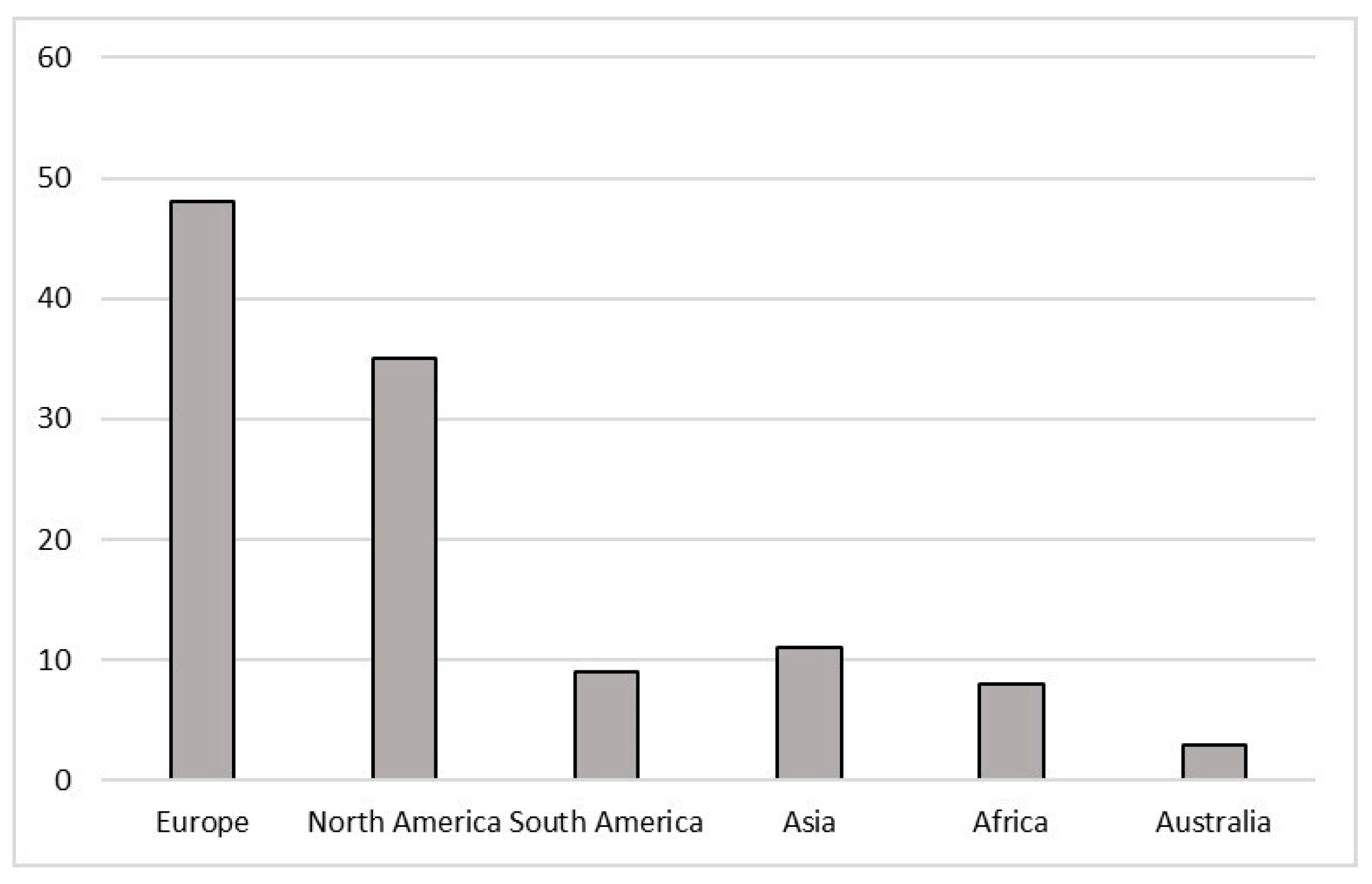
3.1. Where Studies Took Place
3.2. Methodological Approaches
3.3. Life Stages
3.4. Studied Compounds
3.5. Effect Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-Pollinator Interactions over 120 Years: Loss of Species, Co-Occurrence, and Function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef] [Green Version]
- de Groot, R.S.; Wilson, M.A.; Boumans, R.M.J. A typology for the classification, description and valuation of ecosystem functions, goods and services. Ecol. Econ. 2002, 41, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Formato, G.; Zilli, R.; Condoleo, R.; Marozzi, S.; Davis, I.; Smulders, F.J.M. Risk management in primary apicultural production. Part 2: A Hazard Analysis Critical Control Point approach to assuring the safety of unprocessed honey. Vet. Q. 2011, 31, 87–97. [Google Scholar] [CrossRef]
- Biesmeijer, J.C. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef] [Green Version]
- Jacques, A.; Laurent, M.; Ribiere-Chabert, M.; Saussac, M.; Bougeard, S.; Hendrikx, P.; Chauzat, M. Statistical analysis on the EPILOBEE dataset: Explanatory variables related to honeybee colony mortality in EU during a 2 year survey. EFSA Support. Publ. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Steinhauer, N.A.; Rennich, K.; Wilson, M.E.; Caron, D.M.; Lengerich, E.J.; Pettis, J.S.; Rose, R.; Skinner, J.A.; Tarpy, D.R.; Wilkes, J.T.; et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2014, 53, 1–18. [Google Scholar] [CrossRef]
- van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–9 and 2009–10. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Carnesecchi, E.; Svendsen, C.; Lasagni, S.; Grech, A.; Quignot, N.; Amzal, B.; Toma, C.; Tosi, S.; Rortais, A.; Cortinas-Abrahantes, J.; et al. Investigating combined toxicity of binary mixtures in bees: Meta-analysis of laboratory tests, modelling, mechanistic basis and implications for risk assessment. Environ. Int. 2019, 133, 105256. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.L.; Ngo, H.T.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. The Assessment Report on Pollinators, Pollination and Food Production: Summary for Policymakers; Secretariat for Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; ISBN 978-92-807-3568-0. [Google Scholar]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Towards an integrated environmental risk assessment of multiple stressors on bees: Review of research projects in Europe, knowledge gaps and recommendations. EFSA J. 2014, 12, 3594. [Google Scholar]
- Rortais, A.; Arnold, G.; Dorne, J.-L.; More, S.J.; Sperandio, G.; Streissl, F.; Szentes, C.; Verdonck, F. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total Environ. 2017, 587–588, 524–537. [Google Scholar] [CrossRef]
- Nazzi, F.; Pennacchio, F. Disentangling multiple interactions in the hive ecosystem. Trends Parasitol. 2014, 30, 556–561. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, G.; Alaux, C.; Le Conte, Y.; Odoux, J.-F.; Pioz, M.; Vaissière, B.E.; Belzunces, L.P.; Decourtye, A. Variations in the Availability of Pollen Resources Affect Honey Bee Health. PLoS ONE 2016, 11, e0162818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, Y.L.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Rev. Sci. Tech. Off. Int. Epizoot. 2008, 27, 499–510. [Google Scholar]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Nieh, J.C.; Tosi, S. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 2019, 237, 124408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.M.; Wright, G.A. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 2013, 216, 1799–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Brunet, J.-L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S.; vanEngelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Renzi, M.T.; Amichot, M.; Pauron, D.; Tchamitchian, S.; Brunet, J.-L.; Kretzschmar, A.; Maini, S.; Belzunces, L.P. Chronic toxicity and physiological changes induced in the honey bee by the exposure to fipronil and Bacillus thuringiensis spores alone or combined. Ecotoxicol. Environ. Saf. 2016, 127, 205–213. [Google Scholar] [CrossRef]
- Robinson, A.; Hesketh, H.; Lahive, E.; Horton, A.A.; Svendsen, C.; Rortais, A.; Dorne, J.L.; Baas, J.; Heard, M.S.; Spurgeon, D.J. Comparing bee species responses to chemical mixtures: Common response patterns? PLoS ONE 2017, 12, e0176289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.; Yang, Y.; Gao, J.; Zhao, D.; Ren, C.; Wang, S.; Zhao, S.; Zhong, Y. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology 2019, 28, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Impacts of Pesticides on Honey Bees. In Beekeeping and Bee Conservation-Advances in Research; Chambo, E.D., Ed.; InTech: Rijeca, Croatia, 2016; ISBN 978-953-51-2411-5. [Google Scholar]
- Johnson, R.M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Tosi, S.; Nieh, J.C. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto ®), on honeybees. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Caliani, I.; Campani, T.; Conti, B.; Cosci, F.; Bedini, S.; D’Agostino, A.; Ammendola, A.; Di Noi, A.; Gori, A.; Casini, S. Multi-biomarker approach and IBR index to evaluate the effects of different contaminants on the ecotoxicological status of Apis mellifera. Ecotoxicol. Environ. Saf. 2021, 208, 111486. [Google Scholar] [CrossRef] [PubMed]
- Lupi, D.; Tremolada, P.; Colombo, M.; Giacchini, R.; Benocci, R.; Parenti, P.; Parolini, M.; Zambon, G.; Vighi, M. Effects of Pesticides and Electromagnetic Fields on Honeybees: A Field Study Using Biomarkers. Int. J. Environ. Res. 2020, 14, 107–122. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; vanEngelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimets, R.; Bontšutšnaja, A.; Bartkevics, V.; Pugajeva, I.; Kaart, T.; Puusepp, L.; Pihlik, P.; Keres, I.; Viinalass, H.; Mänd, M.; et al. Pesticide residues in beehive matrices are dependent on collection time and matrix type but independent of proportion of foraged oilseed rape and agricultural land in foraging territory. Chemosphere 2020, 238, 124555. [Google Scholar] [CrossRef]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of Imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera: Survival and health of European honey bees. Insect Sci. 2017, 24, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, C.; Hill, M.; Bonetti, C.; Mitchell, G.C.; Sharma, B. The effects of iprodione fungicide on survival, behavior, and brood development of honeybees (Apis mellifera L.) after one foliar application during flowering on mustard: Effects of iprodione application on honeybees. Environ. Toxicol. Chem. 2018, 37, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Nasr, H.M.; Badawy, M.E.I. Toxic Effect and Biochemical Study of Chlorfluazuron, Oxymatrine, and Spinosad on Honey Bees (Apis mellifera). Arch. Environ. Contam. Toxicol. 2010, 58, 722–732. [Google Scholar] [CrossRef]
- Prado, A.; Pioz, M.; Vidau, C.; Requier, F.; Jury, M.; Crauser, D.; Brunet, J.-L.; Le Conte, Y.; Alaux, C. Exposure to pollen-bound pesticide mixtures induces longer-lived but less efficient honey bees. Sci. Total Environ. 2019, 650, 1250–1260. [Google Scholar] [CrossRef]
- Hladun, K.R.; Kaftanoglu, O.; Parker, D.R.; Tran, K.D.; Trumble, J.T. Effects of selenium on development, survival, and accumulation in the honeybee (Apis mellifera L.): Selenium’s impact on survival in honeybees. Environ. Toxicol. Chem. 2013, 32, 2584–2592. [Google Scholar] [CrossRef]
- Mixson, T.A.; Abramson, C.I.; Nolf, S.L.; Johnson, G.; Serrano, E.; Wells, H. Effect of GSM cellular phone radiation on the behavior of honey bees (Apis mellifera). Sci. Bee Cult. 2009, 1, 22–27. [Google Scholar]
- Badiou-Bénéteau, A.; Carvalho, S.M.; Brunet, J.-L.; Carvalho, G.A.; Buleté, A.; Giroud, B.; Belzunces, L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 2012, 82, 22–31. [Google Scholar] [CrossRef]
- Badiou-Bénéteau, A.; Benneveau, A.; Géret, F.; Delatte, H.; Becker, N.; Brunet, J.L.; Reynaud, B.; Belzunces, L.P. Honeybee biomarkers as promising tools to monitor environmental quality. Environ. Int. 2013, 60, 31–41. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.-L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides: Biomarker responses in honeybees exposed to pesticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- El-Saad, A.M.A.; Kheirallah, D.A.; El-Samad, L.M. Biochemical and histological biomarkers in the midgut of Apis mellifera from polluted environment at Beheira Governorate, Egypt. Environ. Sci. Pollut. Res. 2017, 24, 3181–3193. [Google Scholar] [CrossRef]
- Dabour, K.; Al Naggar, Y.; Masry, S.; Naiem, E.; Giesy, J.P. Cellular alterations in midgut cells of honey bee workers (Apis mellifera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 2019, 651, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Schmehl, D.R.; Wedde, A.E.; Godoy, R.S.M.; Ravaiano, S.V.; Guedes, R.N.C.; Martins, G.F.; Ellis, J.D. Frequently encountered pesticides can cause multiple disorders in developing worker honey bees. Environ. Pollut. 2020, 256, 113420. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Niu, X.; hui Wang, D.; Wang, C.; Zhu, L.; Xue, X.; Zhang, Z.; Wu, L. Flumethrin at sublethal concentrations induces stresses in adult honey bees (Apis mellifera L.). Sci. Total Environ. 2020, 700, 134500. [Google Scholar] [CrossRef] [PubMed]
- Arthidoro de Castro, M.B.; Martinez, L.C.; Cossolin, J.F.S.; Serra, R.S.; Serrão, J.E. Cytotoxic effects on the midgut, hypopharyngeal, glands and brain of Apis mellifera honey bee workers exposed to chronic concentrations of lambda-cyhalothrin. Chemosphere 2020, 248, 126075. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Domingues, C.E.C.; de Melo, N.F.S.; Roat, T.C.; Malaspina, O.; Jones-Costa, M.; Silva-Zacarin, E.C.M.; Fraceto, L.F. Nanopesticide based on botanical insecticide pyrethrum and its potential effects on honeybees. Chemosphere 2019, 236, 124282. [Google Scholar] [CrossRef]
- AL Naggar, Y.; Dabour, K.; Masry, S.; Sadek, A.; Naiem, E.; Giesy, J.P. Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis mellifera L.). Environ. Sci. Pollut. Res. 2020, 27, 19004–19015. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Genecque, E.; Menach, K.L.; Budzinski, H.; Cluzeau, S.; Pham-Delegue, M.H. Comparative Sublethal Toxicity of Nine Pesticides on Olfactory Learning Performances of the Honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 2005, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Helmer, S.H.; Kerbaol, A.; Aras, P.; Jumarie, C.; Boily, M. Effects of realistic doses of atrazine, metolachlor, and glyphosate on lipid peroxidation and diet-derived antioxidants in caged honey bees (Apis mellifera). Environ. Sci. Pollut. Res. 2015, 22, 8010–8021. [Google Scholar] [CrossRef] [PubMed]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium Toxicity to Honey Bee (Apis mellifera L.) Pollinators: Effects on Behaviors and Survival. PLoS ONE 2012, 7, e34137. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, S.; Xue, X.; Niu, X.; Wu, L. Nitenpyram disturbs gut microbiota and influences metabolic homeostasis and immunity in honey bee (Apis mellifera L.). Environ. Pollut. 2020, 258, 113671. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delègue, M.-H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef]
- Hladun, K.R.; Di, N.; Liu, T.-X.; Trumble, J.T. Metal contaminant accumulation in the hive: Consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.): Impact of metal contaminants on honey bee health. Environ. Toxicol. Chem. 2016, 35, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Herbert, L.T.; Vazquez, D.E.; Arenas, A.; Farina, W.M. Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J. Exp. Biol. 2014, 217, 3457–3464. [Google Scholar] [CrossRef] [Green Version]
- Morfin, N.; Goodwin, P.H.; Correa-Benitez, A.; Guzman-Novoa, E. Sublethal exposure to clothianidin during the larval stage causes long-term impairment of hygienic and foraging behaviours of honey bees. Apidologie 2019, 50, 595–605. [Google Scholar] [CrossRef]
- Schmuck, R.; Stadler, T.; Schmidt, H.-W. Field relevance of a synergistic effect observed in the laboratory between an EBI fungicide and a chloronicotinyl insecticide in the honeybee (Apis mellifera L, Hymenoptera): Synergistic effect between fungicide and thiacloprid in honeybee. Pest Manag. Sci. 2003, 59, 279–286. [Google Scholar] [CrossRef]
- Søvik, E.; Perry, C.J.; LaMora, A.; Barron, A.B.; Ben-Shahar, Y. Negative impact of manganese on honeybee foraging. Biol. Lett. 2015, 11, 20140989. [Google Scholar] [CrossRef] [Green Version]
- Guez, D.; Zhu, H.; Zhang, S.W.; Srinivasan, M.V. Enhanced cholinergic transmission promotes recall in honeybees. J. Insect Physiol. 2010, 56, 1341–1348. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Z.; Huang, Q.; Zhang, X.W.; Ke, L.; Yan, W.Y.; Zhang, L.Z.; Zeng, Z.J. Deltamethrin Impairs Honeybees (Apis mellifera) Dancing Communication. Arch. Environ. Contam. Toxicol. 2020, 78, 117–123. [Google Scholar] [CrossRef]
- Chaimanee, V.; Evans, J.D.; Chen, Y.; Jackson, C.; Pettis, J.S. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 2016, 89, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dai, P.-L.; Wang, Q.; Sun, J.-H.; Liu, F.; Wang, X.; Wu, Y.-Y.; Zhou, T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Wiseman, S.; Sun, J.; Cutler, G.C.; Aboul-Soud, M.; Naiem, E.; Mona, M.; Seif, A.; Giesy, J.P. Effects of environmentally-relevant mixtures of four common organophosphorus insecticides on the honey bee (Apis mellifera L.). J. Insect Physiol. 2015, 82, 85–91. [Google Scholar] [CrossRef]
- Imran, M.; Sheikh, U.A.A.; Nasir, M.; Ghaffar, M.A.; Tamkeen, A.; Iqbal, M.A. Do neonicotinoid insecticides impaired olfactory learning behavior in Apis mellifera? Int. J. Ind. Entomol. 2019, 38, 1–5. [Google Scholar]
- Weick, J.; Thorn, R.S. Effects of Acute Sublethal Exposure to Coumaphos or Diazinon on Acquisition and Discrimination of Odor Stimuli in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 2002, 95, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Softley, S.; Earnshaw, H. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 2015, 5, 15322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.-C.; Chang, H.-C.; Wu, W.-Y.; Chen, Y.-W. Impaired Olfactory Associative Behavior of Honeybee Workers Due to Contamination of Imidacloprid in the Larval Stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef] [Green Version]
- du Rand, E.E.; Human, H.; Smit, S.; Beukes, M.; Apostolides, Z.; Nicolson, S.W.; Pirk, C.W.W. Proteomic and metabolomic analysis reveals rapid and extensive nicotine detoxification ability in honey bee larvae. Insect Biochem. Mol. Biol. 2017, 82, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Almasri, H.; Tavares, D.A.; Pioz, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Mixtures of an insecticide, a fungicide and a herbicide induce high toxicities and systemic physiological disturbances in winter Apis mellifera honey bees. Ecotoxicol. Environ. Saf. 2020, 203, 111013. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Nasr, H.M.; Rabea, E.I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 2015, 46, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Christen, V.; Krebs, J.; Bünter, I.; Fent, K. Biopesticide spinosad induces transcriptional alterations in genes associated with energy production in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 378, 120736. [Google Scholar] [CrossRef]
- Christen, V.; Joho, Y.; Vogel, M.; Fent, K. Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environ. Pollut. 2019, 244, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, A.; Evans, J.D.; Scharf, M.; Ellis, J.D. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 2012, 58, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Wen, Z.; Schuler, M.A.; Berenbaum, M.R. Mediation of Pyrethroid Insecticide Toxicity to Honey Bees (Hymenoptera: Apidae) by Cytochrome P450 Monooxygenases. J. Econ. Entomol. 2006, 99, 5. [Google Scholar] [CrossRef]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic Interactions Between In-Hive Miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; He, J.; Zhao, X.; Chaimanee, V.; Huang, W.-F.; Nie, H.; Zhao, Y.; Su, S. Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017, 140, 1–8. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc. Natl. Acad. Sci. USA 2011, 108, 12657–12662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, A.I.; Polemitou, I.; Laifi, P.; Yiangou, A.; Tananaki, C. Glutathione S-transferase in the insect Apis mellifera macedonica Kinetic characteristics and effect of stress on the expression of GST isoenzymes in the adult worker bee. Comp. Biochem. Physiol. 2004, 139, 93–97. [Google Scholar]
- Yao, J.; Zhu, Y.C.; Adamczyk, J. Responses of Honey Bees to Lethal and Sublethal Doses of Formulated Clothianidin Alone and Mixtures. J. Econ. Entomol. 2018, 111, 1517–1525. [Google Scholar] [CrossRef]
- Zaworra, M.; Nauen, R. New approaches to old problems: Removal of phospholipase A2 results in highly active microsomal membranes from the honey bee, Apis mellifera. Pestic. Biochem. Physiol. 2019, 161, 68–76. [Google Scholar] [CrossRef]
- Badiou, A.; Meled, M.; Belzunces, L.P. Honeybee Apis mellifera acetylcholinesterase—A biomarker to detect deltamethrin exposure. Ecotoxicol. Environ. Saf. 2008, 69, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, N.; Bounias, M.; Fleche, C. Toxicity of Cypermethrin and Fenitrothion on the Hemolymph Carbohydrates, Head Acetylcholinesterase, and Thoracic Muscle Na+, K+-ATPase of Emerging Honeybees (Apis mellifera L.). Ecotoxicol. Environ. Saf. 1999, 44, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Gagnaire, B.; Bonnet, M.; Tchamitchian, S.; Cavalié, I.; Della-Vedova, C.; Dubourg, N.; Adam-Guillermin, C.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of gamma irradiation in the honeybee, Apis mellifera. Ecotoxicol. Environ. Saf. 2019, 174, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glavan, G.; Kos, M.; Božič, J.; Drobne, D.; Sabotič, J.; Kokalj, A.J. Different response of acetylcholinesterases in salt- and detergent-soluble fractions of honeybee haemolymph, head and thorax after exposure to diazinon. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J.H.; Ruvolo-Takasusuki, M.C.C.; Arnaut de Toledo, V. Evaluation of the Use of the Inhibition Esterase Activity on Apis mellifera as Bioindicators of Insecticide Thiamethoxam Pesticide Residues. Sociobiology 2003, 42, 693–699. [Google Scholar]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef]
- Tavares, D.A.; Roat, T.C.; Silva-Zacarin, E.C.M.; Nocelli, R.C.F.; Malaspina, O. Exposure to thiamethoxam during the larval phase affects synapsin levels in the brain of the honey bee. Ecotoxicol. Environ. Saf. 2019, 169, 523–528. [Google Scholar] [CrossRef]
- Bedick, J.C.; Tunaz, H.; Nor Aliza, A.R.; Putnam, S.M.; Ellis, M.D.; Stanley, D.W. Eicosanoids act in nodulation reactions to bacterial infections in newly emerged adult honey bees, Apis mellifera, but not in older foragers. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Christen, V.; Vogel, M.S.; Hettich, T.; Fent, K. A Vitellogenin Antibody in Honey Bees (Apis mellifera): Characterization and Application as Potential Biomarker for Insecticide Exposure. Environ. Toxicol. Chem. 2019, 38, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.Z.; Bitondi, M.M.G.; Simões, Z.L.P. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000, 46, 153–160. [Google Scholar] [CrossRef]
- Bounias, M. Sublethal Effects of a Synthetic Pyrethroid, Deltamethrin, on the Glycemia, the Lipemia, and the Gut Alkaline Phosphatases of Honeybees. Pestic. Biochem. Phys. 1985, 24, 149–160. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid markedly affects hemolymph proteolysis, biomarkers, DNA global methylation, and the cuticle proteolytic layer in western honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Burton, S.; Wang, Y.; Xu, S.; Zhang, W.; Yu, L. Metabolomic analysis of honey bee, Apis mellifera L. response to thiacloprid. Pestic. Biochem. Physiol. 2018, 152, 17–23. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Gauthier, M.; Aras, P.; Jumarie, C.; Boily, M. Low dietary levels of Al, Pb and Cd may affect the non-enzymatic antioxidant capacity in caged honey bees (Apis mellifera). Chemosphere 2016, 144, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T.V.; Kojić, D.; Orčić, S.; Batinić, D.; Vukašinović, E.; Blagojević, D.P.; Purać, J. The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 2016, 164, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hranitz, J.M.; Abramson, C.I.; Carter, R.P. Ethanol increases HSP70 concentrations in honeybee (Apis mellifera L.) brain tissue. Alcohol 2010, 44, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J. Secondary biomarkers of insecticide-induced stress of honey bee colonies and their relevance for overwintering strength. Ecotoxicol. Environ. Saf. 2016, 132, 379–389. [Google Scholar] [CrossRef]
- Cabbri, R.; Ferlizza, E.; Nanetti, A.; Monari, E.; Andreani, G.; Galuppi, R.; Isani, G. Biomarkers of nutritional status in honeybee haemolymph: Effects of different biotechnical approaches for Varroa destructor treatment and wintering phase. Apidologie 2018, 49, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A Method to Quantify and Analyze the Foraging Activity of Honey Bees: Relevance to the Sublethal Effects Induced by Systemic Insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef]
- Ingram, E.M.; Augustin, J.; Ellis, M.D.; Siegfried, B.D. Evaluating sub-lethal effects of orchard-applied pyrethroids using video-tracking software to quantify honey bee behaviors. Chemosphere 2015, 135, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Yang, H.; Yu, L.; Liao, C.; Liu, Y.; Jin, M.; Yan, W.; Wu, X.B. Sublethal acetamiprid doses negatively affect the lifespans and foraging behaviors of honey bee (Apis mellifera L.) workers. Sci. Total Environ. 2020, 738, 139924. [Google Scholar] [CrossRef] [PubMed]
- Monchanin, C.; Henry, M.; Decourtye, A.; Dalmon, A.; Fortini, D.; Bœuf, E.; Dubuisson, L.; Aupinel, P.; Chevallereau, C.; Petit, J.; et al. Hazard of a neonicotinoid insecticide on the homing flight of the honeybee depends on climatic conditions and Varroa infestation. Chemosphere 2019, 224, 360–368. [Google Scholar] [CrossRef]
- Odemer, R.; Alkassab, A.T.; Bischoff, G.; Frommberger, M.; Wernecke, A.; Wirtz, I.P.; Pistorius, J.; Odemer, F. Chronic High Glyphosate Exposure Delays Individual Worker Bee (Apis mellifera L.) Development under Field Conditions. Insects 2020, 11, 664. [Google Scholar] [CrossRef]
- Siede, R.; Faust, L.; Meixner, M.D.; Maus, C.; Grünewald, B.; Büchler, R. Performance of honey bee colonies under a long-lasting dietary exposure to sublethal concentrations of the neonicotinoid insecticide thiacloprid: Testing thiacloprid on bee colonies in a field trial. Pest Manag. Sci. 2017, 73, 1334–1344. [Google Scholar] [CrossRef] [Green Version]
- Balbuena, M.S.; Tison, L.; Hahn, M.-L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.; Overmyer, J.; Feken, M.; Ruddle, N.; Vaughan, S.; Scorgie, E.; Bocksch, S.; Hill, M. Thiamethoxam: Long-term effects following honey bee colony-level exposure and implications for risk assessment. Sci. Total Environ. 2019, 654, 60–71. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Caren, J.; Reddy, G.V.P.; Li, W.; Yao, J. Effect of age on insecticide susceptibility and enzymatic activities of three detoxification enzymes and one invertase in honey bee workers (Apis mellifera). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108844. [Google Scholar] [CrossRef] [PubMed]
- Leonard, R.J.; Wat, K.K.Y.; McArthur, C.; Hochuli, D.F. Urbanisation and wing asymmetry in the western honey bee (Apis mellifera, Linnaeus 1758) at multiple scales. PeerJ 2018, 6, e5940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naggar, Y.A.A.; Naiem, E.-S.A.; Seif, A.I.; Mona, M.H. Honey bees and their products as a bio-in- dicator of environmental pollution with heavy metals. Mellifera 2013, 13, 1–20. [Google Scholar]
- ALNaggar, Y.; Vogt, A.; Codling, G.; Naiem, E.; Mona, M.; Seif, A.; Robertson, A.J.; Giesy, J.P. Exposure of honeybees (Apis mellifera) in Saskatchewan, Canada to organophosphorus insecticides. Apidologie 2015, 46, 667–678. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Codling, G.; Vogt, A.; Naiem, E.; Mona, M.; Seif, A.; Giesy, J.P. Organophosphorus insecticides in honey, pollen and bees (Apis mellifera L.) and their potential hazard to bee colonies in Egypt. Ecotoxicol. Environ. Saf. 2015, 114, 1–8. [Google Scholar] [CrossRef]
- Amorena, M.; Visciano, P.; Giacomelli, A.; Marinelli, E.; Sabatini, A.G.; Medrzycki, P.; Oddo, L.P.; De Pace, F.M.; Belligoli, P.; Di Serafino, G.; et al. Monitoring of levels of polycyclic aromatic hydrocarbons in bees caught from beekeeping: Remark 1. Vet. Res. Commun. 2009, 33, 165–167. [Google Scholar] [CrossRef]
- Amulen, D.R.; Spanoghe, P.; Houbraken, M.; Tamale, A.; de Graaf, D.C.; Cross, P.; Smagghe, G. Environmental contaminants of honeybee products in Uganda detected using LC-MS/MS and GC-ECD. PLoS ONE 2017, 12, e0178546. [Google Scholar] [CrossRef] [Green Version]
- Codling, G.; Al Naggar, Y.; Giesy, J.P.; Robertson, A.J. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 2016, 144, 2321–2328. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and Their Products as Potential Bioindicators of Heavy Metals Contamination. Environ. Monit. Assess. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Fulton, C.A.; Huff Hartz, K.E.; Fell, R.D.; Brewster, C.C.; Reeve, J.D.; Lydy, M.J. An assessment of pesticide exposures and land use of honey bees in Virginia. Chemosphere 2019, 222, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Kump, P.; Nečemer, M.; Šnajder, J. Determination of trace elements in bee honey, pollen and tissue by total reflection and radioisotope X-ray fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1996, 51, 499–507. [Google Scholar] [CrossRef]
- Nikolić, T.V.; Purać, J.; Orčić, S.; Kojić, D.; Vujanović, D.; Stanimirović, Z.; Gržetić, I.; Ilijević, K.; Šikoparija, B.; Blagojević, D.P. Environmental effects on superoxide dismutase and catalase activity and expression in honey bee: Environmental Effect on SOD and CAT in Honey Bee. Arch. Insect Biochem. Physiol. 2015, 90, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Di Serafino, G.; Giacomelli, A.; Medrzycki, P.; Sabatini, A.G.; Persano Oddo, L.; Marinelli, E.; Amorena, M. Monitoring of Polycyclic Aromatic Hydrocarbons in Bees (Apis mellifera) and Honey in Urban Areas and Wildlife Reserves. J. Agric. Food Chem. 2009, 57, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Ponikvar, M.; Šnajder, J.; Sedej, B. Honey as a bioindicator for environmental pollution with SO2. Apidologie 2005, 36, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Ruschioni, S.; Riolo, P.; Minuz, R.L.; Stefano, M.; Cannella, M.; Porrini, C.; Isidoro, N. Biomonitoring with Honeybees of Heavy Metals and Pesticides in Nature Reserves of the Marche Region (Italy). Biol. Trace Elem. Res. 2013, 154, 226–233. [Google Scholar] [CrossRef]
- Tonelli, D.; Gattavecchia, E.; Ghini, S.; Porrini, C.; Celli, G.; Mercuri, A.M. Honey bees and their products as indicators of environmental radioactive pollution. J. Radioanal. Nucl. Chem. Artic. 1990, 141, 427–436. [Google Scholar] [CrossRef]
- van der Steen, J.J.M.; de Kraker, J.; Grotenhuis, T. Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ. Monit. Assess. 2012, 184, 4119–4126. [Google Scholar] [CrossRef] [Green Version]
- Nicewicz, Ł.; Nicewicz, A.W.; Kafel, A.; Nakonieczny, M. Set of stress biomarkers as a practical tool in the assessment of multistress effect using honeybees from urban and rural areas as a model organism: A pilot study. Environ. Sci. Pollut. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Yu, S.J.; Robinson, F.A.; Nation, J.L. Detoxication capacity in the honey bee, Apis mellifera L. Pestic. Biochem. Physiol. 1984, 22, 360–368. [Google Scholar] [CrossRef]
- Bounias, M.; Kruk, I.; Nectoux, M.; Popeskovic, D. Toxicology of Cupric Salts on Honeybees. V. Gluconate and Sulfate Action on Gut Alkaline and Acid Phosphatases. Ecotoxicol. Environ. Saf. 1996, 35, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 2019, 363, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Animal Health and Welfare (AHAW) Assessing the health status of managed honeybee colonies (HEALTHY-B): A toolbox to facilitate harmonised data collection. EFSA J. 2016, 14, e04578. [CrossRef] [Green Version]
- European Food Safety Authority. Specifications for field data collection contributing to honey bee model corroboration and verification. EFSA Support. Publ. 2017, 14, 1234E. [Google Scholar]
- Lazarov, S.; Zhelyazkova, I. Content of Crude Protein in the Body and Total Protein and Lysozyme in the Haemolymph of Worker Bees (Apis mellifera L.) according to their infestation with Varroa destructor. Zhivotnovadni Nauki 2019, 56, 9–15. [Google Scholar]





| Endpoint | Test | N | Contaminants | Reference |
|---|---|---|---|---|
| Morphology | Cellular structure of midgut cells | 2 | CdO and PbO nanoparticles, mixtures | Dabour et al., 2019 [57] |
| Morphologies of antenna and hypopharyngeal glands | Herbicides, fungicides, insecticides, acaricides | Tomè et al., 2020 [58] | ||
| Apoptosis/necrosis | Apoptosis/necrosis | 2 | Trace elements, mixtures | Dabour et al., 2019 [57] |
| Apoptosis | Insecticides | Qi et al., 2020 [59] | ||
| Histopathology | Midgut, hypopharyngeal and brain | 2 | Insecticides | de Castro et al., 2020 [60] |
| Midgut | Insecticides | Oliveira et al., 2019 [61] | ||
| Cytotoxicity | Midgut, hypopharyngeal and brain | 1 | Insecticides | de Castro et al., 2020 [60] |
| Consumption | Food consumption | 7 | CdO and PbO nanoparticles, mixtures | Al Naggar et al., 2020 [62] |
| Food consumption | Insecticides, fungicides, Acaricides | Decourtye et al., 2005 [63] | ||
| Food consumption | Herbicides | Helmer et al., 2015 [64] | ||
| Food consumption | Sodium selenate, seleno-DL-methionine, DL-methionine | Hladun et al., 2012 [65] | ||
| Food consumption | Insecticides | Tong et al., 2019 [27] | ||
| Food consumption | Insecticides, mixtures | Williamson and Wright 2013 [28] | ||
| Food consumption | Insecticides | Zhu et al., 2020 [66] | ||
| Foraging activity/ fitness/ production of matrixes | Foraging activity | 12 | Insecticides | Decourtye et al., 2004 [67] |
| Sucrose response threshold | Sodium selenate, seleno-DL-methionine, DL-methionine | Hladun et al., 2012 [65] | ||
| Foraging activity | Sodium selenate, sodium selenite, seleno-L-cystine | Hladun et al., 2013 [51] | ||
| Fitness and production of wax and honey | Metals, selenium | Hladun et al., 2016 [68] | ||
| Foraging activity | Herbicides | Herbert et al., 2014 [69] | ||
| Foraging activity | Radiation (cell phone) | Mixson et al., 2009 [52] | ||
| Foraging behaviour | Insecticides | Morfin et al., 2019 [70] | ||
| Foraging activity | Mixtures | Prado et al., 2019 [50] | ||
| Foraging activity | Insecticides, Bacillus thurigiensis, mixtures | Renzi et al., 2016 [33] | ||
| Foraging activity | Fungicides, insecticides, mixtures | Schmuck et al., 2003 [71] | ||
| Foraging activity | Trace elements | Søvik et al., 2015 [72] | ||
| Weight, duration of immature development | Herbicides, fungicides, insecticides, acaricides | Tomè et al., 2020 [58] | ||
| Learning ability | Olfactory learning | Insecticides, fungicides, acaricides | Decourtye et al., 2005 [63] | |
| Visual and olfactory learning | 4 | Insecticides | Guez et al., 2010 [73] | |
| Training for olfactory conditioning using proboscis extension reflex | Insecticides, mixtures | Williamson and Wright 2013 [28] | ||
| Learning and memory-related genes | Insecticides | Zhang et al., 2020 [74] | ||
| Other behaviours | Colony strength | 5 | Trace elements, selenium | Hladun et al., 2016 [68] |
| Aggressive behaviour | Radiation (cell phone) | Mixson et al., 2009 [52] | ||
| Hygienic behaviour | Insecticides | Morfin et al., 2019 [70] | ||
| Thermoregulation | Insecticides | Tong et al., 2019 [27] | ||
| Behavioural anomalies (exaggerated motility, discoordinated movements) | Fungicides, insecticides, mixtures | Schmuck et al., 2003 [71] | ||
| Reproduction | Viability of sperm | Insecticides, acaricides | Chaimanee et al., 2016 [75] | |
| Fecundity | 3 | Insecticides | Dai et al., 2010 [76] | |
| Prepupal weight, percentage of prepupation, and pupation, relative growth indices | Sodium selenate, sodium selenite, seleno-L-cystine | Hladun et al., 2013 [51] | ||
| Sensory (gustatory or olfactory) | Olfactory conditioning of Proboscis extension reflex (PER) | 12 | Insecticides | Al Naggar et al., 2015 [77] |
| PER | Insecticides, acaricides | Decourtye et al., 2004 [67] | ||
| PER | Insecticides, fungicides, acaricides | Decourtye et al., 2005 [63] | ||
| PER | Insecticides | Guez et al., 2010 [73] | ||
| Antennal response assays, Proboscis response assays | Sodium selenate, seleno-DL-methionine, DL-methionine | Hladun et al., 2012 [65] | ||
| PER | Herbicides | Herbert et al., 2014 [69] | ||
| PER | Insecticides | Imran et al., 2019 [78] | ||
| PER | Radiation (cell phone) | Mixson et al., 2009 [52] | ||
| PER | Insecticides, acaricides | Weick and Thorn 2002 [79] | ||
| PER | Insecticides, mixtures | Williamson and Wright 2013 [28] | ||
| PER | Insecticides | Wright et al., 2015 [80] | ||
| PER | Insecticides | Yang et al., 2012 [81] | ||
| Flight activity | Flight navigation | 3 | Radiation (cell phone) | Mixson et al., 2009 [52] |
| Flight ability and success | Insecticides | Tong et al., 2019 [27] | ||
| Flight activity | Mixtures | Prado et al., 2019 [50] | ||
| Growth and development/brood production | Growth of adult workers | 5 | Insecticides, Varroa destructor | Abbo et al., 2017 [47] |
| Growth and development | Insecticides | Dai et al., 2010 [76] | ||
| Larval growth and development | Insecticides | du Rand et al., 2017 [82] | ||
| Brood production | Trace elements, selenium | Hladun et al., 2016 [68] | ||
| Duration of immature development | Herbicides, fungicides, insecticides, acaricides | Tomè et al., 2020 [58] | ||
| Accumulation | Chemical analysis | 2 | Sodium selenate, sodium selenite, seleno-L-cystine | Hladun et al., 2013 [51] |
| Chemical analysis | Trace elements, selenium | Hladun et al., 2016 [68] |
| Endpoint | Test | n | Contaminants | Reference |
|---|---|---|---|---|
| Detoxification | CYP genes expression, glutathione-S-transferase (GST) genes expression | 23 | Insecticides | Al Naggar et al., 2015 [77] |
| CYP and GST genes expression | CdO and PbO nanoparticles, mixtures | Al Naggar et al., 2020 [62] | ||
| (GST) | Insecticides, fungicides, herbicides and mixture | Almasri et al., 2020 [83] | ||
| GST | Insecticides | Badawy et al., 2015 [84] | ||
| GST and CaEs | Insecticides | Badiou-Bénéteau et al., 2012 [53] | ||
| GST and CaE | Fungicides, metals, EMS | Caliani et al., 2021 [43] | ||
| GST | Insecticides | Carvalho et al., 2013 [55] | ||
| Detoxification genes expression | Insecticides, acaricides | Chaimanee et al., 2016 [75] | ||
| Genes encoding CYP450 monooxygenases | Insecticides | Christen et al., 2019 [85] | ||
| Genes encoding CYP450 monooxygenases | Insecticides | Christen et al., 2019 [86] | ||
| Proteomic and metabolomic analysis | Insecticides | du Rand et al., 2017 [82] | ||
| Detoxification genes expression | Herbicides, fungicides, insecticides, Varroa destructor | Gregorc et al., 2012 [87] | ||
| cytochrome P450 (CYP450), GST and CaEs | Insecticides, acaricides | Johnson et al., 2006 [88] | ||
| CYP450 | Insecticides, acaricides | Johnson et al., 2009 [89] | ||
| GST and CaE | Insecticides | Li et al., 2017 [90] | ||
| P450 genes expression | Acaricides | Mao et al., 2011 [91] | ||
| GST isoenzymes expression | Papadopoulos et al., 2004 [92] | |||
| GST, GR and gene expressions | Insecticides | Qi et al., 2020 [59] | ||
| GST | Insecticides, Bacillus thurigiensis, mixtures | Renzi et al., 2016 [33] | ||
| P450 genes expression | Herbicides, fungicides, insecticides, acaricides | Tomè et al., 2020 [58] | ||
| Esterase (EST), GST, CYP450. CYPs and GSTs transcript levels | Insecticide | Yao et al., 2018 [93] | ||
| CYP450 and phospholipase A2 | Insecticides | Zaworra and Nauen 2019 [94] | ||
| Detoxification genes expression | Insecticides | Zhu et al., 2020 [66] | ||
| Neurotoxicity | acetylcholinesterase (AChE) | 24 | Insecticides | Al Naggar et al., 2015 [77] |
| AChE | Al Naggar et al., 2020 | |||
| AChE and CaE-3 | Insecticides, fungicides, herbicides and mixture | Almasri et al., 2020 [83] | ||
| AChE | Insecticides | Badawy et al., 2015 [84] | ||
| AChE | Acaricides, mixtures | Badiou et al., 2008 [95] | ||
| AChE and CaEs | Insecticides | Badiou-Bénéteau et al., 2012 [53] | ||
| AChE | Insecticides | Bendahou et al., 1999 [96] | ||
| AChE | Herbicides, insecticides | Boily et al., 2013 [97] | ||
| AChE and CaE | Fungicides, trace elements, EMS | Caliani et al., 2021 [43] | ||
| AChE and CaEs | Insecticides | Carvalho et al., 2013 [55] | ||
| Genes encoding acetylcholine receptors | Insecticides | Christen et al., 2019 [85] | ||
| Genes encoding acetylcholine receptors | Insecticides | Christen et al., 2019 [86] | ||
| Trembling and paralysis | Insecticides, acaricides | Decourtye et al., 2004 [67] | ||
| AChE and CaEs | Gamma irradiation | Gagnaire et al., 2019 [98] | ||
| AChE | Insecticides | Glavan et al., 2018 [99] | ||
| Esterase | Insecticides | Hashimoto et al., 2003 [100] | ||
| AChE and CaE | Insecticides | Li et al., 2017 [90] | ||
| AChE | Insecticides | Qi et al., 2020 [59] | ||
| AChE | Insecticides | Rabea et al., 2010 [49] | ||
| Octopamine, serotonin, dopamine | Trace elements | Søvik et al., 2015 [72] | ||
| Hyperresponsiveness, hyperactivity and trembling | Insecticides | Suchail et al., 2001 [101] | ||
| Protein level of synapsin | Insecticides | Tavares et al., 2019 [102] | ||
| AChE | Insecticides, acaricides | Weick and Thorn 2002 [79] | ||
| AChE | Insecticide | Yao et al., 2018 [93] | ||
| Immunity | Vtg expression | 13 | Insecticides, Varroa destructor | Abbo et al., 2017 [47] |
| Defensin 1, Abaecin, Hymenoptaecin expressions | Insecticides | Al Naggar et al., 2015 [77] | ||
| Nodulation | Dexamethasone (eicosanoid biosynthesis inhibitor) | Bedick et al., 2001 [103] | ||
| Hemocytes density, encapsulation response and antimicrobic activity | Insecticides | Brandt et al., 2016 [104] | ||
| Lysozyme (LYS) and granulocytes count | Fungicides, metals, EMS | Caliani et al., 2021 [43] | ||
| Immune response genes expression | Insecticides, acaricides | Chaimanee et al., 2016 [75] | ||
| Vtg gene expression | Insecticides | Christen et al., 2019 [105] | ||
| Vtg gene expression | Insecticides | Christen et al., 2019 [86] | ||
| Phenoloxydase (PO) | Gamma irradiation | Gagnaire et al., 2019 [98] | ||
| Immune genes expression | Herbicides, fungicides, insecticides, Varroa destructor | Gregorc et al., 2012 [87] | ||
| Immune gene expression | Insecticides | Li et al., 2017 [90] | ||
| Vtg synthesis | Insecticides | Pinto et al., 2000 [106] | ||
| Immune genes expression | Insecticides | Zhu et al., 2020 [66] | ||
| Metabolism | Alkaline phosphatase (ALP) and GST | Insecticides, fungicides, herbicides and mixture | Almasri et al., 2020 [83] | |
| Alkaline phosphatase (ALP) and GST | 17 | Insecticides | Badiou-Bénéteau et al., 2012 [53] | |
| Na+, K+ -ATPase assay | Insecticides | Bendahou et al., 1999 [96] | ||
| ALP | Insecticides | Bounias, 1985 [107] | ||
| ALP and GST | Fungicides, metals, EMS | Caliani et al., 2021 [43] | ||
| ALP and GST | Insecticides | Carvalho et al., 2013 [55] | ||
| Genes encoding for enzymes involved in phosphorylation | Insecticides | Christen et al., 2019 [85] | ||
| Proteomic and metabolomic analysis | Insecticides | du Rand et al., 2017 [82] | ||
| GST, CaEs and ALP | Gamma irradiation | Gagnaire et al., 2019 [98] | ||
| GST and CaE | Insecticides | Li et al., 2017 [90] | ||
| Aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP | Insecticides | Paleolog et al., 2020 [108] | ||
| ATP assays and GADPH activity | Mixtures | Prado et al., 2019 [50] | ||
| ATPase | Insecticides | Rabea et al., 2010 [49] | ||
| GST, ALP | Insecticides, Bacillus thurigiensis, mixtures | Renzi et al., 2016 [33] | ||
| Metabolic profile | Insecticides | Shi et al., 2018 [109] | ||
| AST, ALT, ALP | Acaricides | Strachecka et al., 2016 [110] | ||
| Abundance of gut microbiota for metabolic homeostasis, metabolic genes expression | Insecticides | Zhu et al., 2020 [66] | ||
| Oxidative stress | GST, G6PDH | Insecticides, fungicides, herbicides and mixture | Almasri et al., 2020 [83] | |
| GST, superoxide dismutase (SOD) and catalase (CAT) genes expression | CdO and PbO nanoparticles, mixtures | Al Naggar et al., 2020 [62] | ||
| polyphenol oxidase (PPO) | 14 | Insecticides | Badawy et al., 2015 [84] | |
| CAT | Insecticides | Badiou-Bénéteau et al., 2012 [53] | ||
| CAT | Insecticides | Carvalho et al., 2013 [55] | ||
| CAT, SOD, glutathione peroxidase (GPx), GST | Gamma irradiation | Gagnaire et al., 2019 [98] | ||
| α-tocopherol and metallothionein-like proteins (MTLPs) | Trace elements | Gauthier et al., 2016 [111] | ||
| LPO, lutein, zeaxanthin, α-Cryptoxanthin, β-Cryptoxanthin, β-Carotene, at-ROH, α-Tocopherol | Herbicides | Helmer et al., 2015 [64] | ||
| GST and PPO | Insecticides | Li et al., 2017 [90] | ||
| SOD, CAT, reduced glutathione (GSH), protein thiol groups (SH), malondialdehyde (MDA) | Trace elements | Nikolić et al., 2016 [112] | ||
| DNA methylation | Insecticides | Paleolog et al., 2020 [108] | ||
| Peroxidase (POD), malondialdehyde (MDA), lipid peroxide (LPO), SOD, CAT | Insecticides | Qi et al., 2020 [59] | ||
| GAPD, G6PD | Insecticides, Bacillus thurigiensis, mixtures | Renzi et al., 2016 [33] | ||
| SOD, GPx, CAT, GST | Acaricides | Strachecka et al., 2016 [110] | ||
| Genotoxicity | Nuclear abnormalities (NA) assay | 1 | Fungicides, metals, EMS | Caliani et al., 2021 [43] |
| Primary stress response | HSP70 | 1 | Ethanol | Hranitz et al., 2010 [113] |
| Carbohydrates assay | 2 | Insecticides | Bendahou et al., 1999 [96] | |
| Insecticides | Bounias, 1985 [107] | |||
| Protein amount | 3 | Herbicides | Helmer et al., 2015 [64] | |
| Insecticides | Li et al., 2017 [90] | |||
| Insecticides | Pinto et al., 2000 [106] | |||
| Lipid amount | 1 | Bounias, 1985 [107] | ||
| Endpoint | Test | n | Contaminants | Reference |
|---|---|---|---|---|
| Morphology | Asymmetry of wing nervature, diameter of forager bee hypopharyngeal gland, asymmetry of left and right branches of ovary | 1 | Insecticides | Wegener et al., 2016 [114] |
| Foraging activity/ fitness/production of matrixes | Colony nutritional status | 6 | Acaricides | Cabbri et al., 2018 [115] |
| Foraging activity | Insecticides | Colin et al., 2004 [116] | ||
| Foraging activity | Insecticides, acaricides | Decourtye et al., 2004 [67] | ||
| Time spent near a food source | Insecticides | Ingram et al., 2015 [117] | ||
| Foraging activity | Fungicides, insecticides | Schmuck et al., 2003 [71] | ||
| Foraging behaviour | Insecticides | Shi et al., 2020 [118] | ||
| Learning ability | Learning capacity and long-term memory of presumed forager bees | 1 | Insecticides | Wegener et al., 2016 [114] |
| Other behaviours | Intensive cleaning, trembling, cramping, locomotion problems, inactive bees, aggressiveness | 6 | Fungicides, insecticides | Berg et al., 2018 [48] |
| Bee locomotion and social interactions | Insecticides | Ingram et al., 2015 [117] | ||
| Homing performances | Insecticides | Monchanin et al., 2019 [119] | ||
| Overwintering success | Herbicides | Odemer et al., 2020 [120] | ||
| Overwintering success | Insecticides | Siede et al., 2017 [121] | ||
| Behavioural anomalies (exaggerated motility, discoordinated movements, trembling, shaking, apathy) | Fungicides, insecticides | Schmuck et al., 2003 [71] | ||
| Reproduction | Number of capped brood cells | 1 | Insecticides | Wegener et al., 2016 [114] |
| Sensory (gustatory or olfactory) | PER | 1 | Insecticides, acaricides | Decourtye et al., 2004 [67] |
| Flight activity | Homeward flight path | 2 | Herbicides | Balbuena et al., 2015 [122] |
| Flight activity | Fungicides, insecticides | Berg et al., 2018 [48] | ||
| Growth and development/brood production | Development of bee brood | 4 | Fungicides, insecticides | Berg et al., 2018 [48] |
| Brood and colony development, colony weight | Herbicides | Odemer et al., 2020 [120] | ||
| Number of brood cells, weight gain and production of drones | Insecticides | Siede et al., 2017 [121] | ||
| Reduction in bees and brood | Insecticides | Thompson et al., 2019 [123] | ||
| Accumulation | Chemical analysis | 1 | Insecticides | Siede et al., 2017 [121] |
| Endpoint | Test | n | Contaminants | Reference |
|---|---|---|---|---|
| Detoxification | GST | 2 | Insecticides | Wegener et al., 2016 [114] |
| CYP450, CaEs, GST | Insecticides | Zhu et al., 2020 [124] | ||
| Neurotoxicity | Trembling and paralysis | 1 | Insecticides | Decourtye et al., 2004 [67] |
| Immunity | Vtg and apolipophorin (APO) | 3 | Acaricides | Cabbri et al., 2018 [115] |
| Hymenoptaecin gene expression | Insecticide | Siede et al., 2017 [121] | ||
| Vtg | Insecticides | Wegener et al., 2016 [114] | ||
| Metabolism | Phosphofructokinase | 1 | Insecticides | Wegener et al., 2016 [114] |
| Oxidative stress | GST, phenoloxydase, glucose oxidase | 1 | Insecticides | Wegener et al., 2016 [114] |
| Protein amount | 3 | Acaricides | Cabbri et al., 2018 [115] | |
| Insecticides | Wegener et al., 2016 [114] | |||
| Insecticides | Zhu et al., 2020 [124] | |||
| Endpoint | Test | n | Contaminants | Reference |
|---|---|---|---|---|
| Morphology | Wing asymmetry | 1 | Urbanisation | Leonard et al., 2018 [125] |
| Accumulation | Chemical analysis | 18 | Metals | Al Naggar et al., 2013 [126] |
| Chemical analysis | Insecticides | Al Naggar et al., 2015 [127] | ||
| Chemical analysis | Insecticides | Al Naggar et al., 2015 [128] | ||
| Chemical analysis | PAHs | Amorena et al., 2009 [129] | ||
| Chemical analysis | Fungicides, insecticides | Amulen et al., 2017 [130] | ||
| Chemical analysis | Insecticides | Codling et al., 2016 [131] | ||
| Chemical analysis | Metals | Conti and Botrè, 2001 [132] | ||
| Chemical analysis | Insecticides | El-Saad et al., 2017 [56] | ||
| Chemical analysis | Herbicides, insecticides | Fulton et al., 2019 [133] | ||
| Chemical analysis | Metals | Kump et al., 1996 [134] | ||
| Chemical analysis | Herbicides, fungicides, insecticides, acaricides | Mullin et al., 2010 [45] | ||
| Chemical analysis | Trace elements | Nikolić et al., 2015 [135] | ||
| Chemical analysis | PAHs | Perugini et al., 2009 [136] | ||
| Chemical analysis | SO2 | Ponikvar et al., 2005 [137] | ||
| Chemical analysis | Herbicides, fungicides, insecticides | Raimets et al., 2020 [46] | ||
| Chemical analysis | Herbicides, insecticides, metals | Ruschioni et al., 2013 [138] | ||
| Gamma spectrometry | Radiations | Tonelli et al., 1990 [139] | ||
| Chemical analysis | Trace elements | van der Steen et al., 2012 [140] |
| Endpoint | Test | n | Contaminants | Reference |
|---|---|---|---|---|
| Detoxification | GST and metallothioneins (MT) | 4 | Trace elements | Badiou-Bénéteau et al., 2013 [54] |
| GST | Herbicides, fungicides, insecticides, electromagnetic fields | Lupi et al., 2020 [44] | ||
| GST | suspended dust and heavy metals | Nicewicz et al., 2020 [141] | ||
| GST, esterases, epoxyde hydrolase and DDT-dehydrochlorinase | Insecticides | Yu et al., 1984 [142] | ||
| Neurotoxicity | AChE | 4 | Trace elements | Badiou-Bénéteau et al., 2013 [54] |
| AChE | Herbicides, fungicides, insecticides, electromagnetic fields | Lupi et al., 2020 [44] | ||
| AChE | suspended dust and heavy metals | Nicewicz et al., 2020 [141] | ||
| Esterases | Insecticides | Yu et al., 1984 [142] | ||
| Immunity | Defensin | 1 | suspended dust and heavy metals | Nicewicz et al., 2020 [141] |
| Metabolism | ALP and GST | 5 | Trace elements | Badiou-Bénéteau et al., 2013 [54] |
| ALP and Acidic phosphatase | Trace elements | Bounias et al., 1996 [143] | ||
| ALP and GST | Herbicides, fungicides, insecticides, electromagnetic fields | Lupi et al., 2020 [44] | ||
| GST | suspended dust and heavy metals | Nicewicz et al., 2020 [141] | ||
| GST | Yu et al., 1984 [142] | |||
| Oxidative stress | SOD, CAT, GPx, GR | 4 | Insecticides | El-Saad et al., 2017 [56] |
| SOD and CAT | Trace elements | Nikolić et al., 2015 [135] | ||
| CAT and GST | Herbicides, fungicides, insecticides, electromagnetic fields | Lupi et al., 2020 [44] | ||
| GST and total antioxidant capacity (TAC) | suspended dust and heavy metals | Nicewicz et al., 2020 [141] | ||
| Primary stress response | HSP70 | 1 | suspended dust and heavy metals | Nicewicz et al., 2020 [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Noi, A.; Casini, S.; Campani, T.; Cai, G.; Caliani, I. Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 1863. https://doi.org/10.3390/ijerph18041863
Di Noi A, Casini S, Campani T, Cai G, Caliani I. Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives. International Journal of Environmental Research and Public Health. 2021; 18(4):1863. https://doi.org/10.3390/ijerph18041863
Chicago/Turabian StyleDi Noi, Agata, Silvia Casini, Tommaso Campani, Giampiero Cai, and Ilaria Caliani. 2021. "Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives" International Journal of Environmental Research and Public Health 18, no. 4: 1863. https://doi.org/10.3390/ijerph18041863








