Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae
Abstract
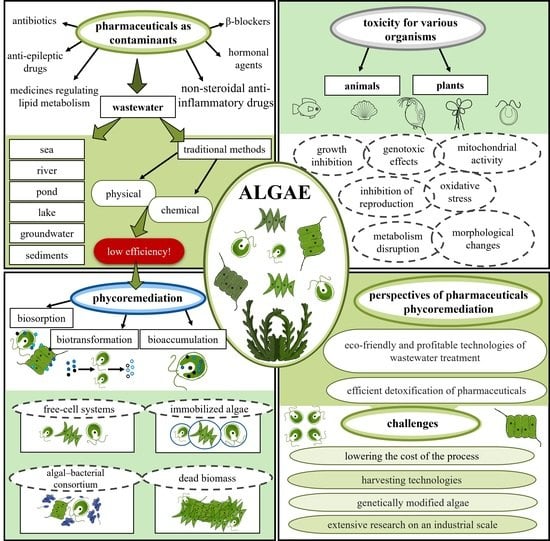
1. Introduction
2. Pharmaceuticals as Contaminants of Emerging Concern
3. Conventional Wastewater Treatment and Pharmaceutical Removal Methods
4. Phycoremediation of Pharmaceuticals in Wastewater
|
Type of Contamination | Substance | Algae Species | Removal Rate | Time | References |
|---|---|---|---|---|---|
| Antibiotic | Enrofloxacin (ENR) | Platymonas subcordiformis Isochrysis galbana Scenedesmus obliquus Chlamydomonas mexicana Chlorella vulgaris Ourococcus multisporus Micractinium resseri | 75–85% *,1 40–70% *,1 23% 25% 26% 20% 26% | 11 d 11 d 11 d 11 d 11 d | [179] [179] [180] [180] [180] [180] [180] |
| Ciprofloxacin hydrochloride (CIP) | Platymonas subcordiformis Isochrysis galbana Chlamydomonas mexicana | 65–85% *,1 40–76% *,1 13–56% 2 | 11 d | [179] [179] [180] | |
| 7-amino cephalosporanic acid (7-ACA) | Chlorella sp. Cha-01 Chlamydomonas sp. Tai-03 Mychonastes sp. YL-02 | >70% 70% * 65% * | 24 h 24 h 24 h | [181] [181] [181] | |
| Cefradine (CFD) | Chlamydomonas reinhardtii Chlorella pyrenoidosa | 5–14% 41% | 8 h 24 h | [182] [183] | |
| Amoxicillin | Chlorella pyrenoidosa | 91% | 6 h | [183] | |
| Clarithromycine | A mixed population of wild freshwater green algal species (Dictyosphaerium) | 90% | 7 d | [184] | |
| NSAID | Ibuprofen | Chlorella pyrenoidosa Chlorella sorokiniana Nannochloropsis sp. Scenedesmus obliquus | 29–31% 100% * 51–100% - | 42 d 31 d 10 d - | [185] [186] [187] [188] |
| Diclofenac | Chlorella sorokiniana Chlorella sorokiniana Chlorella vulgaris Picocystis sp. Graesiella sp. Scenedesmus obliquus | 40–60% 30% 22% 73%, 43% and 25% (25, 50 and 100 mg L−1) 52%, 28% and 24% (25, 50 and 100 mg L−1) 79% | 31 d 9 d 9 d 9 d | [186] [189] [189] [189] [190] [189] | |
| Naproxen | Scenedesmus quadricauda | 59%, 73%, 2% (1, 10 and 100 mg L−1 | 30 d | [109] | |
| Paracetamol | Chlorella sorokiniana Chlorella sorokiniana Nannochloropsis sp | 100% * >67% from 50.5 to 44.4 μg mL−1 | 31 d 8–9 d 24 h | [186] [191] [187] | |
| β-blocker | Atenolol | A mixed population of wild freshwater green algal species (Dictyosphaerium) | 99% | 7 d | [184] |
| Bisoprolol | A mixed population of wild freshwater green algal species (Dictyosphaerium) | 97% | 7 d | [184] | |
| Metoprolol | A mixed population of wild freshwater green algal species (Dictyosphaerium)Chlorella sorokiniana | 99% 100% * | 7 d 31 d | [184] [186] | |
| Other drug | Alfuzosin Atracurium Bupropion Citalopram Clonazepam Dicycloverin Diltiazem Diphenhydramin Hydroxyzine Memantin Miconazole Pizotifen Terbutalin | A mixed population of wild freshwater green algal species (Dictyosphaerium) | 64% 97% 93% 98% 88% 71% 94% 89% 87% 81% 65% 80% 98% | 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d 7 d | [184] |
| Carbamazepine | Chlorella sorokiniana | 30% | 7 d | [186] | |
| Trimethoprim | Chlorella sorokiniana | 60% | 7 d | [186] | |
| Salicylic acid | Chlorella sorokiniana | >73% | 8-9 d | [191] |
4.1. Mechanisms of Phycoremediation
4.2. Selected Factors Affecting Phycoremediation Efficiency
4.2.1. Light
4.2.2. pH Value
4.2.3. Temperature
4.2.4. Other Factors
5. Algae-Based Remediation Systems
| Cultivation System | Mixing | Temperature | Gas Exchange | Limitations | Advantages | References |
|---|---|---|---|---|---|---|
| Open systems | ||||||
| Open ponds | Paddle wheel | None | Limited, through surface aeration | Less control over culturing conditions; temperature fluctuations; poor light utilization by the cells; inefficient stirring; diffusion of carbon dioxide to the atmosphere; lower biomass productivity; risk of contamination; large land space requirement | Simple design; cost-efficient; low investment costs; not difficult to maintain | [256] |
| Closed systems | ||||||
| Vertical column photobioreactors(bubble column photobioreactors and airlift columns) | Airlift or bubble | - | Open gas exchange at head space | Expensive construction materials; limited scale-up opportunities due to design constraints and inhomogeneous distribution of light inside the culture; productivity negatively affected by light-deprived zones; limited surface area for illumination; shading effect issues; photosynthetic efficiency depends on gas flow rate | Efficient mixing; high volumetric mass transfer rates; relatively homogenous culture environment; low photoinhibition; controllable growth conditions; lack of moving parts; no internal structures | [260,263] |
| Stirred-tank photobioreactors | Mechanical agitator | Heat exchanger | Injection through sparger | Not cost-efficient; mechanical agitation requires extra energy; low surface-to-volume ratio; low harvesting efficiency; heating issues due to agitation | Appropriate light dispersion; appropriate heat and mass transfer; simple design; moderate biomass; low contamination issues; productive | [257,263] |
| Flat-panel photobioreactors | Airlift or bubble from bottoms or side or rotating mechanically through motor | Heat exchangecoils | Open gas exchange at head space | Requires many components; short light penetration depth; frequent fouling and clean up issues; not scalable; poor temperature regulation | Cost-efficient; low space requirement; high surface-to-volume ratio; high photosynthetic efficiency; low oxygen build-up | [255,257,263] |
| Horizontal tubular photobioreactors | Recirculation via pumps | Water spraying; shading; overlapping | Injection into feed | Large space requirement; high energy consumption; susceptible to photo inhibition; dissolved oxygen buildup; fouling due to algal growth; poor temperature regulation | Cost-efficient; harnessing sun light efficiently; suitable for outdoor cultivation; high surface-to-volume ratio; low hydrodynamic stress; good biomass productivity; low mutual shading effect | [257,263] |
| Helical-type photobioreactors | Centrifugal pump (injection from bottom) | Heat exchanger | - | Limited commercial use associated with shear stress; fouling on the inside of the reactor | High photosynthetic efficiency through the light dilution effect and light absorbing capacity; high CO2 transfer; balance between energy input and photosynthetic efficiency; low energy requirement; low mechanical stress to cells | [257,264] |
5.1. Free Cell Cultures and Immobilized Algae
5.2. Algal–Bacterial Consortiums
| Consortium | Class of Compounds | Compound | Cultivation System | Removal Rate | Contaminated Matrix | References |
|---|---|---|---|---|---|---|
| Pharmaceuticals | ||||||
| Chlorella vulgaris with heterotrophs | Antibiotics | Tetracycline | High-rate algal ponds | 69% | Urban wastewater | [226] |
| Chlorella sp., Pseudomonas aeuroginosa, Pseudominas sp. with Stenotrophomonas | A/A A/A NSAID NSAID | Paracetamol P-aminophenol Ketoprofen Salycilic acid | Stirred-tank packed-bed reactor | 100% 100% 98% 95% | Urban wastewater | [295] |
| Artemia sp., Spirulina sp. with bacterial consortium | NSAID | Ketoprofen | 5 mM | Wastewater effluents | [296] | |
| Algal–bacterial consortium from high-rate algal ponds | NSAID NSAID NSAID | Ibuprofen Naproxen Salicylic acid Triclosan Propylparaben | Photobioreactor operating at a hydraulic retention time | 94% 52% 98% 100% 100% | Urban wastewater | [292] |
| Nutrients | ||||||
| Chlorella vulgaris with Bacillus licheniformis and Microcystis aeruginosa with Bacillus licheniformis | - | TDN TDP COD | Reactor (conical flask) | 89% 80% 87% | Synthetic wastewater | [288] |
| Scenedesmus quadricauda with bacteria from nitrogen-enriched activated sludge | - | NH4+ | 100% | Synthetic wastewater | [289] | |
| Chlorella vulgaris with bacteria | - | P DOC NH4+ | Tabular photobioreactor | 98% 26% 97% | Municipal wastewater | [297] |
| Scenedesmus sp. with bacteria | - | COD TN TP | 92% 95% 98% | Municipal wastewater | [298] | |
| Chlamydomonas and Euglena with cyanobacteria, Microcystis aeruginosa | COD TN NH4+ TP BOD5 | Waste stabilization pond | 78% 87% 99% 97% 89% | Domestic wastewater | [299] | |
| Chlorella vulgaris with bacteria | - | N TP COD | 100% 100% 90–95% | Synthetically made municipal wastewater | [300] | |
| Metals | ||||||
| Ulothrix sp. with bacteria consortium | - | Cu Ni Mn Zn | Laboratory-scale photo-rotating biological contactor | 50% 50% 40–45% 35% | Drainage wastewater | [11] |
| Chlorella sp., Chlorella sp. and Scenedesmus obliquus with Rhodococcus sp. and Kibdelosporangium | - | Cu Ni Mn | - | 62% 62% 70% | Industrial wastewater | [12] |
| Chlorella sorokiniana with Ralstonia basilensis | - | Cu | - | 8.5 mg/g | Synthetic wastewater | [301] |
5.3. Dead Biomass as a Biosorbent
6. Advantages, Challenges and Future Perspectives on Pharmaceutical Phycoremediation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Acetylsalicylic Acid, Paracetamol, Diclofenac, Ibuprofen and Naproxen towards Freshwater Invertebrates: A Review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic Effects of NSAIDs in Non-Target Species: A Review from the Perspective of the Aquatic Environment. Environ. Pollut. 2021, 273, 115891. [Google Scholar] [CrossRef]
- Abdel-Hameed, M.; Hameed, A.; Ebrahim, O. Biotechnological Potential Uses of Immobilized Algae. Int. J. Agric. Biol. 2007, 9, 183–192. [Google Scholar]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B.M. Potential Use of Green Algae as a Biosorbent for Hexavalent Chromium Removal from Aqueous Solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef]
- Awasthi, M.; Das, D.N. Heavy Metal Stress on Growth, Photosynthesis and Enzymatic Activities of Free and Immobilized Chlorella Vulgaris. Ann. Microbiol. 2005, 55, 1–7. [Google Scholar]
- Chekroun, K.B.; Baghour, M. The Role of Algae in Phytoremediation of Heavy Metals: A Review. J. Mater Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Erkaya, İ.A.; Arica, M.Y.; Akbulut, A.; Bayramoglu, G. Biosorption of Uranium(VI) by Free and Entrapped Chlamydomonas Reinhardtii: Kinetic, Equilibrium and Thermodynamic Studies. J. Radioanal. Nucl. Chem. 2014, 299, 1993–2003. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chew, K.W.; Chen, W.-H.; Chang, J.-S.; Show, P.L. Reuniting the Biogeochemistry of Algae for a Low-Carbon Circular Bioeconomy. Trends Plant Sci. 2021, 26, 729–740. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Campana, O.; Lubián, L.; Blasco, J. Calcium Alginate Immobilized Marine Microalgae: Experiments on Growth and Short-Term Heavy Metal Accumulation. Mar. Pollut. Bull. 2005, 51, 823–829. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Orandi, S.; Lewis, D.M.; Moheimani, N.R. Biofilm Establishment and Heavy Metal Removal Capacity of an Indigenous Mining Algal-Microbial Consortium in a Photo-Rotating Biological Contactor. J. Ind. Microbiol. Biotechnol. 2012, 39, 1321–1331. [Google Scholar] [CrossRef]
- Safonova, E.; Kvitko, K.v.; Iankevitch, M.i.; Surgko, L.f.; Afti, I.a.; Reisser, W. Biotreatment of Industrial Wastewater by Selected Algal-Bacterial Consortia. Eng. Life Sci. 2004, 4, 347–353. [Google Scholar] [CrossRef]
- Shamshad, I.; Khan, S.; Waqas, M.; Ahmad, N.; Ur-Rehman, K.; Khan, K. Removal and Bioaccumulation of Heavy Metals from Aqueous Solutions Using Freshwater Algae. Water Sci. Technol. 2015, 71, 38–44. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Hejna, M.; Kovanda, L.; Rossi, L.; Liu, Y. Mint Oils: In Vitro Ability to Perform Anti-Inflammatory, Antioxidant, and Antimicrobial Activities and to Enhance Intestinal Barrier Integrity. Antioxidants 2021, 10, 1004. [Google Scholar] [CrossRef]
- Jones, O.; Voulvoulis, N.; Lester, J. Human Pharmaceuticals in the Aquatic Environment a Review. Environ. Technol. 2001, 22, 1383–1394. [Google Scholar] [CrossRef]
- Taylor, D.; Senac, T. Human Pharmaceutical Products in the Environment—The “Problem” in Perspective. Chemosphere 2014, 115, 95–99. [Google Scholar] [CrossRef]
- Kress, H.G.; Baltov, A.; Basiński, A.; Berghea, F.; Castellsague, J.; Codreanu, C.; Copaciu, E.; Giamberardino, M.A.; Hakl, M.; Hrazdira, L.; et al. Acute Pain: A Multifaceted Challenge—The Role of Nimesulide. Curr. Med. Res. Opin. 2016, 32, 23–36. [Google Scholar] [CrossRef]
- Daughton, C.G. Cradle-to-Cradle Stewardship of Drugs for Minimizing Their Environmental Disposition While Promoting Human Health. I. Rationale for and Avenues toward a Green Pharmacy. Environ. Health Perspect. 2003, 111, 757–774. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital Effluents as a Source of Emerging Pollutants: An Overview of Micropollutants and Sustainable Treatment Options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of Pharmaceuticals, Cosmetics and Hormones in a Sewage Treatment Plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Pharmaceuticals and the Environment (PiE): Evolution and Impact of the Published Literature Revealed by Bibliometric Analysis. Sci. Total Environ. 2016, 562, 391–426. [Google Scholar] [CrossRef] [PubMed]
- Holm, G.; Snape, J.R.; Murray-Smith, R.; Talbot, J.; Taylor, D.; Sörme, P. Implementing Ecopharmacovigilance in Practice: Challenges and Potential Opportunities. Drug Saf. 2013, 36, 533–546. [Google Scholar] [CrossRef]
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A Review of the Pharmaceutical Exposome in Aquatic Fauna. Environ. Pollut. 2018, 239, 129–146. [Google Scholar] [CrossRef]
- Prichard, E.; Granek, E.F. Effects of Pharmaceuticals and Personal Care Products on Marine Organisms: From Single-Species Studies to an Ecosystem-Based Approach. Environ. Sci. Pollut. Res. 2016, 23, 22365–22384. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence Patterns of Pharmaceuticals in Water and Wastewater Environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment—Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: https://eur-lex.europa.eu/eli/reg/2003/1831/oj (accessed on 10 January 2022).
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional Ecology of Heavy Metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef]
- Mo, W.Y.; Chen, Z.; Leung, H.M.; Leung, A.O.W. Application of Veterinary Antibiotics in China’s Aquaculture Industry and Their Potential Human Health Risks. Environ. Sci. Pollut. Res. 2017, 24, 8978–8989. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.S.U.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.-I. Global Risk of Pharmaceutical Contamination from Highly Populated Developing Countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical Metabolites in the Environment: Analytical Challenges and Ecological Risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Carlsson, C.; Johansson, A.-K.; Alvan, G.; Bergman, K.; Kühler, T. Are Pharmaceuticals Potent Environmental Pollutants? Part II: Environmental Risk Assessments of Selected Pharmaceutical Excipients. Sci. Total Environ. 2006, 364, 88–95. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lai, F.Y.; Kim, H.-Y.; Thai, P.K.; Mueller, J.F.; Oh, J.-E. The First Application of Wastewater-Based Drug Epidemiology in Five South Korean Cities. Sci. Total Environ. 2015, 524–525, 440–446. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Baker, D.R. Estimation of Community-Wide Drugs Use via Stereoselective Profiling of Sewage. Sci. Total Environ. 2012, 423, 142–150. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Removal of Pharmaceuticals, Personal Care Products, Endocrine Disruptors and Illicit Drugs during Wastewater Treatment and Its Impact on the Quality of Receiving Waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of Pharmaceuticals and Personal Care Products in the Water Environment of Poland: A Review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of Hospital Effluents to the Load of Pharmaceuticals in Urban Wastewaters: Identification of Ecologically Relevant Pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Lindim, C.; van Gils, J.; Georgieva, D.; Mekenyan, O.; Cousins, I.T. Evaluation of Human Pharmaceutical Emissions and Concentrations in Swedish River Basins. Sci. Total Environ. 2016, 572, 508–519. [Google Scholar] [CrossRef]
- Petrović, M.; Škrbić, B.; Živančev, J.; Ferrando-Climent, L.; Barcelo, D. Determination of 81 Pharmaceutical Drugs by High Performance Liquid Chromatography Coupled to Mass Spectrometry with Hybrid Triple Quadrupole-Linear Ion Trap in Different Types of Water in Serbia. Sci. Total Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Analysis of 100 Pharmaceuticals and Their Degradates in Water Samples by Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2012, 1259, 148–157. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Y.-X.; Kang, J.; Gan, X.-M.; Peng, X.-Y.; Guo, J.-S.; Gao, X. A Preliminary Study on the Occurrence of Pharmaceutically Active Compounds in the River Basins and Their Removal in Two Conventional Drinking Water Treatment Plants in Chongqing, China. CLEAN Soil Air Water 2015, 43, 794–803. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ta, T.T. A Preliminary Study on the Occurrence of Pharmaceutically Active Compounds in Hospital Wastewater and Surface Water in Hanoi, Vietnam. CLEAN Soil Air Water 2014, 42, 267–275. [Google Scholar] [CrossRef]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and Quantification of Acidic Drug Residues in South African Surface Water Using Gas Chromatography-Mass Spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef]
- Subedi, B.; Codru, N.; Dziewulski, D.M.; Wilson, L.R.; Xue, J.; Yun, S.; Braun-Howland, E.; Minihane, C.; Kannan, K. A Pilot Study on the Assessment of Trace Organic Contaminants Including Pharmaceuticals and Personal Care Products from On-Site Wastewater Treatment Systems along Skaneateles Lake in New York State, USA. Water Res. 2015, 72, 28–39. [Google Scholar] [CrossRef]
- Cai, M.-Q.; Wang, R.; Feng, L.; Zhang, L.-Q. Determination of Selected Pharmaceuticals in Tap Water and Drinking Water Treatment Plant by High-Performance Liquid Chromatography-Triple Quadrupole Mass Spectrometer in Beijing, China. Environ. Sci. Pollut. Res. Int 2015, 22, 1854–1867. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lai, W.W.-P.; Tung, H.; Lin, A.Y.-C. Occurrence of Pharmaceuticals, Hormones, and Perfluorinated Compounds in Groundwater in Taiwan. Environ. Monit. Assess. 2015, 187, 256. [Google Scholar] [CrossRef] [PubMed]
- Lolić, A.; Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of Non-Steroidal Anti-Inflammatory and Analgesic Pharmaceuticals in Seawaters of North of Portugal: Occurrence and Environmental Risk. Sci. Total Environ. 2015, 508, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Yu, T.-H.; Lin, C.-F. Pharmaceutical Contamination in Residential, Industrial, and Agricultural Waste Streams: Risk to Aqueous Environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, Y.; Wang, C.; Zhang, C.; Ge, Y. Review of Arsenic Speciation, Toxicity and Metabolism in Microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Loraine, G.A.; Pettigrove, M.E. Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef]
- Snyder, S.A. Occurrence, Treatment, and Toxicological Relevance of EDCs and Pharmaceuticals in Water. Ozone Sci. Eng. 2008, 30, 65–69. [Google Scholar] [CrossRef]
- Lv, M.; Sun, Q.; Hu, A.; Hou, L.; Li, J.; Cai, X.; Yu, C.-P. Pharmaceuticals and Personal Care Products in a Mesoscale Subtropical Watershed and Their Application as Sewage Markers. J. Hazard. Mater. 2014, 280, 696–705. [Google Scholar] [CrossRef]
- Dai, X.-R.; Saha, C.K.; Ni, J.-Q.; Heber, A.J.; Blanes-Vidal, V.; Dunn, J.L. Characteristics of Pollutant Gas Releases from Swine, Dairy, Beef, and Layer Manure, and Municipal Wastewater. Water Res. 2015, 76, 110–119. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of Pharmaceuticals in the Lis River (Portugal): Sources, Fate and Seasonal Variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, Sources, and Fate of Pharmaceuticals in Aquatic Environment and Soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Nakada, N.; Hanamoto, S.; Inaba, M.; Katayama, H.; Do, A.T.; Nga, T.T.V.; Oguma, K.; Hayashi, T.; Takizawa, S. Pepper Mild Mottle Virus as an Indicator and a Tracer of Fecal Pollution in Water Environments: Comparative Evaluation with Wastewater-Tracer Pharmaceuticals in Hanoi, Vietnam. Sci. Total Environ. 2015, 506–507, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Hanson, M.L.; Solomon, K.R.; Brooks, B.W. Aquatic Plants Exposed to Pharmaceuticals: Effects and Risks. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2008; pp. 67–115. [Google Scholar]
- Ferrari, B.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological Impact of Pharmaceuticals Found in Treated Wastewaters: Study of Carbamazepine, Clofibric Acid, and Diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The Occurrence and Fate of Anti-Inflammatory and Analgesic Pharmaceuticals in Sewage and Fresh Water: Treatability by Conventional and Non-Conventional Processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the Environment: Assessing Risks of Pharmaceuticals to Wildlife and Ecosystems. Philos. Trans. R. Soc. B 2014, 369, 20130569. [Google Scholar] [CrossRef]
- Arnold, W.A.; McNeill, K. Chapter 3.2 Transformation of Pharmaceuticals in the Environment: Photolysis and Other Abiotic Processes. In Comprehensive Analytical Chemistry; Petrović, M., Barceló, D., Eds.; Analysis, Fate and Removal of Pharmaceuticals in the Water Cycle; Elsevier: Amsterdam, The Netherlands, 2007; Volume 50, pp. 361–385. [Google Scholar] [CrossRef]
- Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Biodegradation and Biotransformation of Polycyclic Non-Steroidal Anti-Inflammatory Drugs. Rev. Environ. Sci. Biotechnol. 2015, 14, 229–239. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture Toxicity of the Anti-Inflammatory Drugs Diclofenac, Ibuprofen, Naproxen, and Acetylsalicylic Acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Hernando, M.; Mezcua, M.; Fernandezalba, A.; Barcelo, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Acuña, V.; Ginebreda, A.; Mor, J.R.; Petrovic, M.; Sabater, S.; Sumpter, J.; Barceló, D. Balancing the Health Benefits and Environmental Risks of Pharmaceuticals: Diclofenac as an Example. Environ. Int. 2015, 85, 327–333. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic Ecotoxicity of Pharmaceuticals Including the Assessment of Combination Effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Islas-Flores, H.; Manuel Gómez-Oliván, L.; Galar-Martínez, M.; Michelle Sánchez-Ocampo, E.; SanJuan-Reyes, N.; Ortíz-Reynoso, M.; Dublán-García, O. Cyto-Genotoxicity and Oxidative Stress in Common Carp (Cyprinus carpio) Exposed to a Mixture of Ibuprofen and Diclofenac. Environ. Toxicol. 2017, 32, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Noya, V.M.; Gómez-Oliván, L.M.; Ramírez-Montero, M.D.C.; Islas-Flores, H.; Galar-Martínez, M.; Dublán-García, O.; Romero, R. Ibuprofen at Environmentally Relevant Concentrations Alters Embryonic Development, Induces Teratogenesis and Oxidative Stress in Cyprinus Carpio. Sci. Total Environ. 2020, 710, 136327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A Review of the Potential Utilisation of Plastic Waste as Adsorbent for Removal of Hazardous Priority Contaminants from Aqueous Environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Frenzilli, G.; Fusco, D.; Peluso, C.; Stingo, V. Evaluation of Zebrafish DNA Integrity after Exposure to Pharmacological Agents Present in Aquatic Environments. Ecotoxicol. Environ. Saf. 2010, 73, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, X.; Lee, S.; Kang, S.; Kho, Y.; Giesy, J.P.; Choi, K. Effects of Non-Steroidal Anti-Inflammatory Drugs on Hormones and Genes of the Hypothalamic-Pituitary-Gonad Axis, and Reproduction of Zebrafish. J. Hazard. Mater. 2013, 254–255, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, K.; Kim, J.; Ji, K.; Kim, S.; Ahn, B.; Yun, J.; Choi, K.; Khim, J.S.; Zhang, X.; et al. Endocrine Disruption and Consequences of Chronic Exposure to Ibuprofen in Japanese Medaka (Oryzias latipes) and Freshwater Cladocerans Daphnia Magna and Moina Macrocopa. Aquat. Toxicol. 2010, 98, 256–264. [Google Scholar] [CrossRef]
- Serrano, M.A.S.; Gonzalez-Rey, M.; Mattos, J.J.; Flores-Nunes, F.; Mello, Á.C.P.; Zacchi, F.L.; Piazza, C.E.; Siebert, M.N.; Piazza, R.S.; Alvarez-Muñoz, D.; et al. Differential Gene Transcription, Biochemical Responses, and Cytotoxicity Assessment in Pacific Oyster Crassostrea Gigas Exposed to Ibuprofen. Environ. Sci. Pollut. Res. Int. 2015, 22, 17375–17385. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Cogni, D.; Riva, C.; Provini, A. An in Vitro Biomarker Approach for the Evaluation of the Ecotoxicity of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Toxicol. In Vitro 2009, 23, 935–942. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Provini, A. Chronic Effects Induced by Ibuprofen on the Freshwater Bivalve Dreissena Polymorpha. Ecotoxicol. Environ. Saf. 2011, 74, 1586–1594. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; Buratti, S.; Fabbr, E.; DelValls, A.T.; Martín-Díaz, M.L. Using Lysosomal Membrane Stability of Haemocytes in Ruditapes Philippinarum as a Biomarker of Cellular Stress to Assess Contamination by Caffeine, Ibuprofen, Carbamazepine and Novobiocin. J. Environ. Sci. 2013, 25, 1408–1418. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; DelValls, T.A.; Martín-Díaz, M.L. General Stress, Detoxification Pathways, Neurotoxicity and Genotoxicity Evaluated in Ruditapes Philippinarum Exposed to Human Pharmaceuticals. Ecotoxicol. Environ. Saf. 2016, 124, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Maranho, L.A.; Moreira, L.B.; Baena-Nogueras, R.M.; Lara-Martín, P.A.; DelValls, T.A.; Martín-Díaz, M.L. A Candidate Short-Term Toxicity Test Using Ampelisca Brevicornis to Assess Sublethal Responses to Pharmaceuticals Bound to Marine Sediments. Arch. Environ. Contam. Toxicol. 2015, 68, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliván, L.M.; Galar-Martínez, M.; García-Medina, S.; Valdés-Alanís, A.; Islas-Flores, H.; Neri-Cruz, N. Genotoxic Response and Oxidative Stress Induced by Diclofenac, Ibuprofen and Naproxen in Daphnia Magna. Drug Chem. Toxicol. 2014, 37, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, L.-H.; Connon, R.; Hutchinson, T.H.; Maund, S.J.; Sibly, R.M.; Callaghan, A. Expression of Target and Reference Genes in Daphnia Magna Exposed to Ibuprofen. BMC Genom. 2006, 7, 175. [Google Scholar] [CrossRef]
- Hayashi, Y.; Heckmann, L.-H.; Callaghan, A.; Sibly, R.M. Reproduction Recovery of the Crustacean Daphnia Magna after Chronic Exposure to Ibuprofen. Ecotoxicology 2008, 17, 246–251. [Google Scholar] [CrossRef]
- Wang, F.; Finnin, J.; Tait, C.; Quirk, S.; Chekhtman, I.; Donohue, A.C.; Ng, S.; D’Souza, A.; Tait, R.; Prankerd, R. The Hydrolysis of Diclofenac Esters: Synthetic Prodrug Building Blocks for Biodegradable Drug–Polymer Conjugates. J. Pharm. Sci. 2016, 105, 773–785. [Google Scholar] [CrossRef]
- Maranho, L.A.; Baena-Nogueras, R.M.; Lara-Martín, P.A.; DelValls, T.A.; Martín-Díaz, M.L. Bioavailability, Oxidative Stress, Neurotoxicity and Genotoxicity of Pharmaceuticals Bound to Marine Sediments. The Use of the Polychaete Hediste Diversicolor as Bioindicator Species. Environ. Res. 2014, 134, 353–365. [Google Scholar] [CrossRef]
- Schmitt-Jansen, M.; Bartels, P.; Adler, N.; Altenburger, R. Phytotoxicity Assessment of Diclofenac and Its Phototransformation Products. Anal. Bioanal. Chem. 2007, 387, 1389–1396. [Google Scholar] [CrossRef]
- Feito, R.; Valcárcel, Y.; Catalá, M. Biomarker Assessment of Toxicity with Miniaturised Bioassays: Diclofenac as a Case Study. Ecotoxicology 2012, 21, 289–296. [Google Scholar] [CrossRef]
- Saravanan, M.; Ramesh, M. Short and Long-Term Effects of Clofibric Acid and Diclofenac on Certain Biochemical and Ionoregulatory Responses in an Indian Major Carp, Cirrhinus Mrigala. Chemosphere 2013, 93, 388–396. [Google Scholar] [CrossRef]
- Saravanan, M.; Karthika, S.; Malarvizhi, A.; Ramesh, M. Ecotoxicological Impacts of Clofibric Acid and Diclofenac in Common Carp (Cyprinus carpio) Fingerlings: Hematological, Biochemical, Ionoregulatory and Enzymological Responses. J. Hazard. Mater. 2011, 195, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Mohebi Derakhsh, P.; Mashinchian Moradi, A.; Sharifpour, I.; Jamili, S. Toxic Effects of Diclofenac on Gills, Liver and Kidney of Cyprinus Carpio. Iran. J. Fish. Sci. 2020, 19, 735–747. [Google Scholar] [CrossRef]
- De Felice, B.; Copia, L.; Guida, M. Gene Expression Profiling in Zebrafish Embryos Exposed to Diclofenac, an Environmental Toxicant. Mol. Biol. Rep. 2012, 39, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Guiloski, I.C.; Ribas, J.L.C.; da Pereira, L.S.; Neves, A.P.P.; Silva de Assis, H.C. Effects of Trophic Exposure to Dexamethasone and Diclofenac in Freshwater Fish. Ecotoxicol. Environ. Saf. 2015, 114, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic Effects of the Non-Steroidal Anti-Inflammatory Drug Diclofenac. Part I: Histopathological Alterations and Bioaccumulation in Rainbow Trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Yokota, H.; Higashi, K.; Hanada, E.; Matsuzaki, E.; Tsuruda, Y.; Suzuki, T.; Nakano, E.; Eguchi, S. Recovery from Reproductive and Morphological Abnormalities in Medaka (Oryzias latipes) Following a 14-Day Exposure to Diclofenac. Environ. Toxicol. Chem 2017, 36, 3277–3283. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Ogo, O.A.; Audu, B.S.; Ugwoegbu, K.C. Chronic Diclofenac (DCF) Exposure Alters Both Enzymatic and Haematological Profile of African Catfish, Clarias Gariepinus. Drug Chem. Toxicol. 2015, 38, 383–390. [Google Scholar] [CrossRef]
- Guiloski, I.C.; Stein Piancini, L.D.; Dagostim, A.C.; de Morais Calado, S.L.; Fávaro, L.F.; Boschen, S.L.; Cestari, M.M.; da Cunha, C.; Silva de Assis, H.C. Effects of Environmentally Relevant Concentrations of the Anti-Inflammatory Drug Diclofenac in Freshwater Fish Rhamdia Quelen. Ecotoxicol. Environ. Saf. 2017, 139, 291–300. [Google Scholar] [CrossRef]
- Quinn, B.; Schmidt, W.; O’Rourke, K.; Hernan, R. Effects of the Pharmaceuticals Gemfibrozil and Diclofenac on Biomarker Expression in the Zebra Mussel (Dreissena polymorpha) and Their Comparison with Standardised Toxicity Tests. Chemosphere 2011, 84, 657–663. [Google Scholar] [CrossRef]
- Schmidt, W.; O’Rourke, K.; Hernan, R.; Quinn, B. Effects of the Pharmaceuticals Gemfibrozil and Diclofenac on the Marine Mussel (Mytilus Spp.) and Their Comparison with Standardized Toxicity Tests. Mar. Pollut. Bull. 2011, 62, 1389–1395. [Google Scholar] [CrossRef]
- Fontes, M.K.; Gusso-Choueri, P.K.; Maranho, L.A.; de Abessa, D.M.S.; Mazur, W.A.; de Campos, B.G.; Guimarães, L.L.; de Toledo, M.S.; Lebre, D.; Marques, J.R.; et al. A Tiered Approach to Assess Effects of Diclofenac on the Brown Mussel Perna Perna: A Contribution to Characterize the Hazard. Water Res. 2018, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Nieto, E.; Blasco, J.; González-Ortegón, E.; Drake, P.; Hampel, M. Is Atyaephyra Desmarestii a Useful Candidate for Lethal and Sub-Lethal Toxicity Tests on Pharmaceutical Compounds? J. Hazard. Mater. 2013, 263, 256–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eades, C.; Waring, C.P. The Effects of Diclofenac on the Physiology of the Green Shore Crab Carcinus Maenas. Mar. Environ. Res. 2010, 69, S46–S48. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zanuri, N.B.; Bentley, M.G.; Caldwell, G.S. Assessing the Impact of Diclofenac, Ibuprofen and Sildenafil Citrate (Viagra®) on the Fertilisation Biology of Broadcast Spawning Marine Invertebrates. Mar. Environ. Res. 2017, 127, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Daniel, D.; Canelas, G.G.; Barros, J.; Correia, A.T. Toxic Effects of Environmentally Realistic Concentrations of Diclofenac in Organisms from Two Distinct Trophic Levels, Hediste Diversicolor and Solea Senegalensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 231, 108722. [Google Scholar] [CrossRef]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J.; Li, W.; Gan, J. Biodegradation of Naproxen by Freshwater Algae Cymbella Sp. and Scenedesmus Quadricauda and the Comparative Toxicity. Bioresour. Technol. 2017, 238, 164–173. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of Naproxen and Its Phototransformation Products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Stancová, V.; Ziková, A.; Svobodová, Z.; Kloas, W. Effects of the Non-Steroidal Anti-Inflammatory Drug(NSAID) Naproxen on Gene Expression of Antioxidant Enzymes in Zebrafish (Danio rerio). Environ. Toxicol. Pharmacol 2015, 40, 343–348. [Google Scholar] [CrossRef]
- Kwak, K.; Ji, K.; Kho, Y.; Kim, P.; Lee, J.; Ryu, J.; Choi, K. Chronic Toxicity and Endocrine Disruption of Naproxen in Freshwater Waterfleas and Fish, and Steroidogenic Alteration Using H295R Cell Assay. Chemosphere 2018, 204, 156–162. [Google Scholar] [CrossRef]
- Lucero, G.-M.A.; Marcela, G.-M.; Sandra, G.-M.; Manuel, G.-O.L.; Celene, R.-E. Naproxen-Enriched Artificial Sediment Induces Oxidative Stress and Genotoxicity in Hyalella Azteca. Water Air Soil Pollut. 2015, 226, 195. [Google Scholar] [CrossRef]
- Gagné, F.; Bérubé, E.; Fournier, M.; Blaise, C. Inflammatory Properties of Municipal Effluents to Elliptio Complanata Mussels–Lack of Effects from Anti-Inflammatory Drugs. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Yamindago, A.; Lee, N.; Woo, S.; Yum, S. Transcriptomic Profiling of Hydra Magnipapillata after Exposure to Naproxen. Environ. Toxicol. Pharmacol 2019, 71, 103215. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of Ketoprofen Toxicity in Two Freshwater Species: Effects on Biochemical, Physiological and Population Endpoints. Environ. Pollut. 2020, 265, 114993. [Google Scholar] [CrossRef] [PubMed]
- Mennillo, E.; Arukwe, A.; Monni, G.; Meucci, V.; Intorre, L.; Pretti, C. Ecotoxicological Properties of Ketoprofen and the S(+)-Enantiomer (Dexketoprofen): Bioassays in Freshwater Model Species and Biomarkers in Fish PLHC-1 Cell Line. Environ. Toxicol. Chem 2018, 37, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; d’Errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-Term Exposure of Mytilus Galloprovincialis to Diclofenac, Ibuprofen and Ketoprofen: Insights into Bioavailability, Biomarkers and Transcriptomic Changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef]
- Żbikowska, E.; Lombardo, P.; Żbikowski, J.; Jabłońska, G.; Marszewska, A.; Cichy, A. Ketoprofen-Induced Inhibition of Symptoms of Behavioural Fever Observed in Wintering Planorbarius corneus (L.) (Gastropoda: Planorbidae). J. Molluscan Stud. 2017, 83, 434–439. [Google Scholar] [CrossRef][Green Version]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and Its Transformation Products: Environmental Occurrence and Toxicity—A Review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Couto, E.; Assemany, P.P.; Assis Carneiro, G.C.; Ferreira Soares, D.C. The Potential of Algae and Aquatic Macrophytes in the Pharmaceutical and Personal Care Products (PPCPs) Environmental Removal: A Review. Chemosphere 2022, 302, 134808. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Environment (European Commission); INERIS; Milieu Ltd.; Kümmerer, K. Options for a Strategic Approach to Pharmaceuticals in the Environment: Final Report; Publications Office: Luxembourg, 2019; Available online: https://data.europa.eu/doi/10.2779/87838 (accessed on 10 February 2022).
- EPA. OW/ORD Emerging Contaminants Workgroup. White Paper: Aquatic Life Criteria for Contaminants of Emerging Concern; US EPA: Washington, DC, USA, 2008. [Google Scholar]
- EPA Science Advisory Board. SAB Advisory on Aquatic Life Water Quality Criteria for Contaminants of Emerging Concern; EPA-SAB-09–007; U.S. EPA: Washington, DC, USA, 2008. [Google Scholar]
- Vieno, N.; Hallgren, P.; Wallberg, P.; Pyhälä, M.; Zandaryaa, S. Pharmaceuticals in the Aquatic Environment of the Baltic Sea Region. A Status Report; International Initiative on Water Quality-IIWQ: Paris, France, 2017. [Google Scholar]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Ferreiro, C.; Gómez-Motos, I.; Lombraña, J.I.; de Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an Effluent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to Decrease Pharmaceuticals in the Environment? A Review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses; OECD Studies on Water; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Res.ource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and Removal of Pharmaceuticals in a Municipal Sewage Treatment System in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Amorim, C.G.; Araújo, A.N.; Montenegro, M.C.B.S.M.; Pena, A.; Delerue-Matos, C. Pilot Monitoring Study of Ibuprofen in Surface Waters of North of Portugal. Environ. Sci. Pollut. Res. 2013, 20, 2410–2420. [Google Scholar] [CrossRef]
- Zaborska, A.; Siedlewicz, G.; Szymczycha, B.; Dzierzbicka-Głowacka, L.; Pazdro, K. Legacy and Emerging Pollutants in the Gulf of Gdańsk (Southern Baltic Sea)—Loads and Distribution Revisited. Mar. Pollut. Bull. 2019, 139, 238–255. [Google Scholar] [CrossRef]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and Biomagnification Potential of Pharmaceuticals with a Focus to the Aquatic Environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar] [CrossRef]
- Freitas, O.M.M.; Martins, R.J.E.; Delerue-Matos, C.M.; Boaventura, R.A.R. Removal of Cd(II), Zn(II) and Pb(II) from Aqueous Solutions by Brown Marine Macro Algae: Kinetic Modelling. J. Hazard. Mater. 2008, 153, 493–501. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Kalavrouziotis, I.K.; Koukoulakis, P.H.; Vasquez, M.I. The Risks Associated with Wastewater Reuse and Xenobiotics in the Agroecological Environment. Sci. Total Environ. 2011, 409, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Rostvall, A.; Zhang, W.; Dürig, W.; Renman, G.; Wiberg, K.; Ahrens, L.; Gago-Ferrero, P. Removal of Pharmaceuticals, Perfluoroalkyl Substances and Other Micropollutants from Wastewater Using Lignite, Xylit, Sand, Granular Activated Carbon (GAC) and GAC+Polonite® in Column Tests—Role of Physicochemical Properties. Water Res. 2018, 137, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Mlunguza, N.Y.; Ncube, S.; Nokwethemba Mahlambi, P.; Chimuka, L.; Madikizela, L.M. Adsorbents and Removal Strategies of Non-Steroidal Anti-Inflammatory Drugs from Contaminated Water Bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Singh Arora, D.; Kumar Sharma, R. Ligninolytic Fungal Laccases and Their Biotechnological Applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788. [Google Scholar] [CrossRef]
- Hoon Chu, K.; Al-Hamadani, Y.a.J.; ChangMin, P.; GooYong, L.; Min, J.; Am, J.; NamGuk, H.; AhJeong, S.; YeoMin, Y. Ultrasonic Treatment of Endocrine Disrupting Compounds, Pharmaceuticals, and Personal Care Products in Water: A Review. Chem. Eng. J. 2017, 327, 629–647. [Google Scholar]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Becerra, J.; Gopalakrishnan, V.N.; Quach, T.; Do, T. Plasmonic Materials: Opportunities and Challenges on Reticular Chemistry for Photocatalytic Applications. ChemCatChem 2021, 13, 1059–1073. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Julcour, C.; Barthe, L. Heterogeneous Fenton Oxidation Using Fe-ZSM5 Catalyst for Removal of Ibuprofen in Wastewater. J. Environ. Chem. Eng. 2018, 6, 5920–5928. [Google Scholar] [CrossRef]
- Apriceno, A.; Astolfi, M.L.; Girelli, A.M.; Scuto, F.R. A New Laccase-Mediator System Facing the Biodegradation Challenge: Insight into the NSAIDs Removal. Chemosphere 2019, 215, 535–542. [Google Scholar] [CrossRef]
- Bilgin Simsek, E.; Kilic, B.; Asgin, M.; Akan, A. Graphene Oxide Based Heterojunction TiO2–ZnO Catalysts with Outstanding Photocatalytic Performance for Bisphenol-A, Ibuprofen and Flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; Geng, J.; Li, S.; Wu, G.; Ren, H. Degradation of Three Nonsteroidal Anti-Inflammatory Drugs by UV/Persulfate: Degradation Mechanisms, Efficiency in Effluents Disposal. Chem. Eng. J. 2019, 356, 1032–1041. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonik) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Le Milbeau, C. Occurrence and Removal Efficiency of Pharmaceuticals in an Urban Wastewater Treatment Plant: Mass Balance, Fate and Consumption Assessment. J. Environ. Chem. Eng. 2017, 5, 2894–2902. [Google Scholar] [CrossRef]
- Mora-Ravelo, S.G. Bioremediation of Wastewater for Reutilization in Agricultural Systems: A Review. Appl. Ecol. Environ. Res. 2017, 15, 33–50. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the Environment: Biodegradation and Effects on Natural Microbial Communities. A Review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Eckenfelder, W.W., Jr.; Staff, U. Wastewater Treatment. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Mehmood, M.K.; Adetutu, E.; Nedwell, D.B.; Ball, A.S. In Situ Microbial Treatment of Landfill Leachate Using Aerated Lagoons. Bioresour. Technol. 2009, 100, 2741–2744. [Google Scholar] [CrossRef]
- Dhir, B. Mechanism of Removal of Contaminants by Aquatic Plants. In Phytoremediation: Role of Aquatic Plants in Environmental Clean-Up; Dhir, B., Ed.; Springer: New Delhi, India, 2013; pp. 51–64. [Google Scholar] [CrossRef]
- Doran, P.M. Application of Plant Tissue Cultures in Phytoremediation Research: Incentives and Limitations. Biotechnol. Bioeng. 2009, 103, 60–76. [Google Scholar] [CrossRef]
- Kumar, P.K.; Vijaya Krishna, S.; Verma, K.; Pooja, K.; Bhagawan, D.; Himabindu, V. Phycoremediation of Sewage Wastewater and Industrial Flue Gases for Biomass Generation from Microalgae. S. Afr. J. Chem. Eng. 2018, 25, 133–146. [Google Scholar] [CrossRef]
- Olguín, E.J. Phycoremediation: Key Issues for Cost-Effective Nutrient Removal Processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B.; Golueke, C.G.; Kellen, W.R.; Gloyna, E.F.; Hermann, E.R. Algae in Waste Treatment [with Discussion]. Sew. Ind. Wastes 1957, 29, 437–457. [Google Scholar]
- Henriques, B.; Lopes, C.B.; Figueira, P.; Rocha, L.S.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. Bioaccumulation of Hg, Cd and Pb by Fucus Vesiculosus in Single and Multi-Metal Contamination Scenarios and Its Effect on Growth Rate. Chemosphere 2017, 171, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Laffont-Schwob, I.; Triboit, F.; Prudent, P.; Soulié-Märsche, I.; Rabier, J.; Despréaux, M.; Thiéry, A. Trace Metal Extraction and Biomass Production by Spontaneous Vegetation in Temporary Mediterranean Stormwater Highway Retention Ponds: Freshwater Macroalgae (Chara spp.) vs. Cattails (Typha spp.). Ecol. Eng. 2015, 81, 173–181. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Phytoremediation of Azo Dye Methyl Red by Macroalgae Chara vulgaris L.: Kinetic and Equilibrium Studies. Environ. Sci. Pollut. Res. 2020, 27, 26406–26418. [Google Scholar] [CrossRef]
- Charrier, B.; Abreu, M.H.; Araujo, R.; Bruhn, A.; Coates, J.C.; De Clerck, O.; Katsaros, C.; Robaina, R.R.; Wichard, T. Furthering Knowledge of Seaweed Growth and Development to Facilitate Sustainable Aquaculture. New Phytol. 2017, 216, 967–975. [Google Scholar] [CrossRef]
- Saunders, R.J.; Paul, N.A.; Hu, Y.; de Nys, R. Sustainable Sources of Biomass for Bioremediation of Heavy Metals in Waste Water Derived from Coal-Fired Power Generation. PLoS ONE 2012, 7, e36470. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and Me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The Effect of Heavy Metals on the Viability of Tetraselmis Marina AC16-MESO and an Evaluation of the Potential Use of This Microalga in Bioremediation. PeerJ 2018, 6, e5295. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution Prevention and Waste Phycoremediation by Algal-Based Wastewater Treatment Technologies: The Applications of High-Rate Algal Ponds (HRAPs) and Algal Turf Scrubber (ATS). J. Environ. Manag. 2021, 296, 113193. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Burki, F.; Wilcox, H.M.; Allam, B.; Allen, E.E.; Amaral-Zettler, L.A.; Armbrust, E.V.; Archibald, J.M.; Bharti, A.K.; Bell, C.J.; et al. The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): Illuminating the Functional Diversity of Eukaryotic Life in the Oceans through Transcriptome Sequencing. PLoS Biol. 2014, 12, e1001889. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in Microalgae. In Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 21–36. [Google Scholar] [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae Based Microbial Fuel Cells for Wastewater Treatment and Recovery of Value-Added Products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Pizarro, C.; Kebede-Westhead, E. Treatment of Dairy Manure Effluent Using Freshwater Algae: Algal Productivity and Recovery of Manure Nutrients Using Pilot-Scale Algal Turf Scrubbers. Bioresour. Technol. 2008, 99, 8137–8142. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Molecular Mechanisms of Plant Metal Tolerance and Homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Arora, N.; Gupta, P.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. Chapter 4—Microalgae: An Emerging Source for Mitigation of Heavy Metals and Their Potential Implications for Biodiesel Production. In Advanced Biofuels; Azad, A.K., Rasul, M., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2019; pp. 97–128. [Google Scholar] [CrossRef]
- Hanikenne, M.; Krämer, U.; Demoulin, V.; Baurain, D. A Comparative Inventory of Metal Transporters in the Green Alga Chlamydomonas Reinhardtii and the Red Alga Cyanidioschizon Merolae. Plant Physiol. 2005, 137, 428–446. [Google Scholar] [CrossRef]
- Ge, L.; Deng, H. Degradation of Two Fluoroquinolone Antibiotics Photoinduced by Fe(III)-Microalgae Suspension in an Aqueous Solution. Photochem. Photobiol. Sci. 2015, 14, 693–699. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Zheng, H.-S.; Li, S.; Du, J.-S.; Feng, X.-C.; Yin, R.-L.; Wu, Q.-L.; Ren, N.-Q.; Chang, J.-S. Removal of Cephalosporin Antibiotics 7-ACA from Wastewater during the Cultivation of Lipid-Accumulating Microalgae. Bioresour. Technol. 2016, 221, 284–290. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Khanal, S.K.; Yin, L.; Lu, H. Insights into Pharmaceuticals Removal in an Anaerobic Sulfate-Reducing Bacteria Sludge System. Water Res. 2019, 161, 191–201. [Google Scholar] [CrossRef]
- Xiao, G.; Chen, J.; Show, P.L.; Yang, Q.; Ke, J.; Zhao, Q.; Guo, R.; Liu, Y. Evaluating the Application of Antibiotic Treatment Using Algae-Algae/Activated Sludge System. Chemosphere 2021, 282, 130966. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.G.; Fick, J. Algal Cultivation in Urban Wastewater: An Efficient Way to Reduce Pharmaceutical Pollutants. J. Appl. Phycol. 2017, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The Influence of Nanoplastics on the Toxic Effects, Bioaccumulation, Biodegradation and Enantioselectivity of Ibuprofen in Freshwater Algae Chlorella Pyrenoidosa. Environ. Pollut. 2020, 263, 114593. [Google Scholar] [CrossRef] [PubMed]
- de Wilt, A.; Butkovskyi, A.; Tuantet, K.; Leal, L.H.; Fernandes, T.V.; Langenhoff, A.; Zeeman, G. Micropollutant Removal in an Algal Treatment System Fed with Source Separated Wastewater Streams. J. Hazard. Mater. 2016, 304, 84–92. [Google Scholar] [CrossRef]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef]
- Larsen, C.; Yu, Z.H.; Flick, R.; Passeport, E. Mechanisms of Pharmaceutical and Personal Care Product Removal in Algae-Based Wastewater Treatment Systems. Sci. Total Environ. 2019, 695, 133772. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Comparative Assessment of Diclofenac Removal from Water by Different Microalgae Strains. Algal Res. 2016, 18, 127–134. [Google Scholar] [CrossRef]
- Ben Ouada, S.; Ben Ali, R.; Cimetiere, N.; Leboulanger, C.; Ben Ouada, H.; Sayadi, S. Biodegradation of Diclofenac by Two Green Microalgae: Picocystis Sp. and Graesiella sp. Ecotoxicol. Environ. Saf. 2019, 186, 109769. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; García, A.I.; Otero, M. Nutrients and Pharmaceuticals Removal from Wastewater by Culture and Harvesting of Chlorella Sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation Mechanisms of Heavy Metals Using Living Green Microalgae: Physicochemical and Molecular Approaches for Enhancing Selectivity and Removal Capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Vahabisani, A.; An, C. Use of Biomass-Derived Adsorbents for the Removal of Petroleum Pollutants from Water: A Mini-Review. Environ. Syst. Res. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial Biosorbents and Biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Orta Ledesma, M.T. Wastewater-Leachate Treatment by Microalgae: Biomass, Carbohydrate and Lipid Production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal Extracellular Polymeric Substances and Their Interactions with Metal(Loid)s: A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Reddy, K.; Renuka, N.; Kumari, S.; Bux, F. Algae-Mediated Processes for the Treatment of Antiretroviral Drugs in Wastewater: Prospects and Challenges. Chemosphere 2021, 280, 130674. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from Aqueous Solution Using Green Alga (Ulva lactuca) Biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.-L.; Wang, J.-H. Genetic and Chemical Modification of Cells for Selective Separation and Analysis of Heavy Metals of Biological or Environmental Significance. TrAC Trends Anal. Chem. 2015, 66, 90–102. [Google Scholar] [CrossRef]
- Sears, M.E. Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Rezania, S.; Nazari, V.M. Pharmaceuticals and Personal Care Products in Aquatic Environments and Their Removal by Algae-Based Systems. Chemosphere 2022, 288, 132580. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: A Potential Tool for Remediating Aquatic Environments from Toxic Metals. Int. J. Environ. Sci. Technol. 2018, 15, 1815–1830. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.-X.; Liu, Y.-S.; Zhao, J.-L.; He, L.-Y.; Ying, G.-G. Microalgae-Based Technology for Antibiotics Removal: From Mechanisms to Application of Innovational Hybrid Systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef] [PubMed]
- Poo, K.-M.; Son, E.-B.; Chang, J.-S.; Ren, X.; Choi, Y.-J.; Chae, K.-J. Biochars Derived from Wasted Marine Macro-Algae (Saccharina Japonica and Sargassum Fusiforme) and Their Potential for Heavy Metal Removal in Aqueous Solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent Developments on Algal Biochar Production and Characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Yun, Y.-S.; Park, D.; Park, J.M.; Volesky, B. Biosorption of Trivalent Chromium on the Brown Seaweed Biomass. Environ. Sci. Technol. 2001, 35, 4353–4358. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal Bioremediation of Emerging Contaminants—Opportunities and Challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Ankit; Bordoloi, N.; Tiwari, J.; Kumar, S.; Korstad, J.; Bauddh, K. Efficiency of Algae for Heavy Metal Removal, Bioenergy Production, and Carbon Sequestration. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment; Bharagava, R., Ed.; Microorganisms for Sustainability; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Nzengung, V.A.; O’Niell, W.L.; McCutcheon, S.C.; Wolfe, N.L. Sequestration and Transformation of Water Soluble Halogenated Organic Compounds Using Aquatic Plants, Algae, and Microbial Mats. In Phytoremediation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 497–528. [Google Scholar] [CrossRef]
- Shackira, A.M.; Jazeel, K.; Puthur, J.T. Chapter 13—Phycoremediation and Phytoremediation: Promising Tools of Green Remediation. In Sustainable Environmental Clean-Up; Kumar Mishra, V., Kumar, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 273–293. [Google Scholar] [CrossRef]
- Stravs, M.A.; Pomati, F.; Hollender, J. Exploring Micropollutant Biotransformation in Three Freshwater Phytoplankton Species. Environ. Sci. Processes Impacts 2017, 19, 822–832. [Google Scholar] [CrossRef]
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S.; et al. Arsenic Speciation and Biotransformation Pathways in the Aquatic Ecosystem: The Significance of Algae. J. Hazard. Mater. 2021, 403, 124027. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Algal Foams Applied in Fixed-Bed Process for Lead(II) Removal Using Recirculation or One-Pass Modes. Mar. Drugs 2017, 15, 315. [Google Scholar] [CrossRef]
- Kelly, D.J.A.; Budd, K.; Lefebvre, D.D. Biotransformation of Mercury in PH-Stat Cultures of Eukaryotic Freshwater Algae. Arch. Microbiol. 2007, 187, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Chen, P.-W.; Hsu, C.-Y.; Lee, L. The Use of Autotrophic Chlorella Vulgaris in Chromium (VI) Reduction under Different Reduction Conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.-J. Biotransformation of Progesterone and Norgestrel by Two Freshwater Microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation Kinetics and Products Identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for Municipal Wastewater Nutrient Remediation: Mechanisms, Reactors and Outlook for Tertiary Treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, Y.; Han, S.-H.; Hwang, S.-J. The Effects of Wavelength and Wavelength Mixing Ratios on Microalgae Growth and Nitrogen, Phosphorus Removal Using Scenedesmus Sp. for Wastewater Treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, Z. Performance of Mixed LED Light Wavelengths on Biogas Upgrade and Biogas Fluid Removal by Microalga Chlorella sp. Appl. Energy 2014, 113, 1008–1014. [Google Scholar] [CrossRef]
- Seel, C.J.; Gulder, T. Biocatalysis Fueled by Light: On the Versatile Combination of Photocatalysis and Enzymes. ChemBioChem 2019, 20, 1871–1897. [Google Scholar] [CrossRef]
- Krauss, U.; Lee, J.; Benkovic, S.J.; Jaeger, K.-E. LOVely Enzymes—Towards Engineering Light-Controllable Biocatalysts. Microb. Biotechnol. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Arbib, Z.; de Godos, I.; Ruiz, J.; Perales, J.A. Optimization of Pilot High Rate Algal Ponds for Simultaneous Nutrient Removal and Lipids Production. Sci. Total Environ. 2017, 589, 66–72. [Google Scholar] [CrossRef]
- Gordon, J.M.; Polle, J.E.W. Ultrahigh Bioproductivity from Algae. Appl. Microbiol. Biotechnol. 2007, 76, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, S.-A.; Ko, S.-R.; Oh, H.-M.; Ahn, C.-Y. Effects of Photoperiod on Nutrient Removal, Biomass Production, and Algal-Bacterial Population Dynamics in Lab-Scale Photobioreactors Treating Municipal Wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of Photoperiods on the Growth Rate and Biomass Productivity of Green Microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; de la Noüe, J. Nitrogen and Phosphorus Removal by High Latitude Mat-Forming Cyanobacteria for Potential Use in Tertiary Wastewater Treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of Wastewater by Microalgae: A Review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Ju, X.; Igarashi, K.; Miyashita, S.; Mitsuhashi, H.; Inagaki, K.; Fujii, S.; Sawada, H.; Kuwabara, T.; Minoda, A. Effective and Selective Recovery of Gold and Palladium Ions from Metal Wastewater Using a Sulfothermophilic Red Alga, Galdieria Sulphuraria. Bioresour. Technol. 2016, 211, 759–764. [Google Scholar] [CrossRef]
- Ratnasari, A.; Syafiuddin, A.; Zaidi, N.S.; Hong Kueh, A.B.; Hadibarata, T.; Prastyo, D.D.; Ravikumar, R.; Sathishkumar, P. Bioremediation of Micropollutants Using Living and Non-Living Algae—Current Perspectives and Challenges. Environ. Pollut. 2022, 292, 118474. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of Carbamazepine by Laccase-Mediator System: Kinetics, by-Products and Toxicity Assessment. Process Biochem. 2018, 67, 147–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U. In Vitro Degradation of Carbamazepine and Diclofenac by Crude Lignin Peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Lambrinidis, G.; Parry, D.L. Effect of Temperature on Growth, Chemical Composition and Fatty Acid Composition of Tropical Australian Microalgae Grown in Batch Cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The Effects of Nitrogen Sources and Temperature on Cell Growth and Lipid Accumulation of Microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Nagy, B.J.; Makó, M.; Erdélyi, I.; Ramirez, A.; Moncada, J.; Gursel, I.V.; Ruiz-Martínez, A.; Seco, A.; Ferrer, J.; Abiusi, F.; et al. MAB2.0 Project: Integrating Algae Production into Wastewater Treatment. EuroBiotech J. 2018, 2, 10–23. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature Effect on Microalgae: A Crucial Factor for Outdoor Production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Cerveny, D.; Fick, J.; Klaminder, J.; McCallum, E.S.; Bertram, M.G.; Castillo, N.A.; Brodin, T. Water Temperature Affects the Biotransformation and Accumulation of a Psychoactive Pharmaceutical and Its Metabolite in Aquatic Organisms. Environ. Int. 2021, 155, 106705. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, Y.; Honda, R.; Noguchi, M.; Hara-Yamamura, H.; Kobayashi, S.; Higashimine, K.; Hasegawa, H. Optimum Conditions of PH, Temperature and Preculture for Biosorption of Europium by Microalgae Acutodesmus acuminatus. Biochem. Eng. J. 2019, 143, 58–64. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Qi, Y.; Chen, G.; Song, Y.; Kansha, Y.; Kitamura, Y. Absorption-Microalgae Hybrid CO2 Capture and Biotransformation Strategy—A Review. Int. J. Greenh. Gas Control 2019, 88, 109–117. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic Cultivation, a Preferable Microalgae Cultivation Mode for Biomass/Bioenergy Production, and Bioremediation, Advances and Prospect. Int. J. Hydrogen Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients Utilization and Contaminants Removal. A Review of Two Approaches of Algae and Cyanobacteria in Wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Lu, W.; Asraful Alam, M.; Liu, S.; Xu, J.; Parra Saldivar, R. Critical Processes and Variables in Microalgae Biomass Production Coupled with Bioremediation of Nutrients and CO2 from Livestock Farms: A Review. Sci. Total Environ. 2020, 716, 135247. [Google Scholar] [CrossRef]
- Béchet, Q.; Sialve, B.; Steyer, J.-P.; Shilton, A.; Guieysse, B. Comparative Assessment of Evaporation Models in Algal Ponds. Algal Res. 2018, 35, 283–291. [Google Scholar] [CrossRef]
- Salama, E.-S.; Roh, H.-S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.-H. Algae as a Green Technology for Heavy Metals Removal from Various Wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef]
- Stroppa, N.; Onelli, E.; Hejna, M.; Rossi, L.; Gagliardi, A.; Bini, L.; Baldi, A.; Moscatelli, A. Typha Latifolia and Thelypteris Palustris Behavior in a Pilot System for the Refinement of Livestock Wastewaters: A Case of Study. Chemosphere 2020, 240, 124915. [Google Scholar] [CrossRef] [PubMed]
- Osundeko, O.; Dean, A.P.; Davies, H.; Pittman, J.K. Acclimation of Microalgae to Wastewater Environments Involves Increased Oxidative Stress Tolerance Activity. Plant Cell Physiol. 2014, 55, 1848–1857. [Google Scholar] [CrossRef]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of Graphene Based Materials for Adsorption of Pharmaceutical Traces from Water and Wastewater—A Review. Desalination Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Zerrouki, D.; Henni, A. Outdoor Microalgae Cultivation for Wastewater Treatment. In Application of Microalgae in Wastewater Treatment: Volume 1: Domestic and Industrial Wastewater Treatment; Gupta, S.K., Bux, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–99. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess Engineering of Microalgae to Produce a Variety of Consumer Products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for Mass Cultivation of Algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Singh, A.; Pant, D.; Olsen, S.I.; Nigam, P.S. Key Issues to Consider in Microalgae Based Biodiesel Production. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 29, 687–700. [Google Scholar]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular Photobioreactor Design for Algal Cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Slegers, P.M.; Wijffels, R.H.; van Straten, G.; van Boxtel, A.J.B. Design Scenarios for Flat Panel Photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Eriksen, N.T. The Technology of Microalgal Culturing. Biotechnol. Lett. 2008, 30, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.L.; Lee, S.-M.; Choi, H.-J. A Mini Review: Photobioreactors for Large Scale Algal Cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, Photobioreactor Design and Harvesting of Microalgae for Biodiesel Production: A Critical Review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Posten, C. Design Principles of Photo-Bioreactors for Cultivation of Microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Morita, M.; Watanabe, Y.; Okawa, T.; Saiki, H. Photosynthetic Productivity of Conical Helical Tubular Photobioreactors Incorporating Chlorella Sp. under Various Culture Medium Flow Conditions. Biotechnol. Bioeng. 2001, 74, 136–144. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of Microbial Cells for the Biotreatment of Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Pei, H. Algal–Bacterial Consortia for Bioproduct Generation and Wastewater Treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. Adv. Food Nutr. Res. 2016, 79, 179–211. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Jaén-Gil, A.; Bello-Laserna, I.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Performance of a Microalgal Photobioreactor Treating Toilet Wastewater: Pharmaceutically Active Compound Removal and Biomass Harvesting. Sci. Total Environ. 2017, 592, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.E.; de Coimbra, R.N.; PaniaguaBermejo, S.; Pérez, A.I.G.; Cabero, M.O. Comparative Assessment of Pharmaceutical Removal from Wastewater by the Microalgae Chlorella Sorokiniana, Chlorella Vulgaris and Scenedesmus Obliquus; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Ying, G.-G.; Liu, S.; Zhou, L.-J.; Chen, Z.-F.; Peng, F.-Q. Simultaneous Removal of Inorganic and Organic Compounds in Wastewater by Freshwater Green Microalgae. Environ. Sci. Process. Impacts 2014, 16, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, M.V.; Sánchez-Castillo, P.; Romera, O.; Fernández-Moreno, D.; Pérez-Martínez, C. Growth and Nutrient Removal in Free and Immobilized Planktonic Green Algae Isolated from Pig Manure. Enzym. Microb. Technol. 2004, 34, 392–398. [Google Scholar] [CrossRef]
- Kaparapu, J.; Geddada, M.N.R. Applications of Immobilized Algae. Available online: https://www.semanticscholar.org/paper/Applications-of-immobilized-algae-Kaparapu-Gedddada/ce4e7e62067bedcd1e147ded9bcfe2a0391c11aa (accessed on 16 February 2022).
- Stolarzewicz, I.; Białecka-Florjańczyk, E.; Majewska, E.; Krzyczkowska, J. Immobilization of Yeast on Polymeric Supports. Chem. Biochem. Eng. Q. 2011, 25, 135–144. [Google Scholar]
- de-Bashan, L.E.; Bashan, Y. Immobilized Microalgae for Removing Pollutants: Review of Practical Aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Das, M.; Adholeya, A. Potential Uses of Immobilized Bacteria, Fungi, Algae, and Their Aggregates for Treatment of Organic and Inorganic Pollutants in Wastewater. In Water Challenges and Solutions on a Global Scale; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1206, pp. 319–337. [Google Scholar] [CrossRef]
- Awasthi, M.; Das, D.N. Heavy Metal Toxicity on Nitrate Reductase Activity of Free and Immobilized Algal Cells. Int. J. Algae 2004, 6, 151–157. [Google Scholar] [CrossRef]
- Ozer, T.B.; Erkaya, I.A.; Udoh, A.U.; Duygu, D.Y.; Akbulut, A.; Bayramoglu, G.; Arica, M.Y. Biosorption of Cr(VI) by Free and Immobilized Pediastrum Boryanum Biomass: Equilibrium, Kinetic, and Thermodynamic Studies. Environ. Sci. Pollut. Res. Int. 2011, 19, 2983–2993. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Removal of Ni and Cu from Single and Binary Metalsolutions by Free and Immobilized Chlorella Vulgaris. Eur. J. Protistol. 2001, 37, 261–271. [Google Scholar] [CrossRef]
- Wilkinson, S.C.; Goulding, K.H.; Robinson, P.K. Mercury Removal by Immobilized Algae in Batch Culture Systems. J. Appl. Phycol. 1990, 2, 223–230. [Google Scholar] [CrossRef]
- Kadimpati, K.K.; Mondithoka, K.P.; Bheemaraju, S.; Challa, V.R.M. Entrapment of Marine Microalga, Isochrysis Galbana, for Biosorption of Cr(III) from Aqueous Solution: Isotherms and Spectroscopic Characterization. Appl. Water Sci. 2013, 3, 85–92. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, H.D. Nitrate, Ammonium, and Phosphate Uptake by the Immobilized Cells of Dunaliella Salina. Bull. Environ. Contam. Toxicol. 1999, 62, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fierro, S.; del Sánchez-Saavedra, M.P.; Copalcúa, C. Nitrate and Phosphate Removal by Chitosan Immobilized Scenedesmus. Bioresour. Technol. 2008, 99, 1274–1279. [Google Scholar] [CrossRef]
- Travieso, L.; Cañizares, R.O.; Borja, R.; Benítez, F.; Domínguez, A.R.; Dupeyrón, R.; Valiente, V. Heavy Metal Removal by Microalgae. Bull. Environ. Contam. Toxicol. 1999, 62, 144–151. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C. Removal of Inorganic Ions from Wastewaters by Immobilized Microalgae. World J. Microbiol. Biotechnol. 1994, 10, 439–443. [Google Scholar] [CrossRef]
- Tajes-Martínez, P.; Beceiro-González, E.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Micro-Columns Packed with Chlorella Vulgaris Immobilised on Silica Gel for Mercury Speciation. Talanta 2006, 68, 1489–1496. [Google Scholar] [CrossRef]
- Banerjee, M.; Mishra, S.; Chatterjee, J. Scavenging of Nickel and Chromium Toxicity in Aulosira Fertilissima by Immobilization: Effect on Nitrogen Assimilating Enzymes. Electron. J. Biotechnol. 2004, 7, 13–14. [Google Scholar] [CrossRef][Green Version]
- Ji, X.; Jiang, M.; Zhang, J.; Jiang, X.; Zheng, Z. The Interactions of Algae-Bacteria Symbiotic System and Its Effects on Nutrients Removal from Synthetic Wastewater. Bioresour. Technol. 2018, 247, 44–50. [Google Scholar] [CrossRef]
- Karya, N.G.a.I.; van der Steen, N.P.; Lens, P.N.L. Photo-Oxygenation to Support Nitrification in an Algal-Bacterial Consortium Treating Artificial Wastewater. Bioresour. Technol. 2013, 134, 244–250. [Google Scholar] [CrossRef]
- Arora, K.; Kaur, P.; Kumar, P.; Singh, A.; Patel, S.K.S.; Li, X.; Yang, Y.-H.; Bhatia, S.K.; Kulshrestha, S. Valorization of Wastewater Resources Into Biofuel and Value-Added Products Using Microalgal System. Front. Energy Res. 2021, 9, 646571. [Google Scholar] [CrossRef]
- You, X.; Xu, N.; Yang, X.; Sun, W. Pollutants Affect Algae-Bacteria Interactions: A Critical Review. Environ. Pollut. 2021, 276, 116723. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Posadas, E.; García-Encina, P.A.; Muñoz, R. Removal of Contaminants of Emerging Concern from Urban Wastewater in Novel Algal-Bacterial Photobioreactors. Sci. Total Environ. 2019, 662, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Hernández, D.; García-González, M.C. Microalgal-Based Systems for Wastewater Treatment: Effect of Applied Organic and Nutrient Loading Rate on Biomass Composition. Ecol. Eng. 2012, 49, 112–117. [Google Scholar] [CrossRef]
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R.P. Review of Challenges for Algae-Based Wastewater Treatment: Strain Selection, Wastewater Characteristics, Abiotic, and Biotic Factors. J. Hazard. Toxic Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Ismail, M.M.; Essam, T.M.; Ragab, Y.M.; El-Sayed, A.E.-K.B.; Mourad, F.E. Remediation of a Mixture of Analgesics in a Stirred-Tank Photobioreactor Using Microalgal-Bacterial Consortium Coupled with Attempt to Valorise the Harvested Biomass. Bioresour. Technol. 2017, 232, 364–371. [Google Scholar] [CrossRef]
- Ismail, M.M.; Essam, T.M.; Ragab, Y.M.; Mourad, F.E. Biodegradation of Ketoprofen Using a Microalgal–Bacterial Consortium. Biotechnol. Lett. 2016, 38, 1493–1502. [Google Scholar] [CrossRef]
- He, P.J.; Mao, B.; Lü, F.; Shao, L.M.; Lee, D.J.; Chang, J.S. The Combined Effect of Bacteria and Chlorella Vulgaris on the Treatment of Municipal Wastewaters. Bioresour. Technol. 2013, 146, 562–568. [Google Scholar] [CrossRef]
- Lee, C.S.; Oh, H.-S.; Oh, H.-M.; Kim, H.-S.; Ahn, C.-Y. Two-Phase Photoperiodic Cultivation of Algal-Bacterial Consortia for High Biomass Production and Efficient Nutrient Removal from Municipal Wastewater. Bioresour. Technol. 2016, 200, 867–875. [Google Scholar] [CrossRef]
- Amengual-Morro, C.; Moyà Niell, G.; Martínez-Taberner, A. Phytoplankton as Bioindicator for Waste Stabilization Ponds. J. Environ. Manag. 2012, 95, S71–S76. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of Real Wastewater Using Co-Culture of Immobilized Chlorella Vulgaris and Suspended Activated Sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef]
- Muñoz, R.; Alvarez, M.T.; Muñoz, A.; Terrazas, E.; Guieysse, B.; Mattiasson, B. Sequential Removal of Heavy Metals Ions and Organic Pollutants Using an Algal-Bacterial Consortium. Chemosphere 2006, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Gursel, I.; Tunali, Y.; Arica, M.Y. Biosorption of Phenol and 2-Chlorophenol by Funalia Trogii Pellets. Bioresour. Technol. 2009, 100, 2685–2691. [Google Scholar] [CrossRef]
- López Errasquín, E.; Vázquez, C. Tolerance and Uptake of Heavy Metals by Trichoderma Atroviride Isolated from Sludge. Chemosphere 2003, 50, 137–143. [Google Scholar] [CrossRef]
- Cid, H.; Ortiz, C.; Pizarro, J.; Barros, D.; Castillo, X.; Giraldo, L.; Moreno-Piraján, J.C. Characterization of Copper (II) Biosorption by Brown Algae Durvillaea Antarctica Dead Biomass. Adsorption 2015, 21, 645–658. [Google Scholar] [CrossRef]
- Huang, S.; Lin, G. Biosorption of Hg(II) and Cu(II) by Biomass of Dried Sargassum Fusiforme in Aquatic Solution. J. Environ. Health Sci. Eng. 2015, 13, 21. [Google Scholar] [CrossRef]
- Dinnebier, H.C.F.; Matthiensen, A.; Michelon, W.; Tápparo, D.C.; Fonseca, T.G.; Favretto, R.; Steinmetz, R.L.R.; Treichel, H.; Antes, F.G.; Kunz, A. Phycoremediation and Biomass Production from High Strong Swine Wastewater for Biogas Generation Improvement: An Integrated Bioprocess. Bioresour. Technol. 2021, 332, 125111. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Kumar, D. 12—Biodegradable Polymer-Based Nanoadsorbents for Environmental Remediation. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–278. [Google Scholar] [CrossRef]
- Yusuf, M. Cellulose-Based Nanomaterials for Water Pollutant Remediation: Review. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 213–228. [Google Scholar] [CrossRef]
- Wang, D. A Critical Review of Cellulose-Based Nanomaterials for Water Purification in Industrial Processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, M.; He, Z.; Liu, G.; Ramakrishnan, M.; Thangavel, P.; Pugazhendhi, A.; Raja, R.; Carvalho, I.S. Current Strategies and Prospects in Algae for Remediation and Biofuels: An Overview. Biocatal. Agric. Biotechnol. 2021, 35, 102045. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Enhancing Biomass Energy Yield from Pilot-Scale High Rate Algal Ponds with Recycling. Water Res. 2013, 47, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Rosli, S.S.; Amalina Kadir, W.N.; Wong, C.Y.; Han, F.Y.; Lim, J.W.; Lam, M.K.; Yusup, S.; Kiatkittipong, W.; Kiatkittipong, K.; Usman, A. Insight Review of Attached Microalgae Growth Focusing on Support Material Packed in Photobioreactor for Sustainable Biodiesel Production and Wastewater Bioremediation. Renew. Sustain. Energy Rev. 2020, 134, 110306. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production. Bio. Energy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Znad, H.; Awual, M.R.; Martini, S. The Utilization of Algae and Seaweed Biomass for Bioremediation of Heavy Metal-Contaminated Wastewater. Molecules 2022, 27, 1275. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as Sustainable Renewable Energy Feedstock for Biofuel Production. BioMed Res. Int. 2015, 2015, 519513. [Google Scholar] [CrossRef]
- Moreno-Garrido, I. Microalgae Immobilization: Current Techniques and Uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Roselet, F.; Vandamme, D.; Muylaert, K.; Abreu, P.C. Harvesting of Microalgae for Biomass Production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 211–243. [Google Scholar] [CrossRef]
- Mulbry, W.; Westhead, E.K.; Pizarro, C.; Sikora, L. Recycling of Manure Nutrients: Use of Algal Biomass from Dairy Manure Treatment as a Slow Release Fertilizer. Bioresour. Technol. 2005, 96, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Siripornadulsil, S.; Falcao, V.; Torres, M.; Colepicolo, P.; Sayre, R. Phycoremediation of Heavy Metals Using Transgenic Microalgae. In Transgenic Microalgae as Green Cell Factories; León, R., Galván, A., Fernández, E., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; pp. 99–109. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and Lipid Induction Strategies in Microalgae for Biofuel Production and Other Applications. Microbial Cell Factories 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in Algal-Prokaryotic Wastewater Treatment: A Review of Nitrogen Transformations, Reactor Configurations and Molecular Tools. J. Environ. Manag. 2018, 217, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Sharma, K.; Shah, M.P. Phycoremediation: A Sustainable Alternative in Wastewater Treatment (WWT) Regime. Environ. Technol. Innov. 2022, 25, 102040. [Google Scholar] [CrossRef]
- Kummerer, K. Pharmaceuticals in the Environment—A Brief Summary. In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–21. [Google Scholar] [CrossRef]
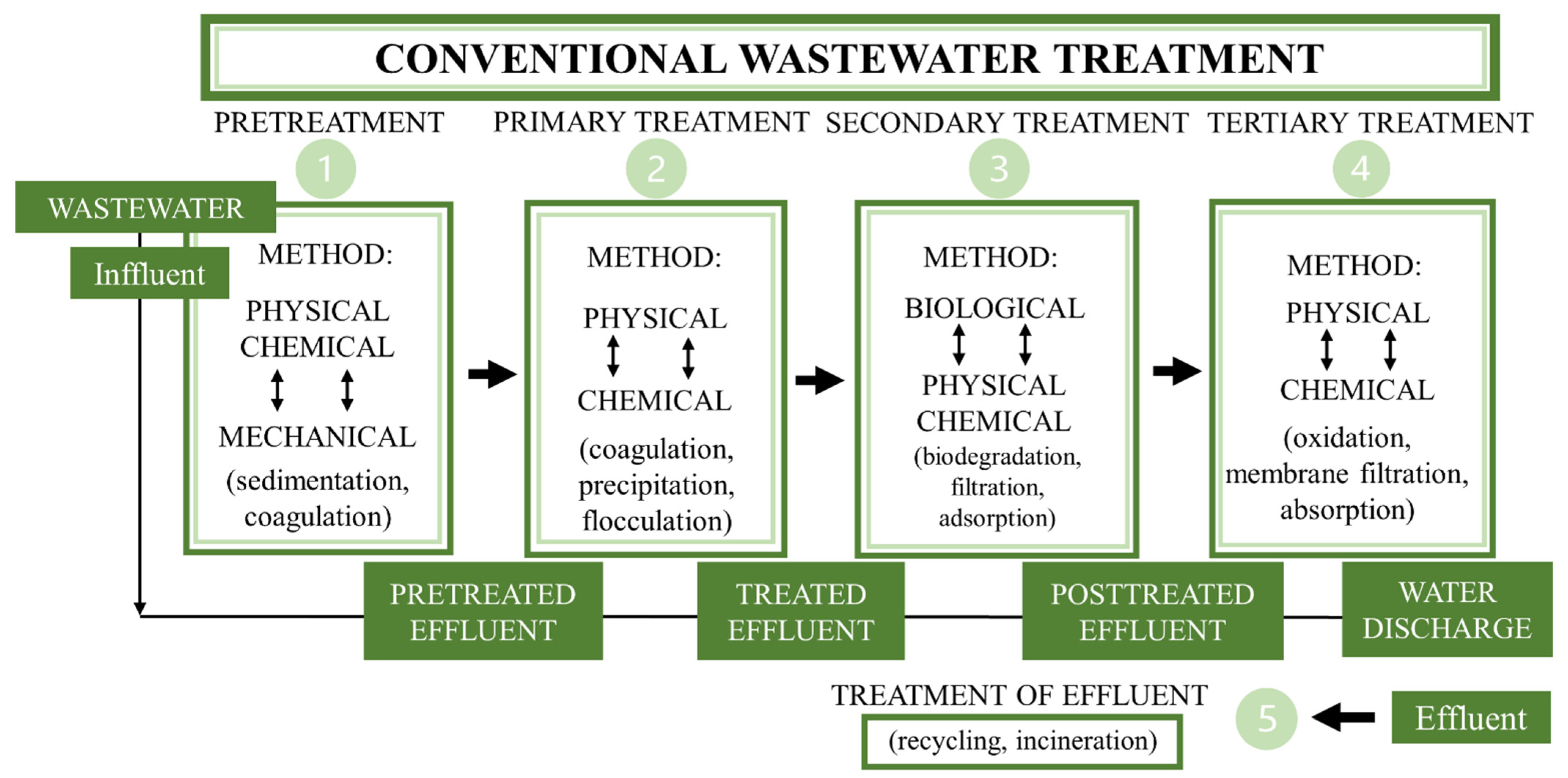
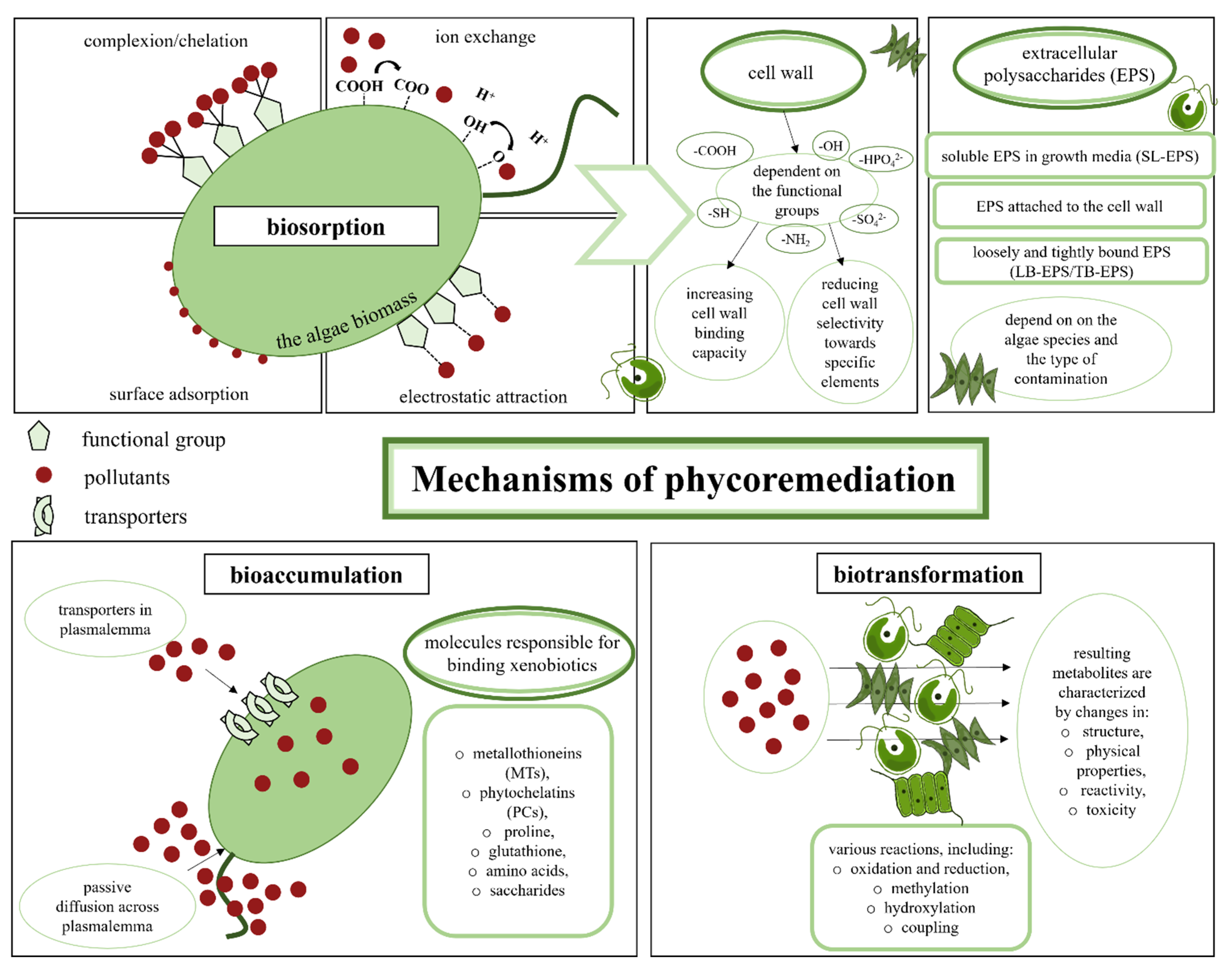

| Surface Water (ng/L) | Reference | Wastewater (Effluents (E)/Influents (I)) (ng/L) | Reference | Drinking Water (DW)/Underground Water (UW) (ng/L) | Reference | Area |
|---|---|---|---|---|---|---|
| Ibuprofen | ||||||
| <0.3–56.0 3730.1 222.0 2.2 0.0−346.0 21.0–2796.0 0.9−115.8 0.1−0.6 524.0−17,600.0 | [42] [43] [44] [45] [46] [47] [48] [49] [50] | 31,250.0 0−4926.0 (I) 20,130.0 0−10,600.0 (E) 0.2−1.9 | [43] [44] [46] [51] [49] | 223.6 (UW)/599.0 (DW) ND−1.2 92 5850.0 <LOD−17.2 7.0−836.7 | [43] [51] [51] [52] [53] | UK Poland Portugal Sweden Serbia North America China Taiwan Vietnam South Africa |
| Diclofenac | ||||||
| <0.5–261.0 5401.5 241.0 1.7−3.6 0−324 17.0–42.0 ND−1.5 0.3−0.4 1010.0−10,200.0 | [42] [43] [54] [45] [46] [47] [48] [49] [50] | 40,570.2 0–269.0 (I) 1338.0 286.0 0.1−1.0 | [43] [44] [46] [55] [49] | 2770.0 (GW)/114.3 (DW) <LOD−2.4 2.1−33.2 | [43] [52] [53] | UK Poland Portugal Sweden Serbia North America China Taiwan Vietnam South Africa |
| Naproxen | ||||||
| <0.3–55.0 1091.9 178.0 0.2 0−74.2 22.0 3.5 0.1−0.4 59,300.0 | [42] [43] [54] [45] [46] [47] [56] [49] [50] | 551,960.0 8.8−1617.0 (I) 208.0 23,210.0 (I) 470.0 0.1−0.6 | [43] [44] [46] [57] [55] [49] | 21.0 (GW)/13.0 (DW) 27.6 44.0 <LOD−3.1 128.0 | [43] [46] [58] [52] [53] | UK Poland Portugal Sweden Serbia North America China Taiwan Vietnam South Africa |
| Ketoprofen | ||||||
| <0.5–4.0 132.2 0.3−89.0 0.3−1.3 1.4−54.5 509 <0.5−0.5 443.0−9220.0 | [42] [43] [54] [45] [59] [60] [49] [50] | 233,630.0 289.0−589.0 (I) 247.0 0.1−1.6 | [43] [44] [46] [49] | 731.8 (GW)/166.9 (DW) 0.09 16.0 (DW) 4.1 (GW) ND | [43] [61] [46] [62] [63] | UK Poland Portugal Sweden Serbia China Vietnam South Africa |
| Compound | Tested Organisms (Taxonomic Group) | Tested Concentration | Exposure Time | Effect (Acute and Chronic) | Reference | |
|---|---|---|---|---|---|---|
| Plants | ||||||
| Ibuprofen | Desmodeus subspicatus | Chlorophyta | 315.0 (mg/L) | Growth inhibition (EC50). | [73] | |
| Lemna minor | Tracheophyta | 22.0 (mg/L) | 7 d | Growth inhibition (EC50). | [73] | |
| Animals | ||||||
| Ibuprofen | Cyprinus carpio | Pisces | 7.1 (mg/L) | 12, 24, 48, 72, 96 h | Genotoxic effects: DNA damage (the intensity of the tail DNA relative to the head). | [74] |
| Cyprinus carpio | 1.5, 3.0, 4.5, 6.0, 7.5, 9.0, 11.5 (mg/L) | 96 h | Teratogenic effect: higher mortality of oocytes and delay in hatching. Delay in embryo development and embryo malformations. | [75] | ||
| Danio rerio | 0.04, 0.2, 1.0, 5.0, 25.0 (mg/L) | 56 h | Reproduction disruption: disruption of cardiac physiology of embryos. | [76] | ||
| Danio rerio | 0.000092 (mg/L) | Genotoxic effects: DNA fragmentation, apoptosis and genomic alterations. | [77] | |||
| Danio rerio | 10.0, 100.0, 1000.0 (mg/L) | 14 d | Genotoxic effects: disruption of gonadotropin production. Increase in the transcription level of genes involved in the acceleration of gametogenesis, maturation of oocytes in females and spermatogenesis in males. | [78] | ||
| Oryzias latipes | 0.0001 (mg/L) | 21 d | Genotoxic effects: influence of sex steroid hormones. Changes in the production of estradiol (E2). Endocrine-disrupting effect: significant increase in vitellogenin (VTG). | [79] | ||
| Oryzias latipes | 0.01, 0.1, 1.0, 10.0, 100.0, 1000.0 (mg/L) | 132 d | Genotoxic effects: disruption of reproduction processes and early life stages. Reproduction disruption: delay in spawning. | [79] | ||
| Crassostrea gigas | Molluscs | 1.0, 100.0 (mg/L) | 7 d | Gene expression disorder: differences in gene transcription in gill tissue. Significant upregulation of CYTP450 genes. | [80] | |
| Dreissena polymorpha | 0.2, 1.0, 3.0 (mM) | 1 h | Acute cytogenotoxic effect: irreversible DNA damage and decrease in LMS. | [81] | ||
| Dreissena polymorpha | 1.0, 9.0, 35.0 (nM) | 96 h | Oxidative stress: increase in activity levels of SOD, CAT, GPx and GST. | [82] | ||
| Ruditapes philippinarum | 0.1, 5.0, 10.0, 50.0 (mg/L) | 35 d | Acute cytogenotoxic effect: decrease in LMS in haemolymph. | [83] | ||
| Ruditapes philippinarum | 0.1, 5.0, 10.0, 50.0 (mg/L) | 14 d | Oxidative stress: increase in GPx activity and LPO. | [84] | ||
| Ampelisca brevicornis | Crustaceans | 0.05, 0.5, 5.0, 50.0, 500.0 (ng/g) | 10 d | Oxidative stress: significant increase in DBF, GST and GPX activity. | [85] | |
| Daphnia magna | 2.9 (mg/L) | 48, 96 h | Genotoxicity effect: DNA damage. | [86] | ||
| Daphnia magna | 20.0, 40.0, 80.0 (mg/L) | 24 h | Endocrine disruption: deregulation of eicosanoid metabolism, the endocrine system and oogenesis. | [87] | ||
| Daphnia magna | 20.0, 40.0, 80.0 (mg/L) | Decrease in reproduction or complete reproduction inhibition. | [88] | |||
| Daphnia magna | 0.0005, 0.005, 0.05 (mg/L) | 21 d, 6 h | Oxidative stress: the induction of antioxidant enzymes (GST, SOD and CAT). Reproduction disruption: significant decrease in the total number of broods per female, body length and intrinsic growth rate. | [89] | ||
| Hediste diversicolor | Polychaeta | 5.0, 500.0 (ng/g) | Genotoxic effect: DNA damage. | [90] | ||
| Plants | ||||||
| Diclofenac | Desmodeus subspicatus | Chlorophyta | 72.0 (mg/L) | Growth inhibition (EC50). | [73] | |
| Dunaliella tertiolecta | 185.7 (mg/L) | 96 h | Growth inhibition (EC50). | [55] | ||
| Pseudokirchneriella subcapitata | 20.0 (mg/L) | 96 h | Growth retardation. | [65] | ||
| Scenedesmus vacuolatus | 23.0 (mg/L) | Inhibition of reproduction. | [91] | |||
| Lemna minor | Tracheophyta | 7.5 (mg/L) | 7 d | Growth inhibition (EC50). | [73] | |
| Polystichum setiferum | 0.0003 (mg/L) | 48 h | Hormetic effects in mitochondrial activity in spores. | [92] | ||
| Animals | ||||||
| Diclofenac | Cirrhinus mrigala | Pisces | 0.001 (mg/L) | 96 h | Oxidative stress: induction of enzymatic activity. | [93] |
| Cyprinus carpio | 0.001 (mg/L) | 96 h | Alterations in hematological and biochemical activities. | [94] | ||
| Cyprinus carpio | 17.6 (mg/L) | 12, 24, 48, 72, 96 h | Genotoxic effects: DNA damage (the intensity of the tail DNA relative to the head). | [74] | ||
| Cyprinus carpio | 1.25, 2.5 and 5.0 (mg/L) | 21 d | Deformations: histopathological changes in gills, liver and kidney. Lesions included necrosis of epithelial cells. | [95] | ||
| Danio rerio embryos | 12.5 (mg/L) | 48 h | Oxidative stress: deregulation of kinase activities. Metabolic disorders: deregulation of gluconeogenesis and lipid metabolism. | [96] | ||
| Hoplias malabaricus | 0.2, 2.0, 20.0 (mg/kg) | Metabolic disorders: interferences with metabolic pathways. Oxidative stress: increase in the activity of SOD, GPx and GSH. | [97] | |||
| Oncorhynchus mykiss | 0.005 (mg/L) | 28 d | Deformations: renal lesions and alterations in the gills. | [98] | ||
| Oryzias latipes | 7.1, 37.0,78.0 (mg/L) | 14 d | Morphological abnormalities. | [99] | ||
| Rhamdia quelen | 25.0 (mg/L) | Behavioral changes: respiratory disorders and loss of balance. | [100] | |||
| Rhamdia quelen | 0.2, 2.0, 20.0 (mg/L) | 21 d | Oxidative stress: significant reduction in SOD activity, increase in activity of GSH and GST. Disruption of antioxidant defense systems in the liver. | [101] | ||
| Brachionus calyciflorus | Rotatoria | 25.0 (mg/L) | 48 h | Reproduction retardation. | [65] | |
| Dreissena polymorpha | Molluscs | 0.2, 0.5, 0.8 (mM) | 1 h | Acute cytogenotoxic effect: significant DNA damage. | [81] | |
| Dreissena polymorpha | 1000.0 (mg/L) | 96 h | Oxidative stress: increase in GST activity, LPO expression and methallothioneins (MTs) alterations. | [102] | ||
| Dreissena polymorpha | 0.001 (mg/L) | 96 h | Oxidative stress: high lipid peroxidation levels. Significant reduction in haemocyte viability. | [81,103] | ||
| Perna perna | 20.0, 200.0, 2000.0 (ng/L) | 48, 96 h | Genotoxic effects: DNA damage. Significant decrease in LMS. Gene expression upregulation. COX inhibition in gill tissue. | [104] | ||
| Atyaephyra desmarestii | Crustaceans | 13.3, 70.6 (mg/L) | 96 h | Metabolism disorder: decrease in respiration under reduced oxygen content. | [105] | |
| Carcinus maenas | 0.00001, 0.0001 (mg/L) | Osmoregulation disturbances. Effect on haemolymph osmolality and osmolality capacity. | [106] | |||
| Ceriodaphnia dubia | 2.0 (mg/L) | 7 d | Reproduction inhibition. | [65] | ||
| Daphnia magna | 32.0 (mg/L) | 21 d | Oxidative stress. | [103] | ||
| Daphnia magna | 9.7 (mg/L) | 48, 96 h | Genotoxicity effect: DNA damage. | [86] | ||
| Arenicola marina | Polychaetes | From 0.6 to 842.0 (ng/L) | Reproduction disruption: decrease in swimming speed of sperm. | [107] | ||
| Hediste diversicolor | 0.5, 1.0, 2.0 (mg/L) | 28 d | Gene expression upregulation: significant effect on the activity of GST enzymes. | [108] | ||
| Plants | ||||||
| Naproxen | Cymbella sp. | Ochrophyta | 102.8 (mg/L) | 72 h | Growth inhibition (EC50). | [109] |
| Desmodeus subspicatus | Chlorophyta | >320.0 (mg/L) | Growth inhibition (EC50). | [73] | ||
| Raphidocelis subcapitata | 0.0318 (mg/L) | 72 h | Growth inhibition (EC50). | [110] | ||
| Scenedesmus quadricauda | 101.5 (mg/L) | 72 h | Growth inhibition (EC50). | [109] | ||
| Scenedesmus subspicatus | 625.5 (mg/L) | 48 h | Growth inhibition (EC50). | [70] | ||
| Lemna minor | Tracheophyta | 24.2 (mg/L) | 7 d | Growth inhibition (EC50). | [70] | |
| Animals | ||||||
| Naproxen | Danio rerio | Pisces | 1.0, 100.0 (mg/L) | 14 d | Gene expression: upregulation of gene expression. Metabolism disorders: upregulation of the activity of GST by affecting glutathione S-transferase P2 (GST P2) mRNA in the intestine. | [111] |
| Oryzias latipes | 0.005, 0.05, 0.5, 5.0, 50.0 (mg/L) | Endocrine disruption: significant increase in the expression of VTG and E2 receptors genes. Reduction in conditions: decrease in the survival of juvenile animals. | [112] | |||
| Daphnia magna | Crustaceans | 46.7 (mg/L) | 48 h | Immobilization (EC50). | [112] | |
| Daphnia magna | 2.9 (mg/L) | 48, 96 h | Genotoxicity effect: DNA damage. Oxidative stress: increase in enzyme activity (SOD, CAT and GPx). | [86] | ||
| Hyalella azteca | 76.6, 339.2 (mg/kg) | 48 h | Genotoxicity effect: DNA damage. Oxidative stress: increase in SOD and CAT activity and decrease in GPX activity. | [113] | ||
| Moina macrocopa | 74.1 (mg/L) | 48 h | Immobilization (EC50). | [112] | ||
| Elliptio complanata | Molluscus | 0.6 to 23.0 (mg/L) | 24 h | Immunotoxic effects. Phagocytic activity, intracellular esterase activity, cell adherence and lipid peroxidation. | [114] | |
| Hydra magnipapillata | Cnidaria | LC50 52.0, 45.0, 43.0 (mg/L) | 24, 48, 72 h | Morphological changes: stimulation of the contraction of the body column and tentacles. Genotoxicity effect: DNA damage or instability. | [115] | |
| Plants | ||||||
| Ketoprofen | Lemna minor | Tracheophyta | 0.2, 1.2, 6.0, 30.0 (mg/L) | 4 d | Oxidative stress: alterations in enzyme activities (CAT, GSTs and CA). | [116] |
| Animals | ||||||
| Ketoprofen | Ceriodaphnia dubia | Crustaceans | From 1.0 to 1000.0 (mg/L) | Chronic toxicity. Effects on reproduction at the highest concentration. | [117] | |
| Daphnia magna | 0.2, 1.2, 6.0, 30.0 (mg/L) | 4 d | Oxidative stress: alterations in enzyme activities (CAT, GSTs and CA). | [116] | ||
| Mytilus galloprovincialis | Molluscus | 0.0025 (mg/L) | 14, 30, 60 d | Alterations in immunological parameters, genotoxic effects and modulation of lipid metabolism. Reduction in lysosomal membrane stability. | [118] | |
| Planorbarius corneus | 100.0 (mg/g) | 48 h | Antipyretic effect. Inhibition of symptoms of behavioral fever and influenced thermal preference. | [119] | ||
| Carrier Used | Group of Carrier | Algae Species | Removed Contaminants | References |
| Alginate | Organic carrier (natural polymers) | Chlorella | Ni, Zn, Cd | [277] |
| Pediastrum boryanum | Cr (VI) | [278] | ||
| Chlorella vulgaris | Cu, Ni | [279] | ||
| Alginate beads | Chlorella emersonii | Hg | [280] | |
| Tetraselmis chui | Cu, Cd | [9] | ||
| Alginate gel | Isochrysis galbana | Cr (III) | [281] | |
| Alginate | Dunaliella salina | P | [282] | |
| Alginate | Chlorella vulgaris Chlamydomonas reinhardtii | Pb | [3] | |
| Alginate | Scenedesmus intermedius | N, P | [272] | |
| Chitosan | Organic carrier (natural polymers) | Scenedesmus spp. | Nitrate, phosphate | [283] |
| Carrageenan beads | Organic carrier (natural polymers) | Scenedesmus acutus Chlorella vulgaris | Zn, Cd, Cr | [284] |
| Carrageenan | Anabaena doliolum Chlorella vulgaris | N, P | [285] | |
| Polyurethane foam | Organic carrier (synthetic polymers) | Scenedesmus acutus Chlorella vulgaris | Zn, Cd, Cr | [284] |
| Carboxymethyl cellulose (CMC) beads | Organic carrier (synthetic polymers) | Chlamydomonas reinhardtii | U (VI) | [7] |
| Silica gel | Organic carrier (synthetic polymers) | Chlorella vulgaris | Hg | [286] |
| Glass beads | Inorganic carrier | Aulosira fertilissima | Ni, Cr | [287] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. https://doi.org/10.3390/ijerph19137717
Hejna M, Kapuścińska D, Aksmann A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. International Journal of Environmental Research and Public Health. 2022; 19(13):7717. https://doi.org/10.3390/ijerph19137717
Chicago/Turabian StyleHejna, Monika, Dominika Kapuścińska, and Anna Aksmann. 2022. "Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae" International Journal of Environmental Research and Public Health 19, no. 13: 7717. https://doi.org/10.3390/ijerph19137717
APA StyleHejna, M., Kapuścińska, D., & Aksmann, A. (2022). Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. International Journal of Environmental Research and Public Health, 19(13), 7717. https://doi.org/10.3390/ijerph19137717