Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease
Abstract
1. Introduction
2. Materials and Methods
- Green: 4 points;
- Green/Blue: 3 points;
- Blue: 2 points;
- Red/Blue: 1 point;
- Red: 0 points.
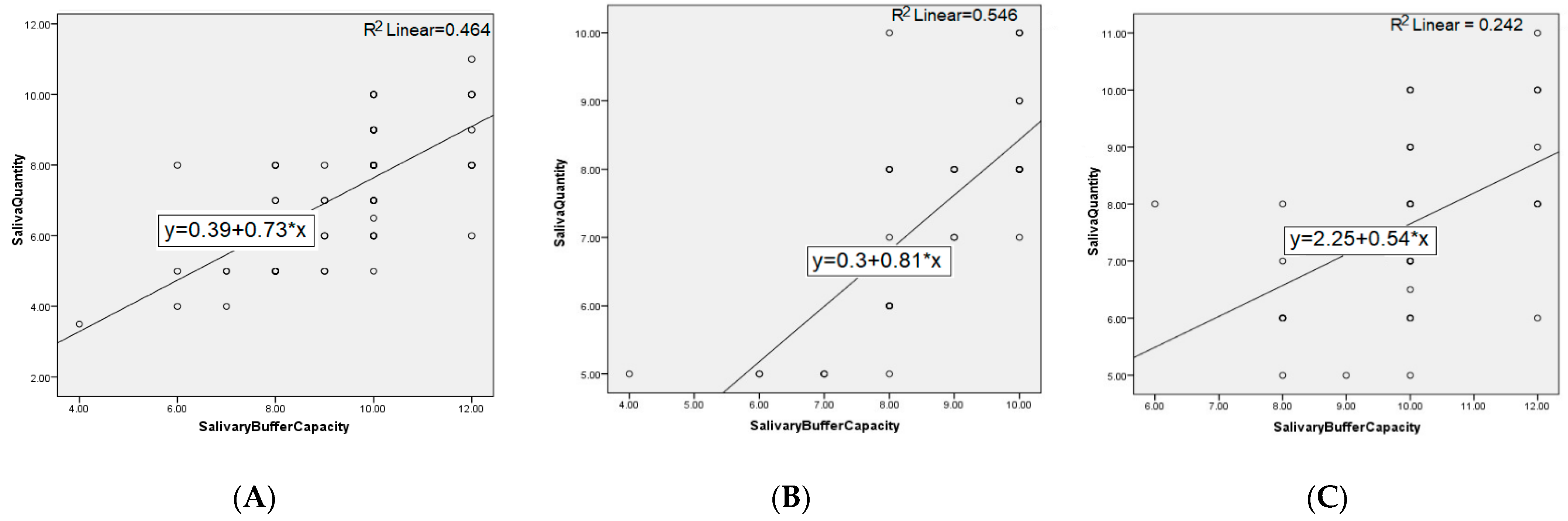
3. Results
4. Discussion
5. Conclusions
- The use of the Saliva-Check Buffer (GC, Tokyo, Japan) kit was a simple, easy, non-invasive and patient-accepted method, and can also be used in the dentist’s office to assess cariogenic risk by testing the quality of pH and buffer capacity of saliva.
- Saliva quantity at 5 min was lower in patients suffering from GERD.
- Salivary pH of patients suffering from GERD turned to acid values, compared to the salivary pH of patients belonging to the control group, without gastrointestinal pathology, where the values were within the normal range.
- In patients with GERD, the determined salivary buffer capacity was low or very low.
- For a correct dental rehabilitation treatment of patients affected by GERD, they should be monitored for a longer duration, and a multidisciplinary approach should be adopted in their treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Staskova, A.; Nemcova, R.; Lauko, S.; Jenca, A. Oral Microbiota from the Stomatology Perspective. In Bacterial Biofilms; Dincer, S., Özdenefe, S.M., Arkut, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Golgovici, F.; Prodana, M.; Ionascu, F.G.; Demetrescu, I. A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures. Metals 2021, 11, 256. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Fang, X. Esophageal Motor Dysfunctions in Gastroesophageal Reflux Disease and Therapeutic Perspectives. J. Neurogastroenterol. Motil. 2019, 25, 499–507. [Google Scholar] [CrossRef]
- Warsi, I.; Ahmed, J.; Younus, A.; Rasheed, A.; Akhtar, T.S.; Ain, Q.U.; Khurshid, Z. Risk factors associated with oral manifestations and oral health impact of gastro-oesophageal reflux disease: A multicenter, cross-sectional study in Pakistan. BMJ Open 2019, 9, e021458. [Google Scholar] [CrossRef]
- Santos Ferreira, P.; Ruivo Biancardi, M.; Lança, M.L.; Foster Lefort Rocha, A.; Morandin Ferrisse, T.; Bufalino, A. Gastroesophageal Reflux Disease in the Oral Cavity: 3 Case Reports. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, e113. [Google Scholar] [CrossRef]
- Wahlqvist, P. Symptoms of gastroesophageal reflux disease, perceived productivity, and health-related quality of life. Am. J. Gastroenterol. 2001, 96, S57–S61. [Google Scholar] [CrossRef]
- Pascu, O. Boala de reflux gastroesofagian. In Gastroenterologie. Hepatologie. Bazele Practicii Clinice, 5th ed.; Pascu, O., Andreica, V., Tanțău, M., Eds.; Editura Medicală Universitară “Iuliu Hațieganu”: Cluj-Napoca, Romania, 2012; pp. 43–48. [Google Scholar]
- Dawood, I.M.; El-Samarrai, S.K. Saliva and Oral Health. Int. J. Adv. Res. Biol. Sci. 2018, 5, 1–45. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- Ghannam, M.G.; Singh, P. Anatomy, Head and Neck, Salivary Glands. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/30855909/ (accessed on 8 October 2021).
- Ok, S.-M.; Ho, D.; Lynd, T.; Ahn, Y.-W.; Ju, H.-M.; Jeong, S.-H.; Cheon, K. Candida Infection Associated with Salivary Gland—A Narrative Review. J. Clin. Med. 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Maddu, N. Functions of Saliva, Saliva and Salivary Diagnostics. In Sridharan Gokul; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/66233 (accessed on 8 October 2021). [CrossRef]
- Dawes, C.; Wong, D.T.W. Role of Saliva and Salivary Diagnostics in the Advancement of Oral Health. J. Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bilbilova, E.Z. Dietary Factors, Salivary Parameters, and Dental Caries, Dental Caries; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/72145 (accessed on 8 October 2021). [CrossRef]
- Meghana Reddy, J.; Gayathri, R.; Vishnu Priya, V. Variation in salivary pH and buffering capacity of saliva in normal and diabetes mellitus patients—A pilot study. Drug Invent. Today 2018, 10, 895. [Google Scholar]
- Makawi, Y.; El-Masry, E.; El-Din, H.M. Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. J. Unexplored Med. Data 2017, 2, 9–15. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, M.-A.; Chae, Y.K.; Nam, O.H. Salivary Characteristics, Individual Casual Parameters, and Their Relationships with the Significant Caries Index among Korean Children Aged 12 Years. Int. J. Environ. Res. Public Health 2021, 18, 3118. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Mancl, L.A.; Presland, R.B.; Rothen, M.L.; Chi, D.L. Unstimulated Saliva-Related Caries Risk Factors in Individuals with Cystic Fibrosis: A Cross-Sectional Analysis of Unstimulated Salivary Flow, pH, and Buffering Capacity. Caries Res. 2017, 51, 1–6. [Google Scholar] [CrossRef]
- Pandey, P.; Reddy, N.V.; Rao, V.A.; Saxena, A.; Chaudhary, C.P. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp. Clin. Dent. 2015, 6, S65–S71. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Bechir, F.; Bataga, S.M.; Ungureanu, E.; Vranceanu, D.M.; Pacurar, M.; Bechir, E.S.; Cotrut, C.M. Experimental Study Regarding the Behavior at Different pH of Two Types of Co-Cr Alloys Used for Prosthetic Restorations. Materials 2021, 14, 4635. [Google Scholar] [CrossRef]
- Bechir, F.; Bataga, S.M.; Tohati, A.; Ungureanu, E.; Cotrut, C.M.; Bechir, E.S.; Suciu, M.; Vranceanu, D.M. Evaluation of the Behavior of Two CAD/CAM Fiber-Reinforced Composite Dental Materials by Immersion Tests. Materials 2021, 14, 7185. [Google Scholar] [CrossRef]
- Caruso, A.; Del Prete, S.; Ferrara, L.; Serra, R.; Telesca, D.; Ruggiero, S.; Russo, T.; Sivero, L. Relationship between gastroesophageal reflux disease and Ph nose and salivary: Proposal of a simple method outpatient in patients adults. Open Med. 2016, 11, 381–386. [Google Scholar] [CrossRef]
- Sujatha, S.; Jalihal, U.; Devi, Y.; Rakesh, N.; Chauhan, P.; Sharma, S. Oral pH in gastroesophageal reflux disease. Indian J. Gastroenterol. 2016, 35, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Balaban, D.P.; Grigorian, M.; Badea, V.; Caraiane, A.; Petcu, L.C. Gastroesophageal reflux disease and oral hygiene—Risk factors for dental erosion. In Proceedings of the 4th International Multidisciplinary Scientific Conference on Social Sciences and Arts SGEM 2017, Sofia, Bulgaria, 24–30 August 2017; 3 SGEM2017 Conference Proceedings; SGEM OnLine Scientific Library: Sofia, Bulgaria, 2017; Volume 3, pp. 293–298. [Google Scholar]
- Mihailopol, C.F.; Codreanu, C.M.; Pancu, G.; Topoliceanu, C.; Ghiorghe, C.A. Correlations between dental erosion severity and salivary factor in patients with gastroesophageal reflux disease. Rom. J. Oral Rehabil. 2011, 3, 63–66. [Google Scholar]
- Rahiotis, C.; Mitropoulos, P.; Kakaboura, A. Comparative Evaluation of Chair-Side Saliva Tests According to Current Dental Status in Adult Patient. Dent. J. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Ichim, D.L.; Sachelarie, L.; Calin, G.; Burlui, A. Are Saliva Tests Important in the Prediction of Carious Disease? Appl. Sci. 2021, 11, 5932. [Google Scholar] [CrossRef]
- Corrêa, M.C.C.S.F.; Lerco, M.M.; Cunha, M.L.R.S.; Henry, M.A.C.A. Salivary parameters and teeth erosions in patients with gastroesophageal reflux disease. Arq. Gastroenterol. 2012, 49, 214–218. [Google Scholar] [CrossRef]
- Burgess, J. Salivary stimulation-could it play a role in GERD management? J. Otolaryngol. Ent. Res. 2018, 10, 127–130. [Google Scholar] [CrossRef][Green Version]
- Tanabe, T.; Koeda, M.; Kitasako, Y.; Momma, E.; Hoshikawa, Y.; Hoshino, S.; Kawami, N.; Kaise, M.; Iwakiri, K. Stimulated saliva secretion is reduced in proton pump inhibitor-resistant severe reflux esophagitis patients. Esophagus Off. J. Jpn. Esophageal Soc. 2021, 18, 676–683. [Google Scholar] [CrossRef]
- Koeda, M.; Tanabe, T.; Kitasako, Y.; Momma, E.; Hoshikawa, Y.; Hoshino, S.; Kawami, N.; Kaise, M.; Iwakiri, K. Saliva secretion is reduced in proton pump inhibitor-responsive non-erosive reflux disease patients. Esophagus Off. J. Jpn. Esophageal Soc. 2021, 18, 900–907. [Google Scholar] [CrossRef]
- Romila, L.; Sachelarie, L.; BurluI, A.; Vasiliu, M.; Farcas, D.M. The salivary factors and dental erosion. Int. J. Med. Dent. 2020, 24, 21–27. [Google Scholar]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 2016, 6, 67–76. [Google Scholar] [CrossRef] [PubMed]
- David, C. Saliva testing. BDJ Pract. 2020, 33, 28–29. [Google Scholar]
- Maldupa, I.; Brinkmane, A.; Mihailova, A. Comparative analysis of CRT Buffer, GC Saliva Check Buffer tests and laboratory titration to evaluate saliva buffering capacity. Stomatol. Balt. Dent. Maxillofac. J. 2011, 13, 55–61. [Google Scholar]
- Kitasako, Y.; Burrow, M.F.; Stacey, M.; Huq, L.; Reynolds, E.C.; Tagami, J. Comparative analysis of three commercial saliva testing kits with a standard saliva buffering test. Aust. Dent. J. 2008, 53, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, S.; Smales, R.J.; Kaidonis, J.A. Oral manifestations of gastroesophageal reflux disease. J. Gastroenterol. Hepatol. 2012, 27, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cokuk, N.; Kaki, G.D.; Zamahay Turk, G.I.; Kara, E. The Effects of pH Changes on the Microhardness of Three Fluoride Releasing Restorative Materials: An In Vitro Study. EC Dent. Sci. 2018, 17, 1645–1651. [Google Scholar]
- Eriwati, Y.K.; Dhiaulfikri, M.; Herda, E. Effect of Salivary pH on Water Absorption and Solubility of Enhanced Resin—Modified Glass Ionomer. J. Dent. Indones. 2020, 27, 164–169. [Google Scholar]
- Liber-Knec, A.; Lagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Barboza-Solís, C.; Acuña-Amador, L.A. The Oral Microbiota: A Literature Review for Updating Professionals in Dentistry. Part I. Odovtos Int. J. Dent. Sci. 2020, 22, 59–68. [Google Scholar] [CrossRef]
- Shahi, S.; Özcan, M.; Maleki, D.S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef]
- Mittal, R.; Tan, K.S.; Wong, M.L.; Allen, P.F. Correlation between microbial host factors and caries among older adults. BMC Oral Health 2021, 21, 47. [Google Scholar] [CrossRef]
- Saramet, V.; Melescanu-Imre, M.; Tâncu, A.M.C.; Albu, C.C.; Ripszky-Totan, A.; Pantea, M. Molecular Interactions between Saliva and Dental Composites Resins: A Way Forward. Materials 2021, 14, 2537. [Google Scholar] [CrossRef]
- Velasco-Ibáñez, R.; Lara-Carrillo, E.; Morales-Luckie, R.A.; Romero-Guzmán, E.T.; Toral-Rizo, V.H.; Ramírez-Cardona, M.; García-Hernández, V.; Medina-Solís, C.E. Evaluation of the release of nickel and titanium under orthodontic treatment. Sci. Rep. 2020, 10, 22280. [Google Scholar] [CrossRef]
- Kanik, O.; Turkun, L.S.; Dasch, W. In vitro abrasion of resin-coated highly viscous glass ionomer cements: A confocal laser scanning microscopy study. Clin. Oral Investig. 2017, 21, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Bechir, E.S.; Bechir, A.; Gioga, C.; Manu, R.; Burcea, A.; Dascalu, I.D. The Advantages of BioHPP Polymer as Superstructure Material in Oral Implantology. Mater. Plast. 2016, 53, 394–398. [Google Scholar]
- Oviya, M.; Pradeep, S.; Ganapathy, D. Biocompatibility of dental restorative materials. Eur. J. Mol. Clin. Med. 2021, 8, 504–512. [Google Scholar]
- Dragus, L.; Ghergic, D.L.; Comaneanu, R.M.; Bechir, A.; Coman, C.; Botoaca, O. In vitro Comparative Tests about the Biocompatibility of Some Dental Alloys. Rev. Chim. 2019, 70, 610–613. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Ferracane, J.; Powers, J. Biocompatibility and Tissue Reaction to Biomaterials, In Craig’s Restorative Dental Materials, 14th ed.; Elselvier: St. Louis, MO, USA, 2019; pp. 91–107. [Google Scholar]
- Ibrahim, M.S.; El-Wassefy, N.A.; Farahat, D. Biocompatibility of dental biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 117–140. [Google Scholar] [CrossRef]
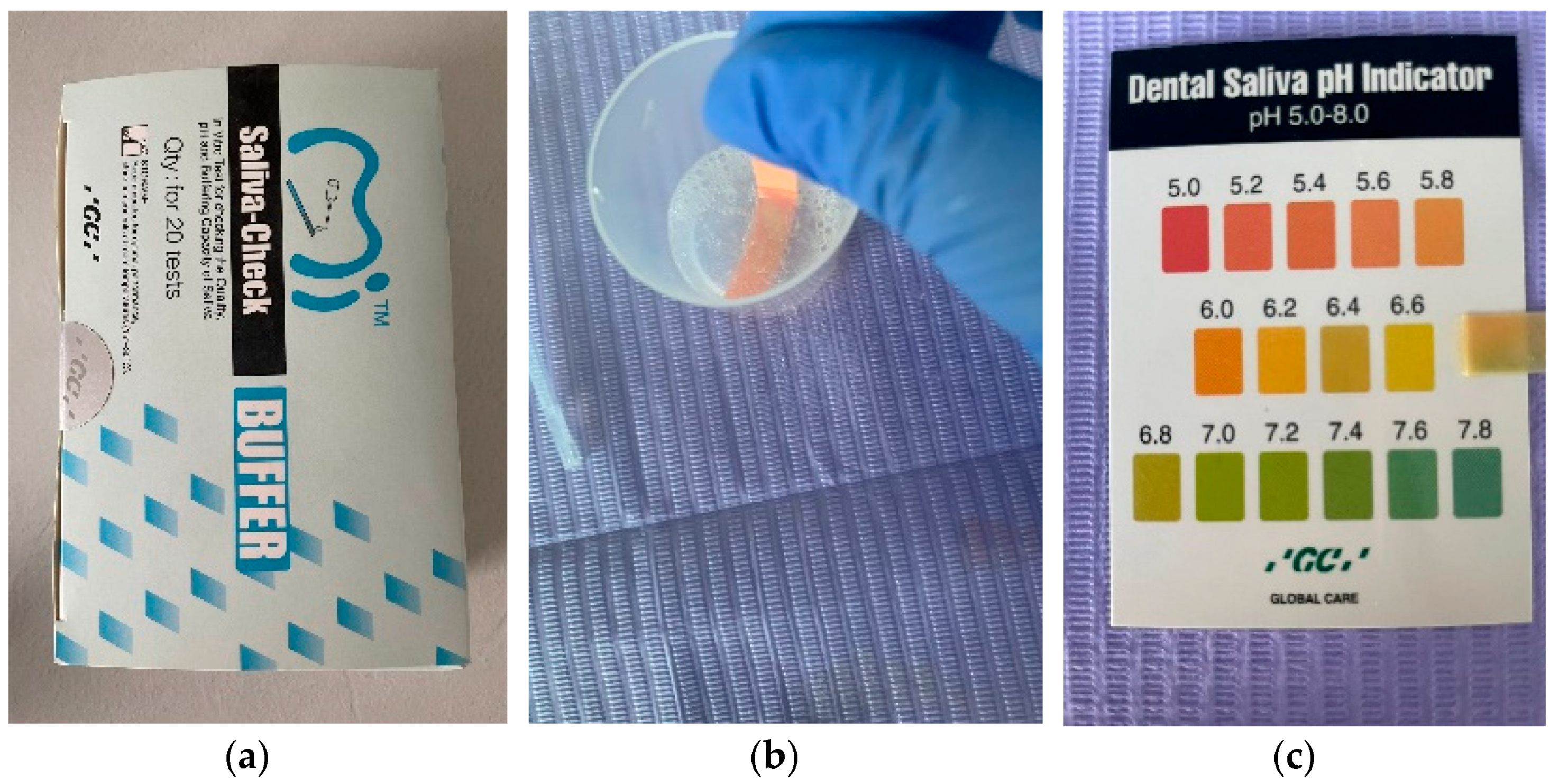


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| GERD positive diagnosis |
|
| Male and female patients, at least 18 years of age | Minors |
| Non-smokers/light smokers | Heavy smokers |
| Patient’s acceptance to participate in the study, with signed informed consent | Uncooperative patients who refused to be included in the study |
| Variable | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|
| Age | 33.46 | 30 | 11.34 | 19 | 63 |
| PH | 6.29 | 6.4 | 0.7 | 5 | 7.6 |
| Quantity at 5 min | 7.1 | 7 | 1.68 | 3.5 | 11 |
| Buffer Capacity | 9.25 | 10 | 1.57 | 4 | 12 |
| GERD Patients | Controls | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Frequency | Median | SD | Min | Max | Mean | Frequency | Median | SD | Min | Max | ||
| Sex | M | 18 (45%) | 14 (35%) | 0.49 | ||||||||||
| F | 22 (55%) | 26 (65%) | ||||||||||||
| Age | 34.45 | 31.5 | 10.32 | 19 | 63 | 32.47 | 30 | 12.32 | 19 | 60 | 0.44 | |||
| PH | 5.71 | 5.8 | 0.41 | 5 | 6.4 | 6.88 | 6.8 | 0.34 | 6.4 | 7.6 | 0 | |||
| Quantity at 5 min | 7.27 | 8 | 1.53 | 5 | 10 | 7.61 | 8 | 1.61 | 5 | 11 | 0.06 | |||
| Buffer Capacity | 8.57 | 8.5 | 1.39 | 4 | 10 | 9.92 | 10 | 1.47 | 6 | 12 | 0 | |||
| Saliva Buffering Capacity | |||
|---|---|---|---|
| 0–5 | 6–9 | 10–12 | |
| Entire population | 1 (1.3%) | 35 (43.75%) | 44 (55%) |
| GERD patients | 1 (2.5%) | 25 (31.25%) | 14 (35%) |
| Controls | 1 (2.5%) | 9 (22.5%) | 30 (75%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechir, F.; Pacurar, M.; Tohati, A.; Bataga, S.M. Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. Int. J. Environ. Res. Public Health 2022, 19, 201. https://doi.org/10.3390/ijerph19010201
Bechir F, Pacurar M, Tohati A, Bataga SM. Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. International Journal of Environmental Research and Public Health. 2022; 19(1):201. https://doi.org/10.3390/ijerph19010201
Chicago/Turabian StyleBechir, Farah, Mariana Pacurar, Adrian Tohati, and Simona Maria Bataga. 2022. "Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease" International Journal of Environmental Research and Public Health 19, no. 1: 201. https://doi.org/10.3390/ijerph19010201
APA StyleBechir, F., Pacurar, M., Tohati, A., & Bataga, S. M. (2022). Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. International Journal of Environmental Research and Public Health, 19(1), 201. https://doi.org/10.3390/ijerph19010201





