Factors Influencing Dechlorane plus Distributions in Various Sheep Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Instrumental Analysis
2.4. Quality Assurance and Quality Control
2.5. Software
3. Results and Discussion
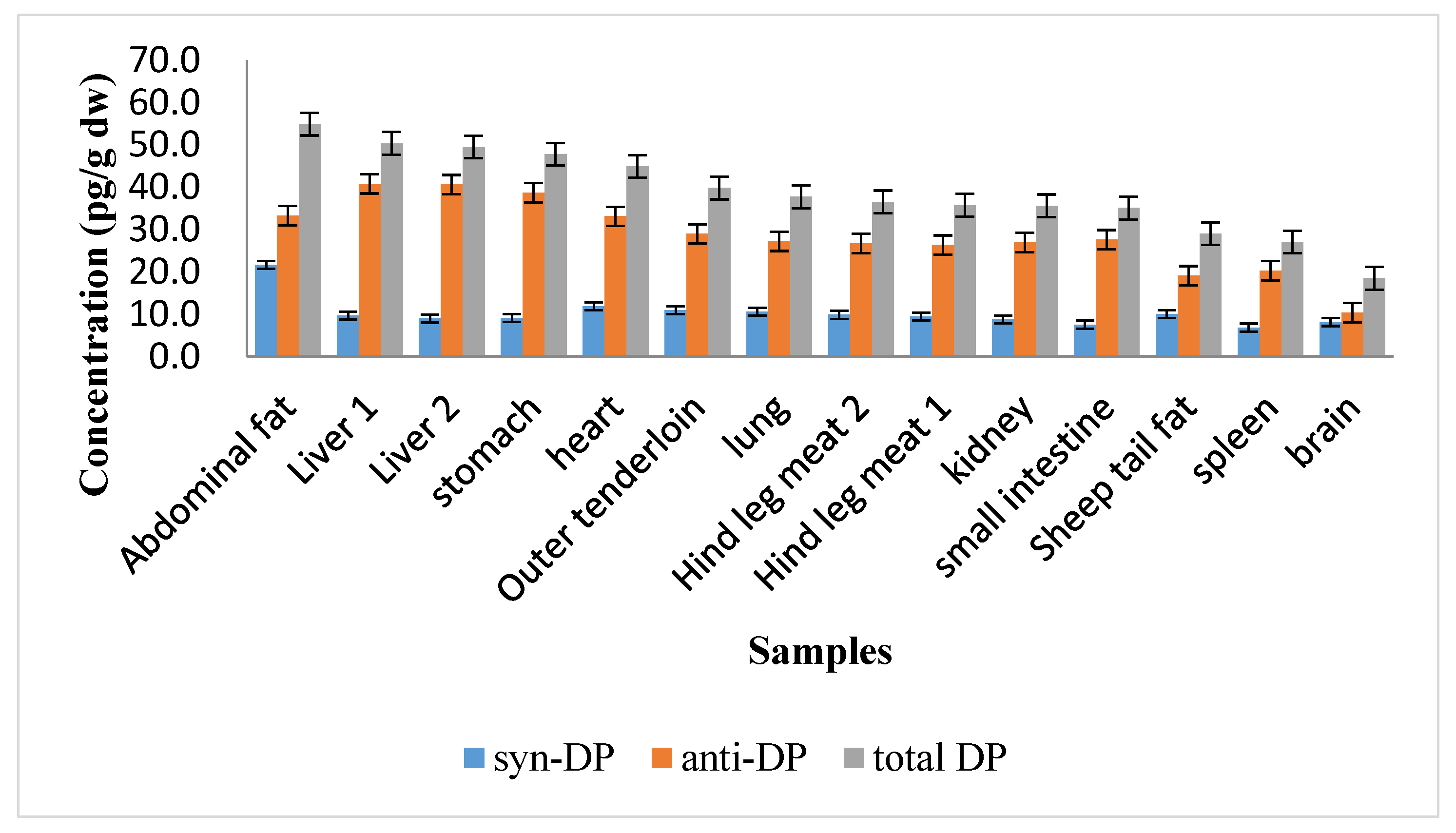
3.1. Concentrations of DP in Sheep Tissues
3.2. Binding between DP and SSA
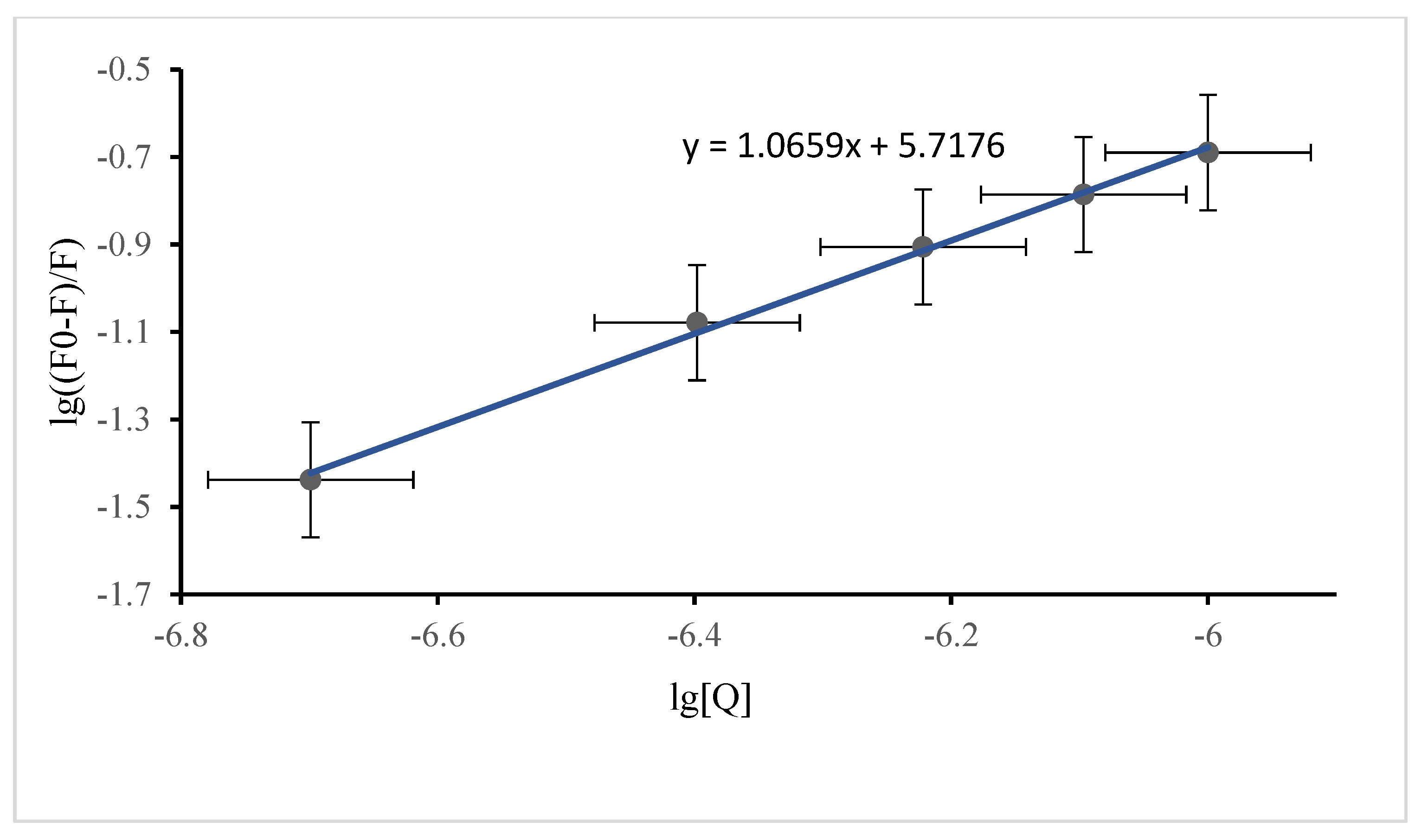
3.2.1. Fluorescence Quenching
3.2.2. Binding Constant for DP and SSA
3.2.3. Ability of DP to Bind to CYP3A24
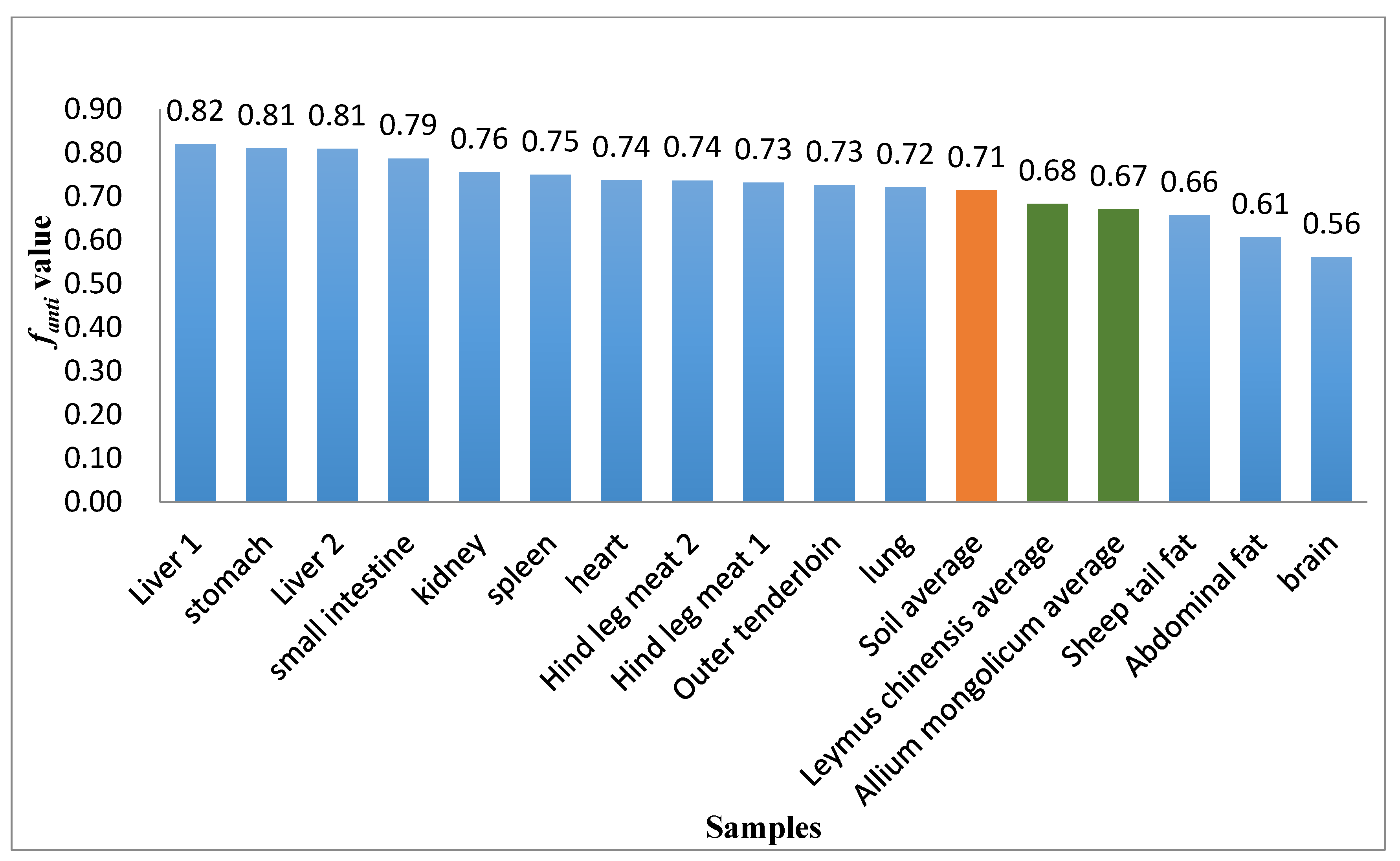
3.3. fanti Values for Sheep Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feo, M.L.; Barón, E.; Eljarrat, E.; Barceló, D. Dechlorane plus and related compounds in aquatic and terrestrial biota: A review. Anal. Bioanal. Chem. 2012, 404, 2625–2637. [Google Scholar] [CrossRef] [PubMed]
- Tomy, G.T.; Pleskach, K.; Ismail, N.; Whittle, D.M.; Helm, P.A.; Sverko, E.; Zaruk, D.; Marvin, C.H. Isomers of Dechlorane plus in Lake Winnipeg and Lake Ontario Food Webs. Environ. Sci. Technol. 2007, 41, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Wang, P.; Chen, W.H.; Ren, D.W.; Zhang, Q.H.; Liang, Y.; Zhang, J. PCBs, PBDEs and PCDD/Fs in the grass samples from the Himalayas, China. Environ. Chem. 2012, 31, 26–30. [Google Scholar]
- Wang, J. The Source, Emission and Human Exposures of Dechlorane plus. Master’s Thesis, Dalian Maritime University, Dalian, China, 2010. [Google Scholar]
- Muir, D.C.; Howard, P.H. Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol. 2006, 40, 7157–7166. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Xie, Z.; Sturm, R.; Ebinghaus, R. Large-Scale Distribution of Dechlorane plus in Air and Seawater from the Arctic to Antarctica. Environ. Sci. Technol. 2010, 44, 8977–8982. [Google Scholar] [CrossRef] [PubMed]
- Tomy, G.T.; Thomas, C.R.; Zidane, T.M.; Murison, K.E.; Pleskach, K.; Hare, J.; Arsenault, G.; Marvin, C.H.; Sverko, E. Examination of Isomer Specific Bioaccumulation Parameters and Potential In vivo Hepatic Metabolites of syn- and anti-Dechlorane plus Isomers in Juvenile Rainbow Trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2008, 42, 5562–5567. [Google Scholar] [CrossRef]
- Qiu, X.; Marvin, C.H.; Hites, R.A. Dechlorane plus and Other Flame Retardants in a Sediment Core from Lake Ontario. Environ. Sci. Technol. 2007, 41, 6014–6019. [Google Scholar] [CrossRef]
- Sverko, E.; Tomy, G.T.; Reiner, E.J.; Li, Y.-F.; McCarry, B.E.; Arnot, J.A.; Law, R.J.; Hites, R.A. Dechlorane plus and Related Compounds in the Environment: A Review. Environ. Sci. Technol. 2011, 45, 5088–5098. [Google Scholar] [CrossRef]
- Wu, J.-P.; Zhang, Y.; Luo, X.-J.; Wang, J.; Chen, S.-J.; Guan, Y.-T.; Mai, B.-X. Isomer-Specific Bioaccumulation and Trophic Transfer of Dechlorane plus in the Freshwater Food Web from a Highly Contaminated Site, South China. Environ. Sci. Technol. 2009, 44, 606–611. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kim, J.-C.; Jin, G.-Z.; Park, H.; Baek, S.-Y.; Chang, Y.-S. Detection of Dechlorane plus in fish from urban-industrial rivers. Chemosphere 2010, 79, 850–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.P.; Luo, X.J.; Mai, B.X. The flame retardant Dechlorane plus (DP) and its dechlorination products in the tissue distribution of fish. In Proceedings of the 6th National Conference on Environmental Chemistry, Shanghai, China, 21–24 September 2011. [Google Scholar]
- Yin, H.Y.; Zheng, X.F.; Cao, H.Y.; Zhang, X.H. Synthesis of 4-Thiothymidine and Its Interaction with Human Serum Albumin. Chin. J. Lumin. 2013, 34, 104–110. [Google Scholar]
- Brasseur, C.; Pirard, C.; Scholl, G.; De Pauw, E.; Viel, J.-F.; Shen, L.; Reiner, E.; Focant, J.-F. Levels of dechloranes and polybrominated diphenyl ethers (PBDEs) in human serum from France. Environ. Int. 2014, 65, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.B.; Luo, X.J.; Zeng, Y.H.; Wu, J.P.; Mai, B.X. Sources, gastrointestinal absorption and stereo-selective and tissue-specific ac-cumulation of Dechlorane plus (DP) in chicken. Chemosphere 2014, 114, 241–246. [Google Scholar] [CrossRef]
- Rang, H.P. Pharmacology; Churchill Living Stone: Edinburgh, NY, USA, 2003. [Google Scholar]
- Li, Y.; Yu, L.; Wang, J.; Wu, J.; Mai, B.; Dai, J. Accumulation pattern of Dechlorane plus and associated biological effects on rats after 90 d of exposure. Chemosphere 2013, 90, 2149–2156. [Google Scholar] [CrossRef]
- Yang, M.L.; Dong, Y.C.; Wang, L.X.; Zhang, J. Spectroscopic Studies of Interaction between Matrine and HSA and BSA. Hubei Agric. Sci. 2018, 57, 96–99. [Google Scholar]
- Jahanban-Esfahlan, A.; Panahi-Azar, V. Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food Chem. 2016, 202, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, G.; Shaghaghi, M.; Sattari, S.; Jouyban, A. Interaction of human serum albumin with Fe(III)–deferasirox studied by multispectroscopic methods. J. Lumin. 2014, 149, 251–257. [Google Scholar] [CrossRef]
- Gowda, J.I.; Nandibewoor, S.T. Binding and conformational changes of human serum albumin upon interaction with 4-aminoantipyrine studied by spectroscopic methods and cyclic voltammetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 397–403. [Google Scholar] [CrossRef]
- Ahrens, I.; Bode, C. Rivaroxaban for stroke prevention in atrial fibrillation and secondary prevention in patients with a recent acute coronary syndrome. Future Cardiol. 2012, 8, 533–541. [Google Scholar] [CrossRef]
- Cao, T.W.; Zhou, K.; Huang, W.B.; Shi, J.W.; Tan, X.P.; Lin, H.C.; Ran, A. Spectroscopic Study of Interaction of Harpagoside and Human Serum Albumin. Chin. J. Anal. Chem. 2017, 5, 700–706. [Google Scholar]
- Gentili, P.L.; Ortica, F.; Favaro, G. Static and Dynamic Interaction of a Naturally Occurring Photochromic Molecule with Bovine Serum Albumin Studied by UV−Visible Absorption and Fluorescence Spectroscopy. J. Phys. Chem. B 2008, 112, 16793–16801. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Al-Asbahy, W.M.; Afzal, M.; Arjmand, F. Synthesis, characterization and interaction studies of copper based drug with Human Serum Albumin (HSA): Spectroscopic and molecular docking investigations. J. Photochem. Photobiol. B Biol. 2012, 114, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zheng, J.; Yan, X.; Yu, L.; Luo, X.; Peng, X.; Yu, Y.; Yang, Z.; Mai, B. Dechlorane plus in paired hair and serum samples from e-waste workers: Correlation and differences. Chemosphere 2014, 123, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hoh, E.; Zhu, L.; Hites, R.A. Dechlorane plus, a Chlorinated Flame Retardant, in the Great Lakes. Environ. Sci. Technol. 2006, 40, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.N.; Siddique, S.; Lavoie, L.; Takser, L.; Abdelouahab, N.; Zhu, J. Hexachloronorbornene-based flame retardants in humans: Levels in maternal serum and milk. Environ. Int. 2014, 66, 11–17. [Google Scholar] [CrossRef]
- Cequier, E.; Marcé, R.M.; Becher, G.; Thomsen, C. Comparing human exposure toemerging and legacy flame retardants from the indo or environment and diet with concentrations measured in serum. Environ. Int. 2015, 74, 54–59. [Google Scholar] [CrossRef]
- Bao, J.; Hou, L.; Wang, Y.; Zhang, Y.; Qiao, J.; Su, M.; Jin, J.; Chen, L. Effect of Affinity between Dechlorane plus and Human Serum Albumin on Its Serum Concentration in a General Population. Environ. Sci. Technol. 2022, 56, 2511–2518. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Li, Y.; Shang, H.; Wang, Y.; Wang, T.; Zhang, Q.; Jiang, G. Assessment on the occupational exposure of manufacturing workers to Dechlorane plus through blood and hair analysis. Environ. Sci. Technol. 2013, 47, 10567–10573. [Google Scholar] [CrossRef]
- Ren, G.; Yu, Z.; Ma, S.; Li, H.; Peng, P.; Sheng, G.; Fu, J. Determination of Dechlorane plus in Serum from Electronics Dismantling Workers in South China. Environ. Sci. Technol. 2009, 43, 9453–9457. [Google Scholar] [CrossRef]
- Ben, Y.-J.; Li, X.-H.; Yang, Y.-L.; Li, L.; Zheng, M.-Y.; Wang, W.-Y.; Xu, X.-B. Placental Transfer of Dechlorane plus in Mother–Infant Pairs in an E-Waste Recycling Area (Wenling, China). Environ. Sci. Technol. 2013, 48, 5187–5193. [Google Scholar] [CrossRef]

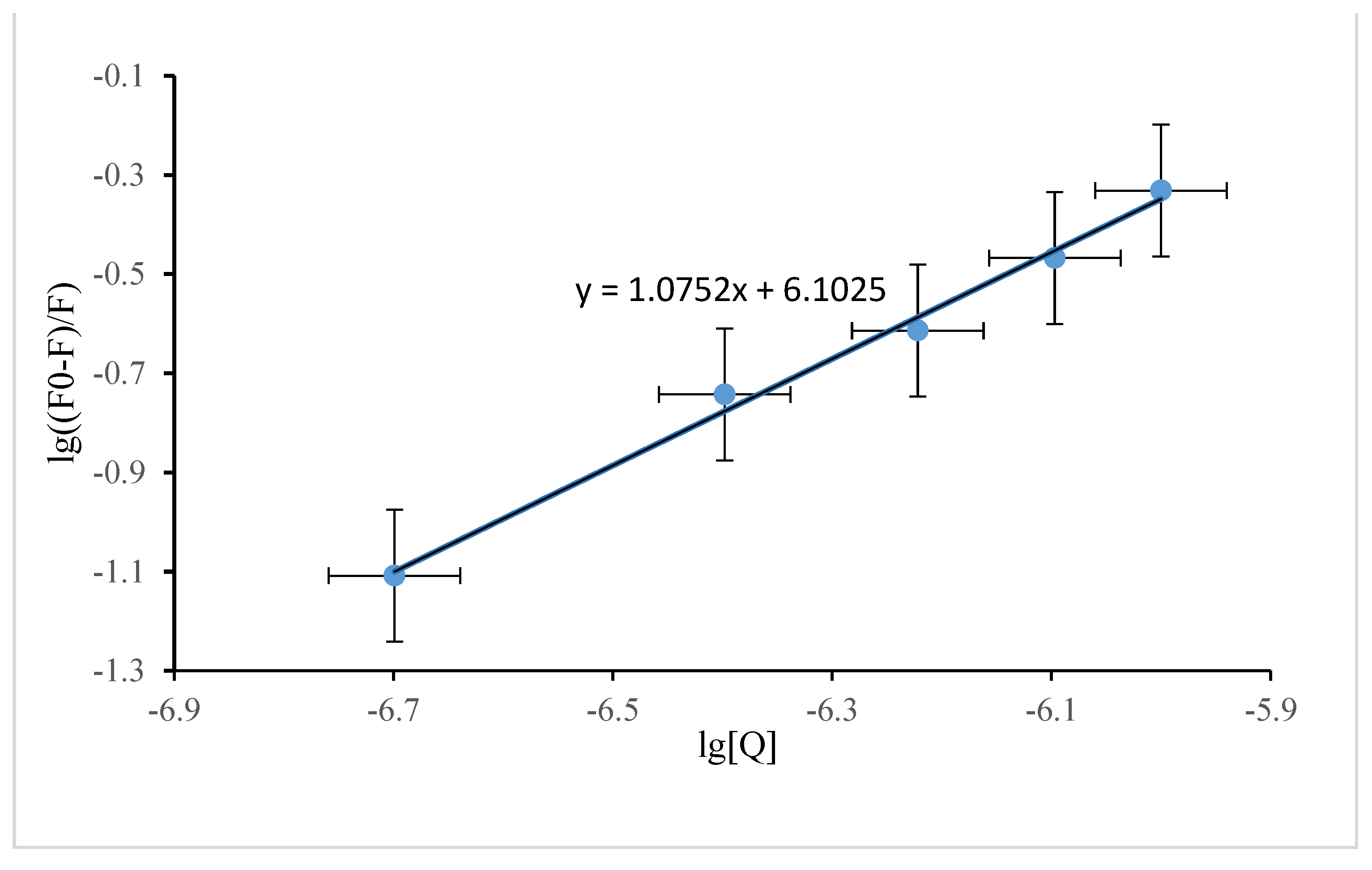
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Chen, W.; Bao, J.; Bu, T.; Li, T.; Liu, Y.; Liu, Y.; Jin, J. Factors Influencing Dechlorane plus Distributions in Various Sheep Tissues. Int. J. Environ. Res. Public Health 2022, 19, 8004. https://doi.org/10.3390/ijerph19138004
Jin H, Chen W, Bao J, Bu T, Li T, Liu Y, Liu Y, Jin J. Factors Influencing Dechlorane plus Distributions in Various Sheep Tissues. International Journal of Environmental Research and Public Health. 2022; 19(13):8004. https://doi.org/10.3390/ijerph19138004
Chicago/Turabian StyleJin, Hongli, Wenming Chen, Junsong Bao, Te Bu, Tianwei Li, Yiming Liu, Yanli Liu, and Jun Jin. 2022. "Factors Influencing Dechlorane plus Distributions in Various Sheep Tissues" International Journal of Environmental Research and Public Health 19, no. 13: 8004. https://doi.org/10.3390/ijerph19138004




