Accidental Hypothermia: 2021 Update
Abstract
:1. Introduction
2. Epidemiology
3. Pathophysiology
4. Diagnosis of Hypothermia
5. Treatment
5.1. Out of Hospital
5.2. In-Hospital Treatment
Extracorporeal cardiopulmonary resuscitation
6. Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Paal, P.; Brugger, H.; Strapazzon, G. Accidental hypothermia. Handb. Clin. Neurol. 2018, 157, 547–563. [Google Scholar]
- Soreide, K. Clinical and translational aspects of hypothermia in major trauma patients: From pathophysiology to prevention, prognosis and potential preservation. Injury 2014, 45, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Paal, P.; Gordon, L.; Strapazzon, G.; Brodmann Maeder, M.; Putzer, G.; Walpoth, B.; Wanscher, M.; Brown, D.; Holzer, M.; Broessner, G.; et al. Accidental hypothermia-an update: The content of this review is endorsed by the International Commission for Mountain Emergency Medicine (ICAR MEDCOM). Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37 (Suppl. 7), S186–S202. [Google Scholar] [CrossRef] [PubMed]
- Kamps, M.; Bisschops, L.A.; van der Hoeven, J.G.; Hoedemaekers, C.W. Hypothermia does not increase the risk of infection: A case control study. Crit. Care 2011, 15, R48. [Google Scholar] [CrossRef] [Green Version]
- Guly, H. History of accidental hypothermia. Resuscitation 2011, 82, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Lechner, R.; Kupper, T.; Tannheimer, M. Challenges of Military Health Service Support in Mountain Warfare. Wilderness Environ. Med. 2018, 29, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Paton, B.C. Cold, casualties, and conquests: The effects ofcold on warfare. In Medical Aspects of Harsh Environments; Pandolf, K.B., Burr, R.E., Eds.; Office of the Surgeon General, Department of the Army: Washington, DC, USA, 2001; pp. 313–349. [Google Scholar]
- Mittermair, C.; Foidl, E.; Wallner, B.; Brugger, H.; Paal, P. Extreme Cooling Rates in Avalanche Victims: Case Report and Narrative Review. High Alt. Med. Biol. 2021, 22, 235–240. [Google Scholar] [CrossRef]
- Zhang, P.; Wiens, K.; Wang, R.; Luong, L.; Ansara, D.; Gower, S.; Bassil, K.; Hwang, S.W. Cold Weather Conditions and Risk of Hypothermia among People Experiencing Homelessness: Implications for Prevention Strategies. Int. J. Environ. Res. Public Health 2019, 16, 3259. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, T.; Morita, S.; Ehara, N.; Miyamae, N.; Okada, Y.; Jo, T.; Sumida, Y.; Okada, N.; Watanabe, M.; Nozawa, M.; et al. Characteristics and outcomes of accidental hypothermia in Japan: The J-Point registry. Emerg. Med. J. 2018, 35, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, E.A.; Belson, M.; Rubin, C.; Patel, M. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995–2004. Wilderness Environ. Med. 2008, 19, 233–237. [Google Scholar] [CrossRef]
- Herity, B.; Daly, L.; Bourke, G.J.; Horgan, J.M. Hypothermia and mortality and morbidity. An epidemiological analysis. J. Epidemiol. Commun. Health 1991, 45, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.A.; Griffiths, R.F.; Cotter, J.D. Epidemiology of hypothermia: Fatalities and hospitalisations in New Zealand. Aust. N. Z. J. Med. 1994, 24, 705–710. [Google Scholar] [CrossRef]
- Vassal, T.; Benoit-Gonin, B.; Carrat, F.; Guidet, B.; Maury, E.; Offenstadt, G. Severe accidental hypothermia treated in an ICU: Prognosis and outcome. Chest 2001, 120, 1998–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandstrom, H.; Johansson, G.; Giesbrecht, G.G.; Angquist, K.A.; Haney, M.F. Accidental cold-related injury leading to hospitalization in northern Sweden: An eight-year retrospective analysis. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hislop, L.J.; Wyatt, J.P.; McNaughton, G.W.; Ireland, A.J.; Rainer, T.H.; Olverman, G.; Laughton, L.M. Urban hypothermia in the west of Scotland. West of Scotland Accident and Emergency Trainees Research Group. BMJ 1995, 311, 725. [Google Scholar] [CrossRef]
- Kosinski, S.; Darocha, T.; Galazkowski, R.; Drwila, R. Accidental hypothermia in Poland—Estimation of prevalence, diagnostic methods and treatment. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haman, F.; Blondin, D.P. Shivering thermogenesis in humans: Origin, contribution and metabolic requirement. Temperature 2017, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Iampietro, P.F.; Vaughan, J.A.; Goldman, R.F.; Kreider, M.B.; Masucci, F.; Bass, D.E. Heat production from shivering. J. Appl. Physiol. 1960, 15, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 2016, 196, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Singer, D. Pediatric Hypothermia: An Ambiguous Issue. Int. J. Environ. Res. Public Health, 2021; in press. [Google Scholar] [CrossRef]
- Elbaz, G.; Etzion, O.; Delgado, J.; Porath, A.; Talmor, D.; Novack, V. Hypothermia in a desert climate: Severity score and mortality prediction. Am. J. Emerg. Med. 2008, 26, 683–688. [Google Scholar] [CrossRef]
- Gallaher, M.M.; Fleming, D.W.; Berger, L.R.; Sewell, C.M. Pedestrian and hypothermia deaths among Native Americans in New Mexico. Between bar and home. JAMA 1992, 267, 1345–1348. [Google Scholar] [CrossRef]
- Oshiro, K.; Yuuichiro, T.; Schweizer, J.; Zafren, K.; Brugger, H.; Paal, P. Prevention of Hypothermia in the Aftermath of Natural Disasters in Areas at risk of Avalanches, Earthquakes, Floods, and Tsunamis. IJERPH, 2021; under review. [Google Scholar]
- Rauch, S.; Dal Cappello, T.; Strapazzon, G.; Palma, M.; Bonsante, F.; Gruber, E.; Strohle, M.; Mair, P.; Brugger, H.; International Alpine Trauma Registry Study Group. Pre-hospital times and clinical characteristics of severe trauma patients: A comparison between mountain and urban/suburban areas. Am. J. Emerg. Med. 2018, 36, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Lampl, L.; Hauke, J.; Bock, K.H. Accidental hypothermia in trauma patients. Is it relevant to preclinical emergency treatment? Anaesthesist 1995, 44, 101–107. [Google Scholar]
- Kander, T.; Schott, U. Effect of hypothermia on haemostasis and bleeding risk: A narrative review. J. Int. Med. Res. 2019, 47, 3559–3568. [Google Scholar] [CrossRef] [Green Version]
- Wallner, B.; Schenk, B.; Hermann, M.; Paal, P.; Falk, M.; Strapazzon, G.; Martini, W.Z.; Brugger, H.; Fries, D. Hypothermia-Associated Coagulopathy: A Comparison of Viscoelastic Monitoring, Platelet Function, and Real Time Live Confocal Microscopy at Low Blood Temperatures, an in vitro Experimental Study. Front. Physiol. 2020, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Weuster, M.; Bruck, A.; Lippross, S.; Menzdorf, L.; Fitschen-Oestern, S.; Behrendt, P.; Iden, T.; Hocker, J.; Lefering, R.; Seekamp, A.; et al. Epidemiology of accidental hypothermia in polytrauma patients: An analysis of 15,230 patients of the TraumaRegister DGU. J. Trauma Acute Care Surg. 2016, 81, 905–912. [Google Scholar] [CrossRef]
- Danzl, D.F.; Pozos, R.S. Accidental hypothermia. N. Engl. J. Med. 1994, 331, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Lankford, H.V.; Fox, L.R. The Wind-Chill Index. Wilderness Environ. Med. 2021, 32, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Proulx, C.I.; Ducharme, M.B.; Kenny, G.P. Effect of water temperature on cooling efficiency during hyperthermia in humans. J. Appl. Physiol. 2003, 94, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lott, C.; Truhlar, A.; Alfonzo, A.; Barelli, A.; Gonzalez-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef] [PubMed]
- Tipton, M.J.; Collier, N.; Massey, H.; Corbett, J.; Harper, M. Cold water immersion: Kill or cure? Exp. Physiol. 2017, 102, 1335–1355. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, M.; Zurron, N.; Weith, B.; Turini, P.; Dami, F.; Carron, P.N.; Paal, P. Deep accidental hypothermia with core temperature below 24 degrees c presenting with vital signs. High Alt. Med. Biol. 2014, 15, 58–63. [Google Scholar] [CrossRef]
- Pasquier, M.; Paal, P. Rescue collapse—A hitherto unclassified killer in accidental hypothermia. Resuscitation 2021, 164, 142–143. [Google Scholar] [CrossRef]
- Frei, C.; Darocha, T.; Debaty, G.; Dami, F.; Blancher, M.; Carron, P.N.; Oddo, M.; Pasquier, M. Clinical characteristics and outcomes of witnessed hypothermic cardiac arrest: A systematic review on rescue collapse. Resuscitation 2019, 137, 41–48. [Google Scholar] [CrossRef]
- Brown, D.J.; Brugger, H.; Boyd, J.; Paal, P. Accidental hypothermia. N. Engl. J. Med. 2012, 367, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Smolen, A.; Kosinski, S.; Hymczak, H.; Waligorski, S.; Witt-Majchrzak, A.; Drobinski, D.; Nowak, E.; Barteczko-Grajek, B.; Toczek, K.; et al. Impact of rescue collapse on mortality rate in severe accidental hypothermia: A matched-pair analysis. Resuscitation 2021, 164, 108–113. [Google Scholar] [CrossRef]
- Musi, M.E.; Sheets, A.; Zafren, K.; Brugger, H.; Paal, P.; Holzl, N.; Pasquier, M. Clinical staging of accidental hypothermia: The Revised Swiss System: Recommendation of the International Commission for Mountain Emergency Medicine (ICAR MedCom). Resuscitation 2021, 162, 182–187. [Google Scholar] [CrossRef]
- Strapazzon, G.; Procter, E.; Paal, P.; Brugger, H. Pre-hospital core temperature measurement in accidental and therapeutic hypothermia. High Alt. Med. Biol. 2014, 15, 104–111. [Google Scholar] [CrossRef]
- Pasquier, M.; Cools, E.; Zafren, K.; Carron, P.N.; Frochaux, V.; Rousson, V. Vital Signs in Accidental Hypothermia. High Alt. Med. Biol. 2021, 22, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.A.; Upex, A.; Bateman, D.N. Comparison of consciousness level assessment in the poisoned patient using the alert/verbal/painful/unresponsive scale and the Glasgow Coma Scale. Ann. Emerg. Med. 2004, 44, 108–113. [Google Scholar] [CrossRef]
- Opatz, O.; Trippel, T.; Lochner, A.; Werner, A.; Stahn, A.; Steinach, M.; Lenk, J.; Kuppe, H.; Gunga, H.C. Temporal and spatial dispersion of human body temperature during deep hypothermia. Br. J. Anaesth. 2013, 111, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, P.R.; Snow, B.W.; Rodrigues, D.B.; Salahi, S.; Oliveira, T.R.; Reudink, D.; Maccarini, P.F. Non-invasive measurement of brain temperature with microwave radiometry: Demonstration in a head phantom and clinical case. Neuroradiol. J. 2014, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Makinen, M.T.; Pesonen, A.; Jousela, I.; Paivarinta, J.; Poikajarvi, S.; Alback, A.; Salminen, U.S.; Pesonen, E. Novel Zero-Heat-Flux Deep Body Temperature Measurement in Lower Extremity Vascular and Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Gobolos, L.; Philipp, A.; Ugocsai, P.; Foltan, M.; Thrum, A.; Miskolczi, S.; Pousios, D.; Khawaja, S.; Budra, M.; Ohri, S.K. Reliability of different body temperature measurement sites during aortic surgery. Perfusion 2014, 29, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strapazzon, G.; Procter, E.; Putzer, G.; Avancini, G.; Dal Cappello, T.; Uberbacher, N.; Hofer, G.; Rainer, B.; Rammlmair, G.; Brugger, H. Influence of low ambient temperature on epitympanic temperature measurement: A prospective randomized clinical study. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 90. [Google Scholar] [CrossRef] [Green Version]
- Bagley, J.R.; Judelson, D.A.; Spiering, B.A.; Beam, W.C.; Bartolini, J.A.; Washburn, B.V.; Carney, K.R.; Munoz, C.X.; Yeargin, S.W.; Casa, D.J. Validity of field expedient devices to assess core temperature during exercise in the cold. Aviat. Space Environ. Med. 2011, 82, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Camboni, D.; Philipp, A.; Schebesch, K.M.; Schmid, C. Accuracy of core temperature measurement in deep hypothermic circulatory arrest. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 922–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, F.; Zehner, W.J.; Terndrup, T.E. The effect of ambient temperature extremes on tympanic and oral temperatures. Am. J. Emerg. Med. 1992, 10, 285–289. [Google Scholar] [CrossRef]
- Skaiaa, S.C.; Brattebo, G.; Assmus, J.; Thomassen, O. The impact of environmental factors in pre-hospital thermistor-based tympanic temperature measurement: A pilot field study. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 72. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.G.; Young, W.L.; Smith, C.R.; Solomon, R.A.; Wald, A.; Ostapkovich, N.; Shrebnick, D.B. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology 1995, 82, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Walpoth, B.H.; Galdikas, J.; Leupi, F.; Muehlemann, W.; Schlaepfer, P.; Althaus, U. Assessment of hypothermia with a new “tympanic” thermometer. J. Clin. Monit. 1994, 10, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Niven, D.J.; Gaudet, J.E.; Laupland, K.B.; Mrklas, K.J.; Roberts, D.J.; Stelfox, H.T. Accuracy of peripheral thermometers for estimating temperature: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 163, 768–777. [Google Scholar] [CrossRef]
- Tuitui, R.L.; Suwal, S.N.; Shrestha, S. Hand-touch method for detection of neonatal hypothermia in Nepal. J. Trop. Pediatr. 2011, 57, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Sund-Levander, M.; Grodzinsky, E. Assessment of body temperature measurement options. Br. J. Nurs. 2013, 22, 882–888. [Google Scholar] [CrossRef]
- Kiekkas, P.; Stefanopoulos, N.; Bakalis, N.; Kefaliakos, A.; Karanikolas, M. Agreement of infrared temporal artery thermometry with other thermometry methods in adults: Systematic review. J. Clin. Nurs. 2016, 25, 894–905. [Google Scholar] [CrossRef]
- Goswami, E.; Batra, P.; Khurana, R.; Dewan, P. Comparison of Temporal Artery Thermometry with Axillary and Rectal Thermometry in Full Term Neonates. Indian J. Pediatr. 2017, 84, 195–199. [Google Scholar] [CrossRef]
- Cox, E.G.M.; Dieperink, W.; Wiersema, R.; Doesburg, F.; van der Meulen, I.C.; Paans, W. Temporal artery temperature measurements versus bladder temperature in critically ill patients, a prospective observational study. PLoS ONE 2020, 15, e0241846. [Google Scholar] [CrossRef]
- Aykanat, V.M.; Broadbent, E.; Peyton, P.J. Reliability of alternative devices for postoperative patient temperature measurement: Two prospective, observational studies. Anaesthesia 2021, 76, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.R.; Dent, S.J.; Hall, K.D.; Smith, W.W. Physiologic reactions during profound hypothermia with cardioplegia. Anesthesiology 1961, 22, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Kimberger, O.; Thell, R.; Schuh, M.; Koch, J.; Sessler, D.I.; Kurz, A. Accuracy and precision of a novel non-invasive core thermometer. Br. J. Anaesth. 2009, 103, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.; Rischall, M.; Durgin, K.; Donoghue, M.; Pham, T.; Wyatt, T.; Stang, J.; DeVries, P.; Driver, B. Non-invasive zero-heat-flux technology compared with traditional core temperature measurements in the emergency department. Am. J. Emerg. Med. 2020, 38, 2383–2386. [Google Scholar] [CrossRef] [PubMed]
- Dahyot-Fizelier, C.; Lamarche, S.; Kerforne, T.; Benard, T.; Giraud, B.; Bellier, R.; Carise, E.; Frasca, D.; Mimoz, O. Accuracy of Zero-Heat-Flux Cutaneous Temperature in Intensive Care Adults. Crit. Care Med. 2017, 45, e715–e717. [Google Scholar] [CrossRef]
- Muth, C.M.; Shank, E.; Hauser, B.; Radermacher, P.; Groger, M.; Ehrmann, U. Infrared ear thermometry in water-related accidents-not a good choice. J. Emerg. Med. 2010, 38, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.R.; Brannigan, D.; Montgomery, A.; Khangure, N.; Williams, A.; Jacobs, I. Tympanic thermometry is unsuitable as a screening tool for hypothermia after open water swimming. Wilderness Environ. Med. 2007, 18, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Ducharme, M.B.; Frim, J.; Bourdon, L.; Giesbrecht, G.G. Evaluation of infrared tympanic thermometers during normothermia and hypothermia in humans. Ann. N. Y. Acad. Sci. 1997, 813, 225–229. [Google Scholar] [CrossRef]
- McCaffrey, T.V.; McCook, R.D.; Wurster, R.D. Effect of head skin temperature on tympanic and oral temperature in man. J. Appl. Physiol. 1975, 39, 114–118. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.; Song, K.; Kwak, Y. Core temperature measurement in therapeutic hypothermia according to different phases: Comparison of bladder, rectal, and tympanic versus pulmonary artery methods. Resuscitation 2013, 84, 810–817. [Google Scholar] [CrossRef]
- Lim, H.; Kim, B.; Kim, D.C.; Lee, S.K.; Ko, S. A comparison of the temperature difference according to the placement of a nasopharyngeal temperature probe. Korean J. Anesthesiol. 2016, 69, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, M.; Paal, P.; Kosinski, S.; Brown, D.; Podsiadlo, P.; Darocha, T. Esophageal Temperature Measurement. N. Engl. J. Med. 2020, 383, e93. [Google Scholar] [CrossRef]
- Whitby, J.D.; Dunkin, L.J. Temperature differences in the oesophagus. Preliminary study. Br. J. Anaesth. 1968, 40, 991–995. [Google Scholar] [CrossRef]
- Rauch, S.; Miller, C.; Brauer, A.; Wallner, B.; Bock, M.; Paal, P. Perioperative Hypothermia—A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 8749. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.; Giesbrecht, G.G.; Danzl, D.F.; Brugger, H.; Sagalyn, E.B.; Walpoth, B.; Auerbach, P.S.; McIntosh, S.E.; Nemethy, M.; McDevitt, M.; et al. Wilderness Medical Society Clinical Practice Guidelines for the out-of-Hospital Evaluation and Treatment of Accidental Hypothermia: 2019 Update. Wilderness Environ. Med. 2019, 30, S47–S69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rischall, M.L.; Rowland-Fisher, A. Evidence-Based Management of Accidental Hypothermia in the Emergency Department. Emerg. Med. Pract. 2016, 18, 1–18. [Google Scholar]
- Zafren, K.; Giesbrecht, G.G.; Danzl, D.F.; Brugger, H.; Sagalyn, E.B.; Walpoth, B.; Weiss, E.A.; Auerbach, P.S.; McIntosh, S.E.; Nemethy, M.; et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia: 2014 update. Wilderness Environ. Med. 2014, 25 (Suppl. 4), S66–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.; Ellerton, J.; Paal, P.; Boyd, J. Hypothermia Evidence, Afterdrop, and Practical Experience. Wilderness Environ. Med. 2015, 26, 437–439. [Google Scholar] [CrossRef] [Green Version]
- Zafren, K.; Giesbrecht, G.G.; Danzl, D.F.; Brugger, H.; Sagalyn, E.B.; Walpoth, B.; Weiss, E.A.; Auerbach, P.S.; McIntosh, S.E.; Nemethy, M.; et al. Hypothermia Evidence, Afterdrop, and Guidelines. Wilderness Environ. Med. 2015, 26, 439–441. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, M.; Blancher, M.; Zen Ruffinen, G.; Hugli, O. Does Rescue Collapse Mandate a Paradigm Shift in the Field Management of Avalanche Victims? High Alt. Med. Biol. 2015, 16, 171–172. [Google Scholar] [CrossRef]
- Blumenberg, A. Dosing Heat: Expected Core Temperature Change with Warmed or Cooled Intravenous Fluids. Ther. Hypothermia Temp. Manag. 2020, 11, 223–229. [Google Scholar] [CrossRef]
- Debaty, G.; Moustapha, I.; Bouzat, P.; Maignan, M.; Blancher, M.; Rallo, A.; Brun, J.; Chavanon, O.; Danel, V.; Carpentier, F.; et al. Outcome after severe accidental hypothermia in the French Alps: A 10-year review. Resuscitation 2015, 93, 118–123. [Google Scholar] [CrossRef]
- Darocha, T.; Kosinski, S.; Jarosz, A.; Podsiadlo, P.; Zietkiewicz, M.; Sanak, T.; Galazkowski, R.; Piatek, J.; Konstanty-Kalandyk, J.; Drwila, R. Should capnography be used as a guide for choosing a ventilation strategy in circulatory shock caused by severe hypothermia? Observational case-series study. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 15. [Google Scholar] [CrossRef] [Green Version]
- Schon, C.A.; Gordon, L.; Holzl, N.; Milani, M.; Paal, P.; Zafren, K. Determination of Death in Mountain Rescue: Recommendations of the International Commission for Mountain Emergency Medicine (ICAR MedCom). Wilderness Environ. Med. 2020, 31, 506–520. [Google Scholar] [CrossRef]
- Maeder, M.B.; Lischke, V.; Berner, A.; Reisten, O.; Pietsch, U.; Pasquier, M. A patient with polytrauma, hypothermia and cardiac arrest after delayed mountain rescue. CMAJ 2018, 190, E1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, M.; Hugli, O.; Paal, P.; Darocha, T.; Blancher, M.; Husby, P.; Silfvast, T.; Carron, P.N.; Rousson, V. Hypothermia outcome prediction after extracorporeal life support for hypothermic cardiac arrest patients: The HOPE score. Resuscitation 2018, 126, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swol, J.; Darocha, T.; Paal, P.; Brugger, H.; Podsiadlo, P.; Kosinski, S.; Puslecki, M.; Ligowski, M.; Pasquier, M. Extracorporeal Life Support in Accidental Hypothermia with Cardiac Arrest-A Narrative Review. ASAIO J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Putzer, G.; Braun, P.; Zimmermann, A.; Pedross, F.; Strapazzon, G.; Brugger, H.; Paal, P. LUCAS compared to manual cardiopulmonary resuscitation is more effective during helicopter rescue-a prospective, randomized, cross-over manikin study. Am. J. Emerg. Med. 2013, 31, 384–389. [Google Scholar] [CrossRef]
- Gordon, L.; Paal, P.; Ellerton, J.A.; Brugger, H.; Peek, G.J.; Zafren, K. Delayed and intermittent CPR for severe accidental hypothermia. Resuscitation 2015, 90, 46–49. [Google Scholar] [CrossRef]
- Putzer, G.; Braun, P.; Martini, J.; Niederstatter, I.; Abram, J.; Lindner, A.K.; Neururer, S.; Mulino, M.; Glodny, B.; Helbok, R.; et al. Effects of head-up vs. supine CPR on cerebral oxygenation and cerebral metabolism—A prospective, randomized porcine study. Resuscitation 2018, 128, 51–55. [Google Scholar] [CrossRef]
- Aslam, A.F.; Aslam, A.K.; Vasavada, B.C.; Khan, I.A. Hypothermia: Evaluation, electrocardiographic manifestations, and management. Am. J. Med. 2006, 119, 297–301. [Google Scholar] [CrossRef]
- Giesbrecht, G.G.; Bristow, G.K.; Uin, A.; Ready, A.E.; Jones, R.A. Effectiveness of three field treatments for induced mild (33.0 °C) hypothermia. J. Appl. Physiol. 1987, 63, 2375–2379. [Google Scholar] [CrossRef]
- Kornberger, E.; Schwarz, B.; Lindner, K.H.; Mair, P. Forced air surface rewarming in patients with severe accidental hypothermia. Resuscitation 1999, 41, 105–111. [Google Scholar] [CrossRef]
- Anadolli, V.; Markovic-Bozic, J.; Benedik, J. Management of hypothermic submersion associated cardiac arrest in a 5-year-old child: A case report. Resusc. Plus 2021, 8, 100161. [Google Scholar] [CrossRef]
- Vince, S.C.; Flint, N.J.; Hall, A.P. A novel non-invasive warming technique in severe accidental hypothermia. Resuscitation 2008, 77, 144–145. [Google Scholar] [CrossRef]
- Cocchi, M.N.; Giberson, B.; Donnino, M.W. Rapid rewarming of hypothermic patient using arctic sun device. J. Intensive Care Med. 2012, 27, 128–130. [Google Scholar] [CrossRef]
- Roser, M.; Martens, F.; Storm, C. Iceman Survived due to Cooling Device. ISRN Cardiol. 2011, 2011, 617912. [Google Scholar] [CrossRef] [Green Version]
- Gregory, J.S.; Bergstein, J.M.; Aprahamian, C.; Wittmann, D.H.; Quebbeman, E.J. Comparison of three methods of rewarming from hypothermia: Advantages of extracorporeal blood warming. J. Trauma 1991, 31, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Willekes, T.; Naunheim, R.; Lasater, M. A novel method of intravascular temperature modulation to treat severe hypothermia. Emerg. Med. J. 2006, 23, e56. [Google Scholar] [CrossRef] [Green Version]
- Ban, L.H.; Leone, M.; Blasco, V.; Visintini, P.; Antonini, F.; Bisbal, M.; Albanese, J.; Martin, C. A novel intravascular rewarming method to treat severe hypothermia. Eur. J. Emerg. Med. 2008, 15, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.Y.; Lundbye, J. Endovascular catheter as a rewarming method for accidental hypothermia. Ther. Hypothermia Temp. Manag. 2012, 2, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Camp-Rogers, T.; Murphy, G.; Dean, A.; Gunnerson, K.; Rossler, D.; Kurz, M.C. Therapeutic hypothermia after profound accidental hypothermia and cardiac arrest. Am. J. Emerg. Med. 2012, 30, 387.e5–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Kiridume, K.; Hifumi, T.; Kawakita, K.; Okazaki, T.; Hamaya, H.; Shinohara, N.; Abe, Y.; Takano, K.; Hagiike, M.; Kuroda, Y. Clinical experience with an active intravascular rewarming technique for near-severe hypothermia associated with traumatic injury. J. Intensive Care 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laniewicz, M.; Lyn-Kew, K.; Silbergleit, R. Rapid endovascular warming for profound hypothermia. Ann. Emerg. Med. 2008, 51, 160–163. [Google Scholar] [CrossRef]
- Hall, K.N.; Syverud, S.A. Closed thoracic cavity lavage in the treatment of severe hypothermia in human beings. Ann. Emerg. Med. 1990, 19, 204–206. [Google Scholar] [CrossRef]
- Turtiainen, J.; Halonen, J.; Syvaoja, S.; Hakala, T. Rewarming a patient with accidental hypothermia and cardiac arrest using thoracic lavage. Ann. Thorac. Surg. 2014, 97, 2165–2166. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P. Use of peritoneal dialysis in severely hypothermic patients. Ann. Emerg. Med. 1986, 15, 104–105. [Google Scholar] [CrossRef]
- Gruber, E.; Beikircher, W.; Pizzinini, R.; Marsoner, H.; Pornbacher, M.; Brugger, H.; Paal, P. Non-extracorporeal rewarming at a rate of 6.8 °C per hour in a deeply hypothermic arrested patient. Resuscitation 2014, 85, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Lomas, A.; Drummond, I.; McGugan, E. Survival after 5-h resuscitation attempt for hypothermic cardiac arrest using CVVH for extracorporeal rewarming. Nephrol. Dial. Transplant. 2009, 24, 1054–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spooner, K.; Hassani, A. Extracorporeal rewarming in a severely hypothermic patient using venovenous haemofiltration in the accident and emergency department. J. Accid. Emerg. Med. 2000, 17, 422–424. [Google Scholar] [CrossRef]
- Hughes, A.; Riou, P.; Day, C. Full neurological recovery from profound (18.0 °C) acute accidental hypothermia: Successful resuscitation using active invasive rewarming techniques. Emerg. Med. J. 2007, 24, 511–512. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, S.; Shimomatsuya, T.; Kobuchi, T.; Nakajima, M.; Amaya, H.; Konishi, S.; Shiraishi, S.; Ono, S.; Maruhashi, K. Severe accidental hypothermia successfully treated by rewarming strategy using continuous venovenous hemodiafiltration system. J. Trauma 2007, 62, 775–776. [Google Scholar] [CrossRef]
- Carr, M.E., Jr.; Wolfert, A.I. Rewarming by hemodialysis for hypothermia: Failure of heparin to prevent DIC. J. Emerg. Med. 1988, 6, 277–280. [Google Scholar] [CrossRef]
- Owda, A.; Osama, S. Hemodialysis in management of hypothermia. Am. J. Kidney Dis. 2001, 38, E8. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Theakston, K.D.; Butler, R.; Suri, R.S. Treatment of severe accidental hypothermia with intermittent hemodialysis. CJEM 2009, 11, 174–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caluwe, R.; Vanholder, R.; Dhondt, A. Hemodialysis as a treatment of severe accidental hypothermia. Artif. Organs 2010, 34, 237–239. [Google Scholar] [CrossRef]
- Rahman, S.; Rubinstein, S.; Singh, J.; Samih, M.; Balsam, L. Early use of hemodialysis for active rewarming in severe hypothermia: A case report and review of literature. Ren. Fail. 2012, 34, 784–788. [Google Scholar] [CrossRef] [Green Version]
- Denk, J.A.; Michel, E.; Clark, A.J.; Thinh Pham, D.; Mehta, C.K. Veno-Venous Extracorporeal Rewarming Using Dual-Lumen Cannula in Accidental Hypothermia. ASAIO J. 2021. [Google Scholar] [CrossRef]
- Waters, D.J.; Belz, M.; Lawse, D.; Ulstad, D. Portable cardiopulmonary bypass: Resuscitation from prolonged ice-water submersion and asystole. Ann. Thorac. Surg. 1994, 57, 1018–1019. [Google Scholar] [CrossRef]
- Bjertnaes, L.J.; Hindberg, K.; Naesheim, T.O.; Suborov, E.V.; Reierth, E.; Kirov, M.Y.; Lebedinskii, K.M.; Tveita, T. Rewarming From Hypothermic Cardiac Arrest Applying Extracorporeal Life Support: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 641633. [Google Scholar] [CrossRef] [PubMed]
- Friess, J.O.; Gisler, F.; Kadner, A.; Jenni, H.; Eberle, B.; Erdoes, G. The use of minimal invasive extracorporeal circulation for rewarming after accidental hypothermia and circulatory arrest. Acta Anaesthesiol. Scand. 2021, 65, 633–638. [Google Scholar] [CrossRef]
- Marino, R.B.; Argudo, E.; Ribas, M.; Robledo, X.R.; Martinez, I.S.; Strapazzon, G.; Darocha, T. Anesthetic Management of Successful Extracorporeal Resuscitation After Six Hours of Cardiac Arrest Due to Severe Accidental Hypothermia. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T.; Gladki, M.; Skalski, J. Successful resuscitation from accidental hypothermia of 11.8 °C: Where is the lower bound for human beings? Eur. J. Cardiothorac. Surg. 2020, 58, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Darocha, T.; Svendsen, O.S.; Kosinski, S.; Silfvast, T.; Blancher, M.; Sawamoto, K.; Pasquier, M. Outcomes of patients suffering unwitnessed hypothermic cardiac arrest rewarmed with extracorporeal life support: A systematic review. Artif. Organs 2021, 45, 222–229. [Google Scholar] [CrossRef]
- Ohbe, H.; Isogai, S.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Extracorporeal membrane oxygenation improves outcomes of accidental hypothermia without vital signs: A nationwide observational study. Resuscitation 2019, 144, 27–32. [Google Scholar] [CrossRef]
- Carlsen, A.W.; Skjaervold, N.K.; Berg, N.J.; Karlsen, O.; Gunnarson, E.; Wahba, A. Swedish-Norwegian co-operation in the treatment of three hypothermia victims: A case report. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 73. [Google Scholar] [CrossRef] [Green Version]
- Niehaus, M.T.; Pechulis, R.M.; Wu, J.K.; Frei, S.; Hong, J.J.; Sandhu, R.S.; Greenberg, M.R. Extracorporeal Membrane Oxygenation (ECMO) for Hypothermic Cardiac Deterioration: A Case Series. Prehosp. Disaster Med. 2016, 31, 570–571. [Google Scholar] [CrossRef]
- Winkler, B.; Jenni, H.J.; Gygax, E.; Schnuriger, B.; Seidl, C.; Erdoes, G.; Kadner, A.; Carrel, T.; Eberle, B. Minimally invasive extracorporeal circulation resuscitation in hypothermic cardiac arrest. Perfusion 2016, 31, 489–494. [Google Scholar] [CrossRef]
- Weuster, M.; Haneya, A.; Panholzer, B.; Kluter, T.; van der Brelie, M.; van Laak, U.; Cremer, J.; Haake, N. The Use of Extracorporeal Membrane Oxygenation Systems in Severe Accidental Hypothermia after Drowning: A Centre Experience. ASAIO J. 2016, 62, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Darocha, T.; Podsiadlo, P.; Polak, M.; Hymczak, H.; Krzych, L.; Skalski, J.; Witt-Majchrzak, A.; Nowak, E.; Toczek, K.; Waligorski, S.; et al. Prognostic Factors for Nonasphyxia-Related Cardiac Arrest Patients Undergoing Extracorporeal—HELP Registry Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 365–371. [Google Scholar] [CrossRef]
- Ruttmann, E.; Weissenbacher, A.; Ulmer, H.; Muller, L.; Hofer, D.; Kilo, J.; Rabl, W.; Schwarz, B.; Laufer, G.; Antretter, H.; et al. Prolonged extracorporeal membrane oxygenation-assisted support provides improved survival in hypothermic patients with cardiocirculatory arrest. J. Thorac. Cardiovasc. Surg. 2007, 134, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.L.; Turtle, M.R.; Chambers, D.J.; James, D.N.; Newman, S.; Venn, G.E. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcome in patients undergoing coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1996, 111, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- Pynnonen, L.; Falkenbach, P.; Kamarainen, A.; Lonnrot, K.; Yli-Hankala, A.; Tenhunen, J. Therapeutic hypothermia after cardiac arrest—Cerebral perfusion and metabolism during upper and lower threshold normocapnia. Resuscitation 2011, 82, 1174–1179. [Google Scholar] [CrossRef]
- Pasquier, M.; Rousson, V.; Darocha, T.; Bouzat, P.; Kosinski, S.; Sawamoto, K.; Champigneulle, B.; Wiberg, S.; Wanscher, M.C.J.; Brodmann Maeder, M.; et al. Hypothermia outcome prediction after extracorporeal life support for hypothermic cardiac arrest patients: An external validation of the HOPE score. Resuscitation 2019, 139, 321–328. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.E.; Freer, L.; Grissom, C.K.; Auerbach, P.S.; Rodway, G.W.; Cochran, A.; Giesbrecht, G.G.; McDevitt, M.; Imray, C.H.; Johnson, E.L.; et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Frostbite: 2019 Update. Wilderness Environ. Med. 2019, 30, S19–S32. [Google Scholar] [CrossRef] [Green Version]
- Zafren, K. Nonfreezing Cold Injury (Trench Foot). Int. J. Environ. Res. Public Health 2021, 18, 10482. [Google Scholar] [CrossRef]
- Regli, I.B.; Strapazzon, G.; Falla, M.; Oberhammer, R.; Brugger, H. Long-Term Sequelae of Frostbite—A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 9655. [Google Scholar] [CrossRef]
- Richardson, A.S.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef]
- Brugger, H.; Paal, P.; Zafren, K.; Strapazzon, G.; Musi, M.E. Are mobile ECMO teams necessary to treat severe accidental hypothermia? Resuscitation 2021, 158, 301–302. [Google Scholar] [CrossRef]
- Riera, J.; Argudo, E.; Ruiz-Rodriguez, J.C.; Rodriguez-Lecoq, R.; Ferrer, R. Full neurological recovery 6 h after cardiac arrest due to accidental hypothermia. Lancet 2020, 395, e89. [Google Scholar] [CrossRef]
- Darocha, T.; Kosinski, S.; Jarosz, A.; Drwila, R. Extracorporeal Rewarming from Accidental Hypothermia of Patient with Suspected Trauma. Medicine 2015, 94, e1086. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.; Kosinski, S.; Darocha, T.; Paal, P.; Galazkowski, R.; Hymczak, H.; Drwila, R. Problems and Pitfalls of Qualification for Extracorporeal Rewarming in Severe Accidental Hypothermia. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1693–1697. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Monika, B.M.; Martin, D.; Balthasar, E.; Stefan, L.; Roland, D.; Lars, E.; Luca, M.; Markus, N.; Mario, S.; Eva, R.K.; et al. The Bernese Hypothermia Algorithm: A consensus paper on in-hospital decision-making and treatment of patients in hypothermic cardiac arrest at an alpine level 1 trauma centre. Injury 2011, 42, 539–543. [Google Scholar] [PubMed]
- Austin, M.A.; Maynes, E.J.; O’Malley, T.J.; Mazur, P.; Darocha, T.; Entwistle, J.W.; Guy, T.S.; Massey, H.T.; Morris, R.J.; Tchantchaleishvili, V. Outcomes of Extracorporeal Life Support Use in Accidental Hypothermia: A Systematic Review. Ann. Thorac. Surg. 2020, 110, 1926–1932. [Google Scholar] [CrossRef]
- Saczkowski, R.; Kuzak, N.; Grunau, B.; Schulze, C. Extracorporeal life support rewarming rate is associated with survival with good neurological outcome in accidental hypothermia. Eur. J. Cardiothorac. Surg. 2021, 59, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Engelman, R.; Baker, R.A.; Likosky, D.S.; Grigore, A.; Dickinson, T.A.; Shore-Lesserson, L.; Hammon, J.W. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical Practice Guidelines for Cardiopulmonary Bypass—Temperature Management during Cardiopulmonary Bypass. Ann. Thorac. Surg. 2015, 100, 748–757. [Google Scholar] [CrossRef]
- Kratzert, W.B.; Gudzenko, V. ECPR or Do Not ECPR-Who and How to Choose. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Saczkowski, R.S.; Brown, D.J.A.; Abu-Laban, R.B.; Fradet, G.; Schulze, C.J.; Kuzak, N.D. Prediction and risk stratification of survival in accidental hypothermia requiring extracorporeal life support: An individual patient data meta-analysis. Resuscitation 2018, 127, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Bird, S.B.; Katayama, Y.; Maekawa, K.; Uemura, S.; Tanno, K.; Narimatsu, E. Outcome from severe accidental hypothermia with cardiac arrest resuscitated with extracorporeal cardiopulmonary resuscitation. Am. J. Emerg. Med. 2014, 32, 320–324. [Google Scholar] [CrossRef]
- Huang, M.; Shoskes, A.; Migdady, I.; Amin, M.; Hasan, L.; Price, C.; Uchino, K.; Choi, C.W.; Hernandez, A.V.; Cho, S.M. Does Targeted Temperature Management Improve Neurological Outcome in Extracorporeal Cardiopulmonary Resuscitation (ECPR)? J. Intensive Care Med. 2021, 8850666211018982. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullen, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Casadio, M.C.; Coppo, A.; Vargiolu, A.; Villa, J.; Rota, M.; Avalli, L.; Citerio, G. Organ donation in cardiac arrest patients treated with extracorporeal CPR: A single centre observational study. Resuscitation 2017, 118, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Walpoth, B.H.; Maeder, M.B.; Courvoisier, D.S.; Meyer, M.; Cools, E.; Darocha, T.; Blancher, M.; Champly, F.; Mantovani, L.; Lovis, C.; et al. Hypothermic Cardiac Arrest—Retrospective cohort study from the International Hypothermia Registry. Resuscitation 2021, 167, 58–65. [Google Scholar] [CrossRef] [PubMed]
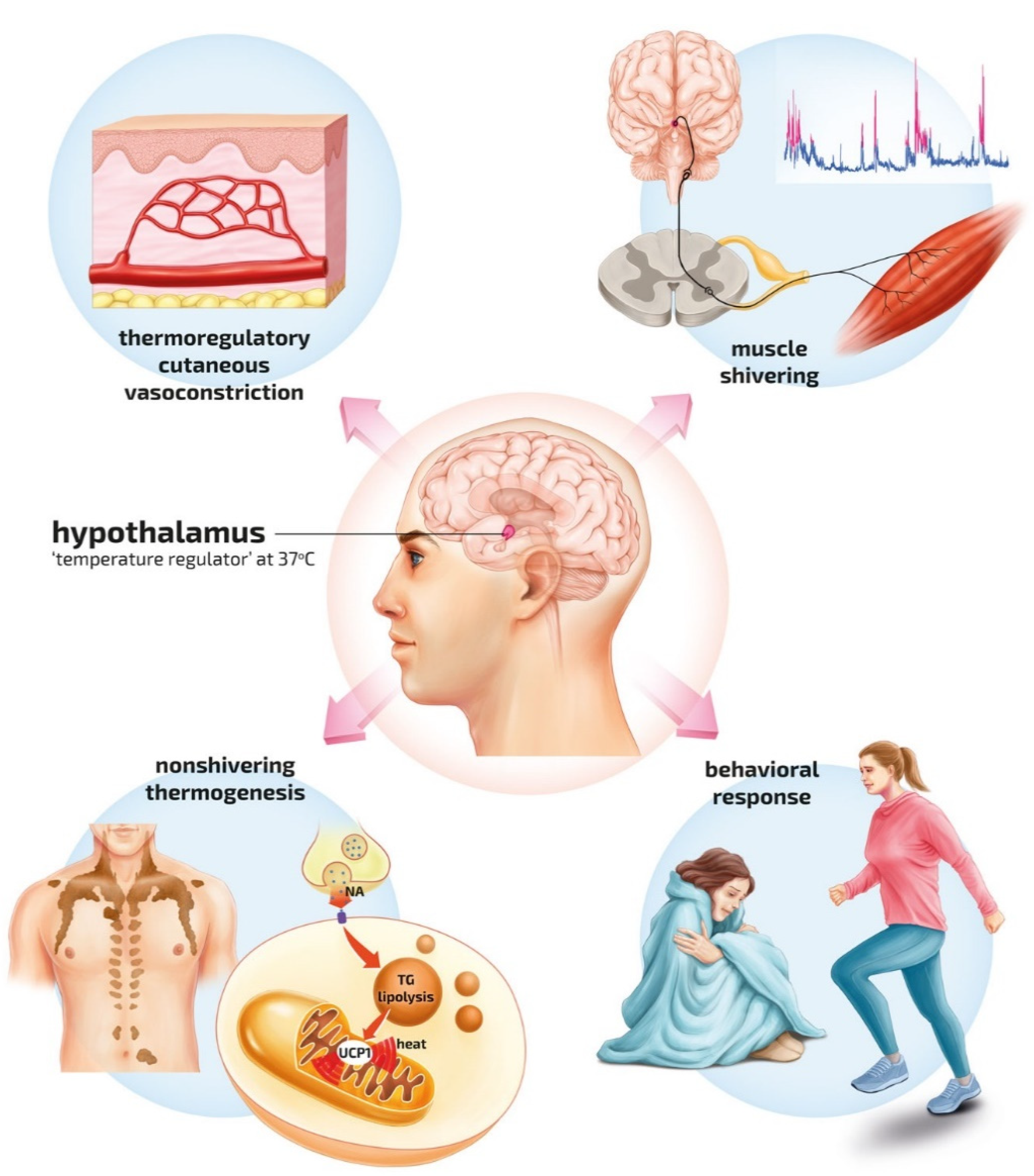
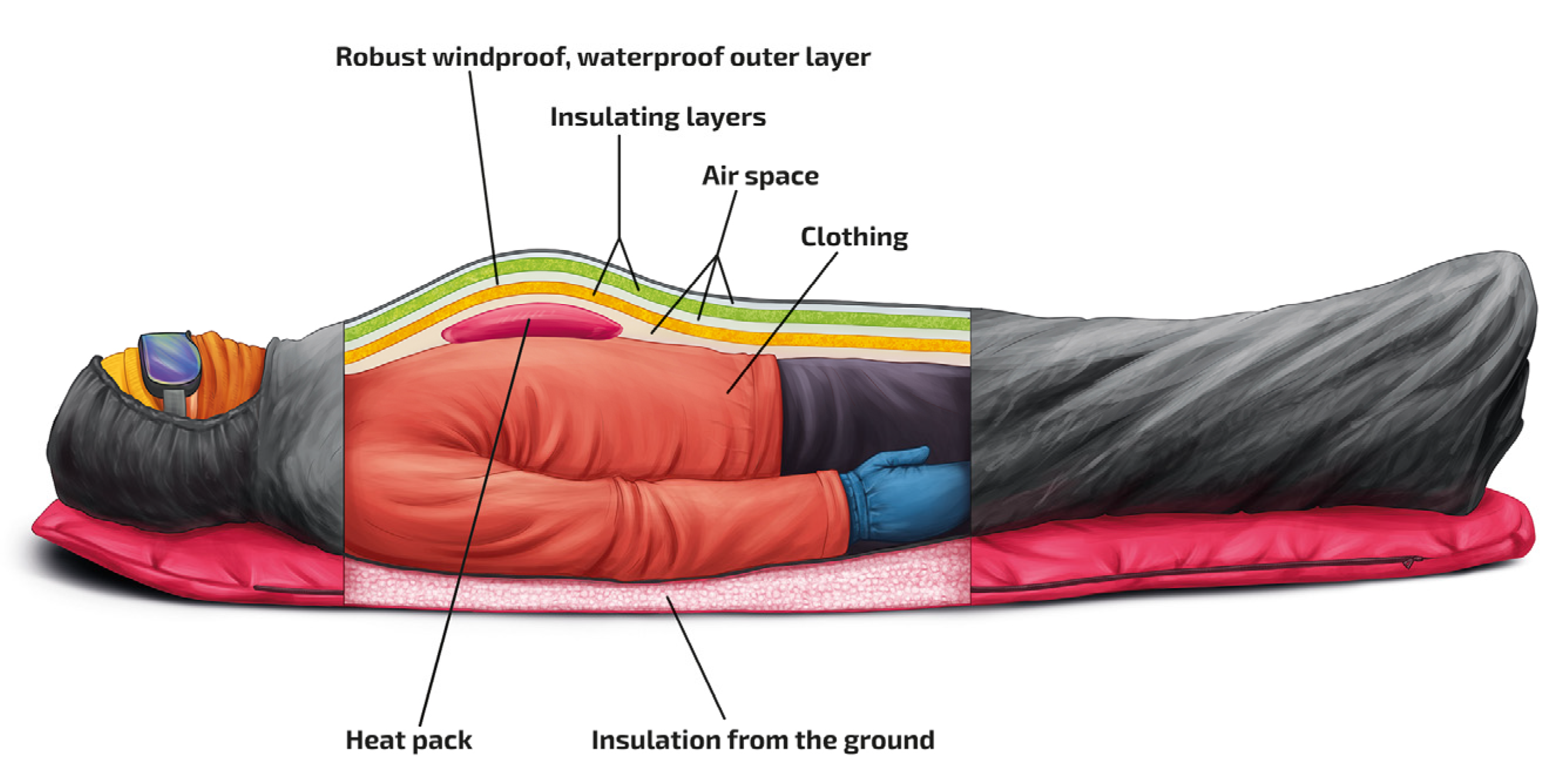

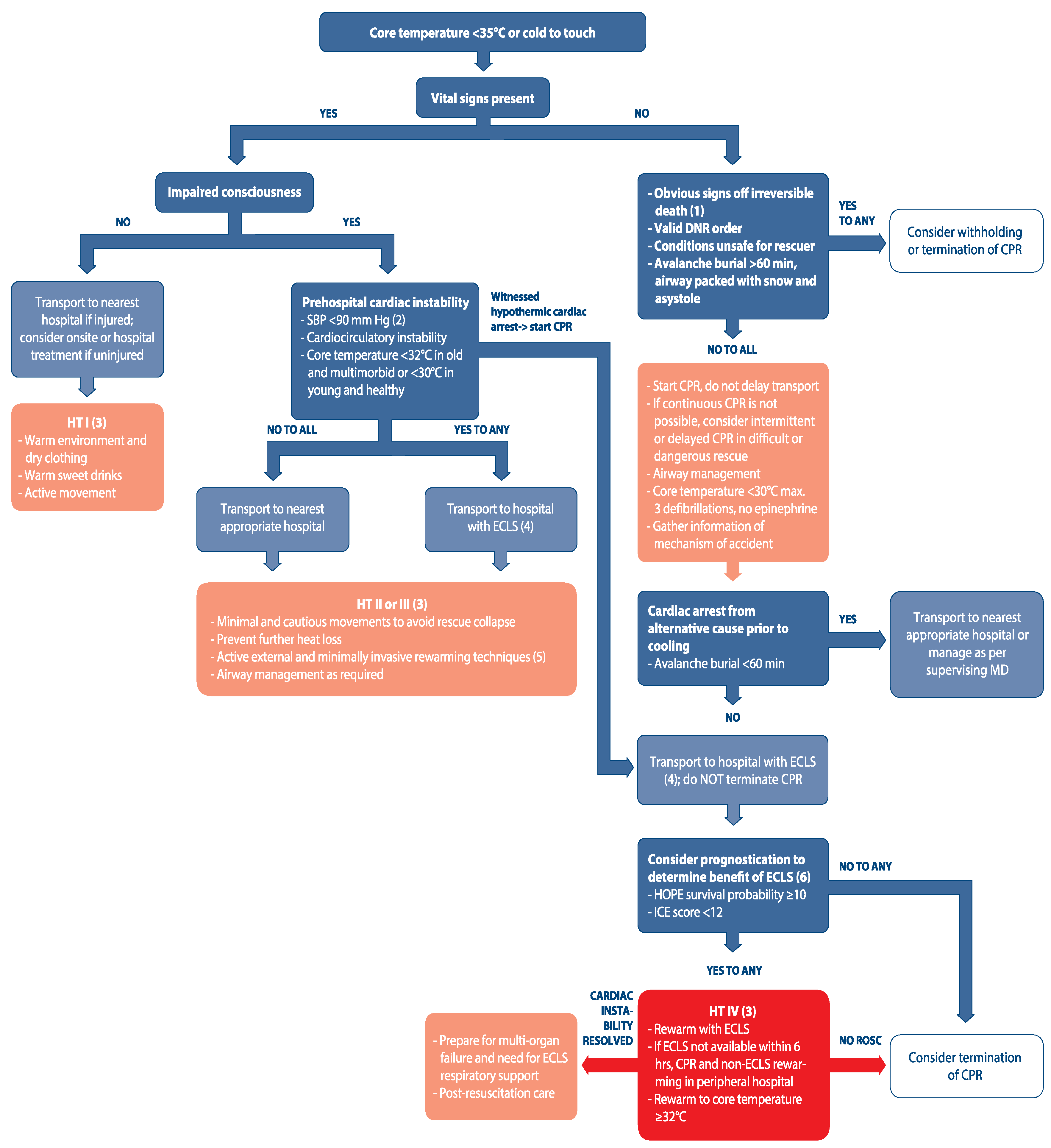
| Impaired Thermoregulation | Decreased Heat Production | Increased Heat Loss |
|---|---|---|
| Central nervous system failure | Endocrine failure | Dermatologic lllness |
| Anorexia nervosa | Alcoholic or diabetic ketoacidosis | Burns |
| Stroke | Hypoadrenalism | Induced vasodilation |
| Traumatic brain injury | Hypopituitarism | Medications and toxins |
| Hypothalamic dysfunction | Lactic acidosis | |
| Metabolic failure | Iatrogenic | |
| Neoplasm | Insufficient fuel | Emergency childbirth (possibly without prevention of hypothermia) |
| Parkinson’s disease | Extreme physical exertion | Cold infusions |
| Pharmacologic effects (anaesthetic drugs) | Hypoglycaemia | Heat-stroke treatment |
| Stroke, haemorrhagic or ischaemic | Malnutrition | |
| Toxins | Other associated clinical states | |
| Neuromuscular compromise | Carcinomatosis | |
| Peripheral failure | Extremes of age | Cardiopulmonary disesae |
| Acute spinal cord transection | Impaired shivering | Major infections |
| Peipheral neuropathy | Inactivity | Multiple trauma |
| Shock |
| Stage | Clinical Findings | Estimated Core Temperature ( °C) |
|---|---|---|
| Hypothermia I (mild) | Conscious, shivering * | 35–32 °C |
| Hypothermia II (moderate) | Impaired consciousness *; may or may not be shivering | <32–28 °C |
| Hypothermia III (severe) | Unconscious *; vital signs present | <28 °C |
| Hypothermia IV (severe) | Apparent death; vital signs absent | Classically < 24 °C ** |
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
|---|---|---|---|---|
| Clinical findings 1 | “Alert” from AVPU | “Verbal” from AVPU | “Painful’’ or “Unconscious” from AVPU Vital signs present | “Unconscious” from AVPU AND No detectable vital signs 2 |
| Risk of cardiac arrest 3 | Low | Moderate | High | Hypothermic cardiac arrest |
| Oxygen according to ususal clinical practice, (goal: SpO2 > 94%) 4 | + | + | + | + |
| Carbohydrates | Warm sweet tea, sweet bars | Glucose IV/IO. 5 | Glucose IV/IO. 5 | − |
| Active movement | + | − 6 | − | − |
| Passive rewarming | + | + | + | + |
| Active rewarming | (+) | + | + | + |
| Cautious mobilisation/horizontal transport if possible | − | + | + | − |
| Defibrillation pads | - | + | + | + |
| Intubation | - | - | Consider | + |
| CPR | - | - | - | + |
| Defibrillation | - | - | - | + 7 |
| Drugs (CPR) | - | - | - | + 8 |
| Hospital with ECLS 9 | - | - | + | + |
| Type of Measurement | Characteristics | Limitations | Suitability for Core Temperature Measurement in Hypothermia | Feasible in Hospital (IH) or Out of Hospital (OH) | References |
|---|---|---|---|---|---|
| Touching the skin of torso torso |
|
| (+) | (OH)/IH | [57] |
| Temporal artery (infrared) |
|
| - | None | [50,56,58,59,60,61,62] |
| (Forehead) skin (infrared radiation, electronic thermistor, liquid crystal strip) |
|
| - | None | [47,50,54,63,64] |
| Temporal microwave thermometer |
|
| (+) | IH | [46] |
| Zero-heat-flux thermometer on the forehead. Deep tissue temperature is measured at the skin by an insulated temperature probe) |
|
| - | IH | [42,47,64,65,66] |
| Axillary (electronic device or glass thermometer +) |
|
| - | None | [50,54,56,60] |
| Tympanic (infrared radiation) |
|
| - | (IH) | [42,50,52,56,58,62,67,68,69] |
| Epitympanic (electronic thermistor) |
|
| + | OH/IH | [42,48,49,51,53,55,70,71] |
| Oral (electronic thermistor or glass thermometer +) |
|
| - | IH | [70] |
| Nasopharyngeal (electronic thermistor) |
|
| + | OH/IH | [72] |
| Gastrointestinal temperature (telemetry temperature sensor) |
|
| - | None | [50,72] |
| Oesophageal (electronic thermistor) |
|
| + | OH/IH | [42,48,54,56,63,73,74] |
| Bladder (electronic thermistor) |
|
| + | IH | [42,47,48,51,56,62,71] |
| Rectal (electronic thermistor or glass thermometer +) |
|
| + | IH | [42,48,51,54,56,63,71] |
| Pulmonary artery catheter (electronic thermistor) |
|
| + | IH | [42,47,48,54,56] |
| Brain temperature |
|
| + | IH | [48,51,54,63] |
| Rewarming Technique | Rewarming Rate | Notes & Controversies | Rewarming Complications |
|---|---|---|---|
| Passive Rewarming | |||
| Passive rewarming [3,92] | 0.5–4 °C /h (dependends on patient’s thermoregulatory function and metabolic reserves) | Protects from further heat loss and allows patient to self-rewarm. | Negligible in isolated mild hypothermia. For colder patients and those with secondary hypothermia or comorbidities, passive rewarming alone is not adequate. |
| Passive rewarming with active movement [93] | 1–5 °C /h | Exercise immediately after rescue increases afterdrop | Increased afterdrop could cause rescue collapse. |
| Active External Rewarming | |||
| Active rewarming including forced-air surface rewarming [94,95], heating pads, e.g. Arctic Sun® [96,97,98],warmed IV fluids (40 °C). | 0.5–4 °C /h | Protects from further heat loss, delivers external heat. Warmed IV fluids are not effective if used as the sole method of rewarming. | Similar to passive rewarming |
| Active Internal Rewarming | |||
| Bladder lavage [31,92,99] | Variable. Adds < 0.5 °C /h | Not recommended Rewarming is intermittent and slow because of small surface area. Poor control of infusate temperature | Negligible unless difficult catheterisation |
| Gastric lavage [31] | May add ~0.5–1 °C /h | Not recommended. Unacceptably high risk to benefit ratio | Potential for aspiration, fluid and electrolyte shifts |
| Intravascular catheter rewarming, e.g., CoolGuard® [36,100,101,102] Quattro® [103] Cool Line® [104] Innercool® [105] | Device specific (adds ~0.5–2.5 °C /h) | Uncertain indications for use. Potential beneficial for colder patients, especially those with comorbidites, with stable circulation | Potential for haemorrhage or thrombosis, potentially worsening arterial hypotension in unstable patients |
| Thoracic [106,107] or peritoneal lavage [108,109] | Variable, depending on tempearture and flow rate of paricardial irrigation. | May be useful in unstable patients when ECLS rewarming is not available. Very invasive. | Potential for haemorrhage, lung or bowel trauma, fluid and electrolyte shifts. Thoracic lavage may interfere with CPR |
| CRRT (including CVVHF, CVVHD, CVVHDF) [92,110,111,112,113] | Adds ~1.5–3 °C /h | Not recommended unless ECLS rewarming not available. Require adequate blood pressure. Heparinisation, citrate anticoagulation, or prostacyclin required | Problems rare. Local vascular complications. Air embolism. Arterial hypotension |
| Haemodialysis [92,114,115,116,117,118] | Adds ~2–3 °C /h | Patient must be able to increase cardiac output to perfuse the external circuit. Heparinisation required | Potential for arterial hypotension, haemorrhage, thrombosis, haemolysis, etc. |
| Veno-venous rewarming (usually with ECMO) [99,119] | ~4–10 °C /h | Provides no circulatory or ventilatory support in case of cardiac arrest. Patient must be able to increase cardiac output to perfuse the external circuit | Potential for arterial hypotension, haemorrhage, thrombosis, haemolysis, etc. |
| Extra-corporeal life support (ECLS; VA-ECMO, CPB including minimally invasive extracorporeal circulation (MiECC)) [83,120,121,122,123,124,125,126,127,128,129,130] | ~4–10 °C /h | Preferred rewarming method for patients in cardiac arrest. ECMO preferred over CPB. ECMO can use femoral route avoiding need for sternotomy. Can be used to treat post-rewarming pulmonary complications, such as ARDS. | Potential for haemorrhage and arterial hypotension, thrombosis, haemolysis, etc., as with all intravascular devices |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paal, P.; Pasquier, M.; Darocha, T.; Lechner, R.; Kosinski, S.; Wallner, B.; Zafren, K.; Brugger, H. Accidental Hypothermia: 2021 Update. Int. J. Environ. Res. Public Health 2022, 19, 501. https://doi.org/10.3390/ijerph19010501
Paal P, Pasquier M, Darocha T, Lechner R, Kosinski S, Wallner B, Zafren K, Brugger H. Accidental Hypothermia: 2021 Update. International Journal of Environmental Research and Public Health. 2022; 19(1):501. https://doi.org/10.3390/ijerph19010501
Chicago/Turabian StylePaal, Peter, Mathieu Pasquier, Tomasz Darocha, Raimund Lechner, Sylweriusz Kosinski, Bernd Wallner, Ken Zafren, and Hermann Brugger. 2022. "Accidental Hypothermia: 2021 Update" International Journal of Environmental Research and Public Health 19, no. 1: 501. https://doi.org/10.3390/ijerph19010501
APA StylePaal, P., Pasquier, M., Darocha, T., Lechner, R., Kosinski, S., Wallner, B., Zafren, K., & Brugger, H. (2022). Accidental Hypothermia: 2021 Update. International Journal of Environmental Research and Public Health, 19(1), 501. https://doi.org/10.3390/ijerph19010501










