Short-Term Effects of PM10, NO2, SO2 and O3 on Cardio-Respiratory Mortality in Cape Town, South Africa, 2006–2015
Abstract
:1. Introduction
2. Method
2.1. Study Area
2.2. Outcome and Exposure Data
2.3. Statistical Analysis
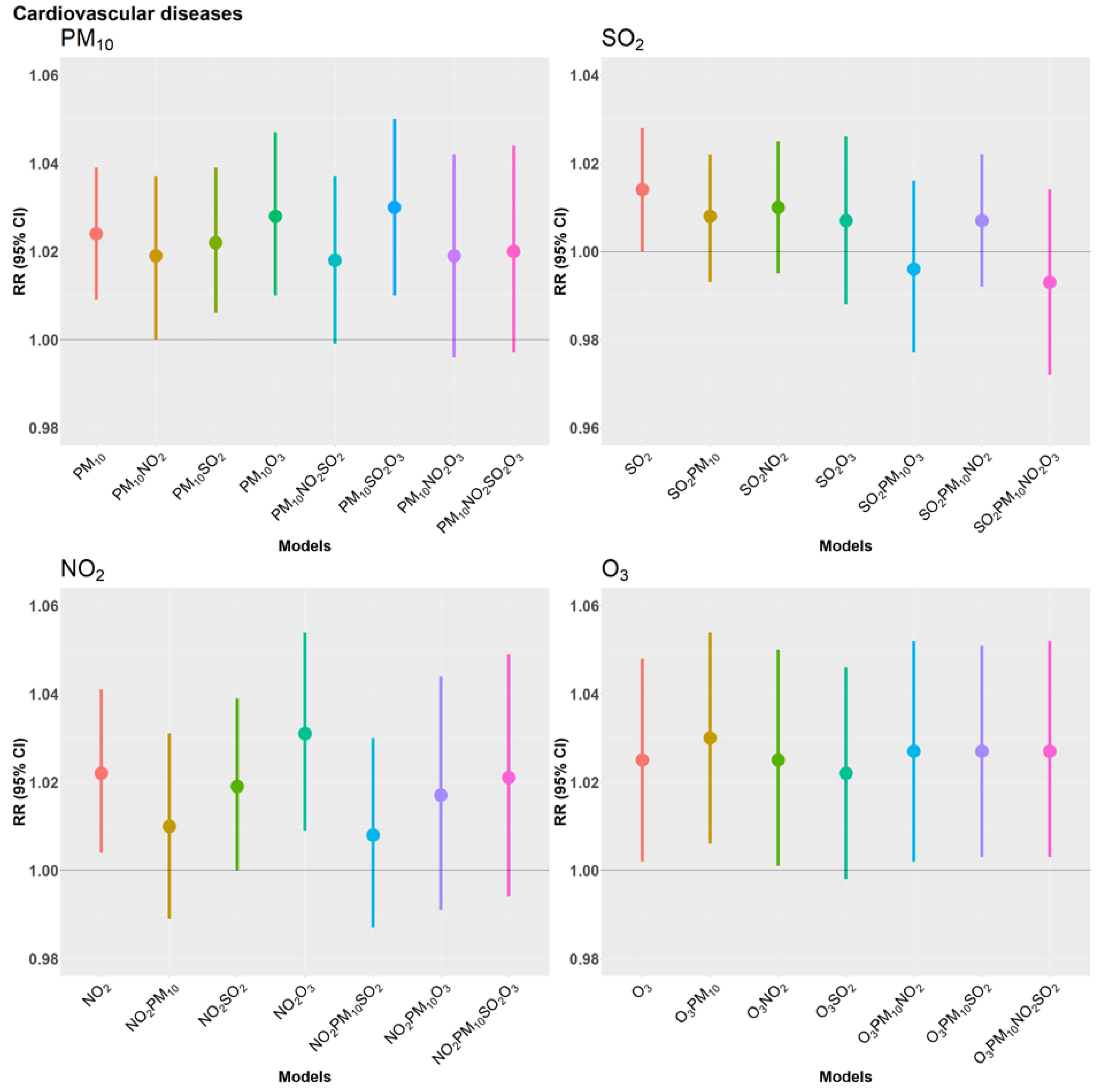
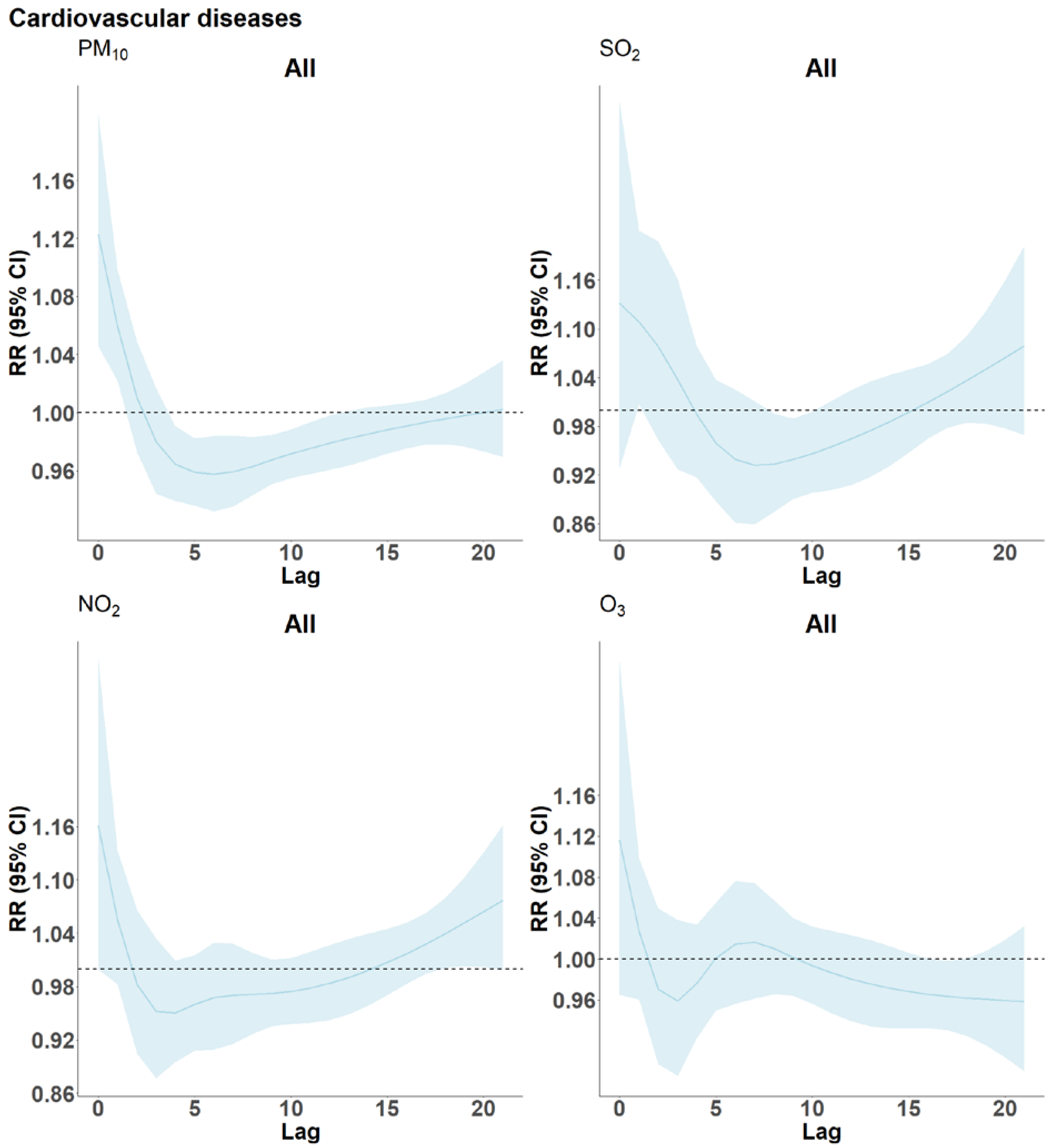
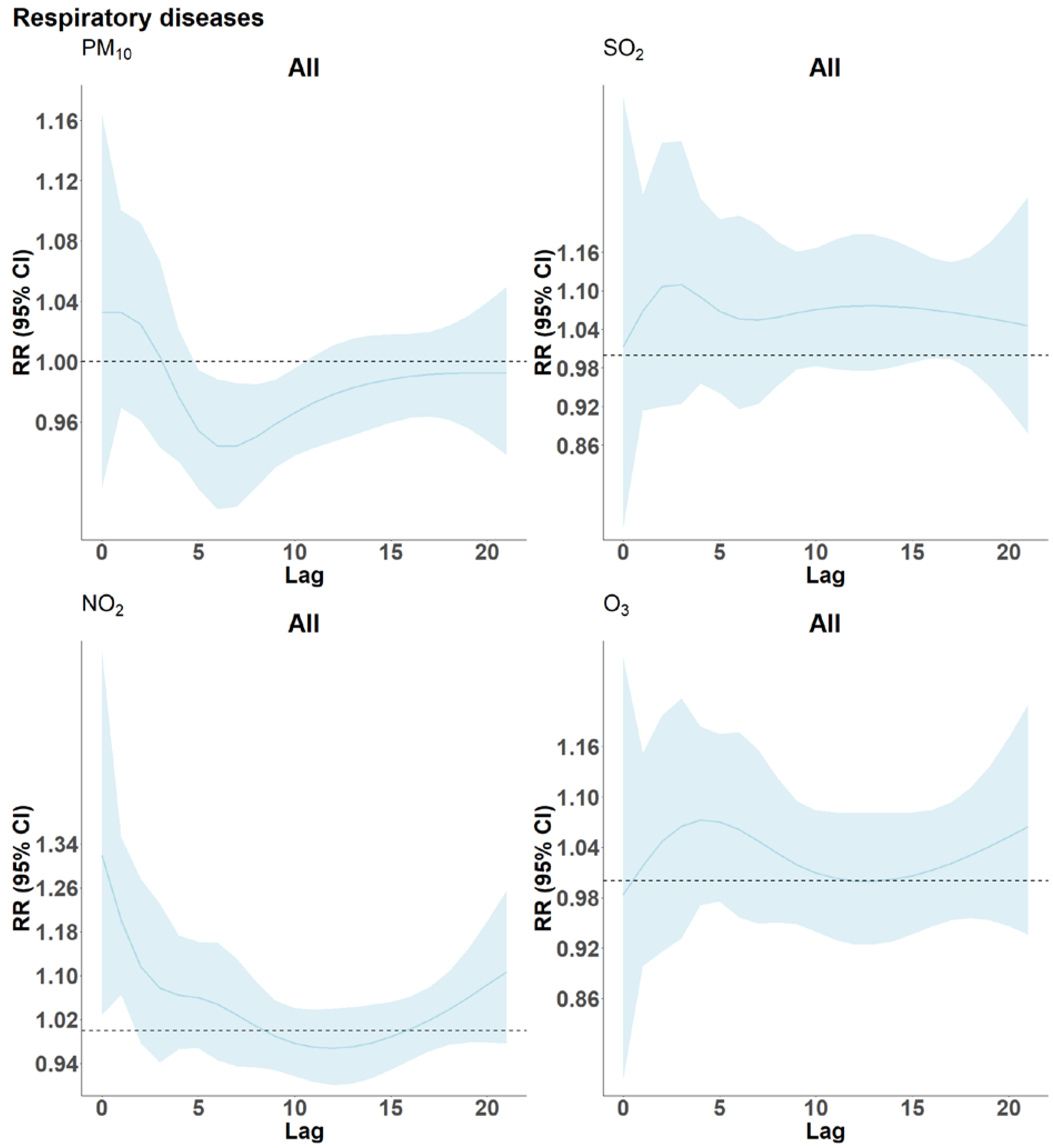
3. Results
4. Discussion
4.1. Brief Overview
4.2. Cardiovascular Disease and Air Pollutants
4.3. Respiratory Disease
4.4. Effect Modification by Sex
4.5. Effect Modification by Age
4.6. Effect Modification by Season
4.7. Harvesting of Frailty by Air Pollution
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. WHO global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Orellano, P.; Reynoso, J.; Quaranta, N. Short-term exposure to sulphur dioxide (SO2) and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2021, 150, 106434. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Voyi, K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int. J. Environ. Res. Public Health 2012, 9, 3978–4016. [Google Scholar] [CrossRef]
- Dominici, F.; McDermott, A.; Daniels, M.; Zeger, S.L.; Samet, J.M. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: Mortality among residents of 90 cities. J. Toxicol. Environ. Health A 2005, 68, 1071–1092. [Google Scholar] [CrossRef]
- Samoli, E.; Peng, R.; Ramsay, T.; Pipikou, M.; Touloumi, G.; Dominici, F.; Burnett, R.; Cohen, A.; Krewski, D.; Samet, J.; et al. Acute effects of ambient particulate matter on mortality in Europe and North America: Results from the APHENA study. Environ. Health Perspect. 2008, 116, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.Y.; Hoek, G.; Eeftens, M.; de Hoogh, K.; Beelen, R.; Beregszászi, T.; Cesaroni, G.; Cirach, M.; Cyrys, J.; De Nazelle, A.; et al. Spatial variation of PM elemental composition between and within 20 European study areas—Results of the ESCAPE project. Environ. Int. 2015, 84, 181–192. [Google Scholar] [CrossRef]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Katsouyanni, K.; Zmirou, D.; Spix, C.; Sunyer, J.; Schouten, J.; Ponka, A.; Anderson, H.; Le Moullec, Y.; Wojtyniak, B.; Vigotti, M.A. Short-term effects of air pollution on health: A European approach using epidemiological time-series data. The APHEA project: Background, objectives, design. Eur. Respir. J. 1995, 8, 1030–1038. [Google Scholar]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Vicedo-Cabrera, A.M.; Sera, F.; Liu, C.; Armstrong, B.; Milojevic, A.; Guo, Y.; Tong, S.; Lavigne, E.; Kyselý, J.; Urban, A.; et al. Short term association between ozone and mortality: Global two stage time series study in 406 locations in 20 countries. BMJ 2020, 368, m108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabethe, N.D.L.; Voyi, K.; Wichmann, J. Association between ambient air pollution and cause-specific mortality in Cape Town, Durban, and Johannesburg, South Africa: Any susceptible groups? Environ. Sci. Pollut. Res. 2021, 28, 42868–42876. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Petrik, L.; Wichmann, J. PM2.5 chemical composition and geographical origin of air masses in Cape Town, South Africa. Air Qual. Atmos. Health 2021, 14, 431–442. [Google Scholar] [CrossRef]
- Hart, J.E.; Garshick, E.; Dockery, D.W.; Smith, T.J.; Ryan, L.; Laden, F. Long-term ambient multipollutant exposures and mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M.L. Protecting Human Health from Air Pollution: Shifting from a Single-Pollutant to a Multipollutant Approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Western Cape Government. 2020 Socio-Economic Profile: City of Cape Town Municipality City of Cape Town; Western Cape Government: Cape Town, South Africa, 2020. [Google Scholar]
- Adebayo-Ojo, T.C.; Wichmann, J.; Arowosegbe, O.O.; Probst-Hensch, N.; Schindler, C.; Künzli, N. Short-Term Joint Effects of PM10, NO2 and SO2 on Cardio-Respiratory Disease Hospital Admissions in Cape Town, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 495. [Google Scholar] [CrossRef]
- South Africa Air Quality Information System. National Air Quality Indicator—Monthly Data Report for the Western Cape Province; South Africa Air Quality Information System: Pretoria, South Africa, 2022. [Google Scholar]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. Cardiovascular Disease. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cardiovascular-disease (accessed on 23 February 2022).
- Nawrot, T.S.; Perez, L.; Künzli, N.; Munters, E.; Nemery, B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet 2011, 377, 732–740. [Google Scholar] [CrossRef]
- Committee on the Medical Effects of Air Pollutants, Department of Health. Cardiovascular Disease and Air Pollution; United Kingdom Department of Health: London, UK, 2006. [Google Scholar]
- Newell, K.; Kartsonaki, C.; Lam, K.B.H.; Kurmi, O.P. Cardiorespiratory health effects of particulate ambient air pollution exposure in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e368–e380. [Google Scholar] [CrossRef] [Green Version]
- Perez, L.; Grize, L.; Infanger, D.; Künzli, N.; Sommer, H.; Alt, G.-M.; Schindler, C. Associations of daily levels of PM10 and NO2 with emergency hospital admissions and mortality in Switzerland: Trends and missed prevention potential over the last decade. Environ. Res. 2015, 140, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Milojevic, A.; Guo, Y.; Tong, S.; Coelho, M.d.S.Z.S.; Saldiva, P.H.N.; et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ 2021, 372, n534. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Aga, E.; Touloumi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the APHEA project. Eur. Respir. J. 2006, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Medical Effects of Air Pollutants, Department of Health. Quantification of Mortality and Hospital Admissions Associated with Ground-level Ozone; Public Health England: London, UK, 2015. [Google Scholar]
- Newell, K.; Kartsonaki, C.; Lam, K.B.H.; Kurmi, O. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: A systematic review and meta-analysis. Environ. Health 2018, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Chiusolo, M.; Cadum, E.; Stafoggia, M.; Galassi, C.; Berti, G.; Faustini, A.; Bisanti, L.; Vigotti, M.A.; Dessì, M.P.; Cernigliaro, A.; et al. Short-Term Effects of Nitrogen Dioxide on Mortality and Susceptibility Factors in 10 Italian Cities: The EpiAir Study. Environ. Health Perspect. 2011, 119, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieb, D.M.; Judek, S.; Burnett, R.T. Meta-analysis of time-series studies of air pollution and mortality: Effects of gases and particles and the influence of cause of death, age, and season. J. Air Waste Manag. Assoc. 2002, 52, 470–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, M.L.; McDermott, A.; Zeger, S.L.; Samet, J.M.; Dominici, F. Ozone and Short-term Mortality in 95 US Urban Communities, 1987–2000. JAMA 2004, 292, 2372–2378. [Google Scholar] [CrossRef] [Green Version]
- Stafoggia, M.; Forastiere, F.; Faustini, A.; Biggeri, A.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Pandolfi, P.; Serinelli, M.; et al. Susceptibility factors to ozone-related mortality: A population-based case-crossover analysis. Am. J. Respir. Crit. Care Med. 2010, 182, 376–384. [Google Scholar] [CrossRef]
- Wong, C.-M.; Vichit-Vadakan, N.; Kan, H.; Qian, Z. Public Health and Air Pollution in Asia (PAPA): A multicity study of short-term effects of air pollution on mortality. Environ. Health Perspect. 2008, 116, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Morakinyo, O.M.; Adebowale, A.S.; Mokgobu, M.I.; Mukhola, M.S. Health risk of inhalation exposure to sub-10 µm particulate matter and gaseous pollutants in an urban-industrial area in South Africa: An ecological study. BMJ Open 2017, 7, e013941. [Google Scholar] [CrossRef]
- Engelbrecht, J.; Mostert, J.; Reddy, V.; Swanepoel, L. Aerosol Monitoring and Source Apportionment in the Vaal Triangle; Mintek: Randburg, South Africa, 1998. [Google Scholar]
- Perez, L.; Tobias, A.; Querol, X.; Künzli, N.; Pey, J.; Alastuey, A.; Viana, M.; Valero, N.; González-Cabré, M.; Sunyer, J. Coarse Particles From Saharan Dust and Daily Mortality. Epidemiology 2008, 19, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, F.D.; Bekiswa, S.; Von Holdt, J.R.; Jack, C.; Kuhn, N.J.; Mogane, F.; Murray, J.E.; Ndara, N.; Palmer, A.R. South Africa’s agricultural dust sources and events from MSG SEVIRI. Aeolian Res. 2020, 47, 100637. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Mills, I.C.; Walton, H.A.; Anderson, H.R. Fine particle components and health—A systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Faustini, A.; Stafoggia, M.; Colais, P.; Berti, G.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Scarnato, C.; Forastiere, F. Air pollution and multiple acute respiratory outcomes. Eur. Respir. J. 2013, 42, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olstrup, H.; Johansson, C.; Forsberg, B.; Åström, C. Association between Mortality and Short-Term Exposure to Particles, Ozone and Nitrogen Dioxide in Stockholm, Sweden. Int. J. Environ. Res. Public Health 2019, 16, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, A.; Khanjani, N.; Bahrampour, A.; Goudarzi, G.; Yunesian, M. The relation between air pollution and respiratory deaths in Tehran, Iran-using generalized additive models. BMC Pulm. Med. 2018, 18, 49. [Google Scholar] [CrossRef]
- Bell, M.L.; Zanobetti, A.; Dominici, F. Who is More Affected by Ozone Pollution? A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2014, 180, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Boss, G.R.; Seegmiller, J.E. Age-related physiological changes and their clinical significance. West. J. Med. 1981, 135, 434–440. [Google Scholar]
- Zeka, A.; Zanobetti, A.; Schwartz, J. Individual-Level Modifiers of the Effects of Particulate Matter on Daily Mortality. Am. J. Epidemiol. 2006, 163, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Western Cape Government Department of Health. Western Cape Burden of Disease 2019; Western Cape Government Department of Health: Cape Town, South Africa, 2019. [Google Scholar]
- Statistics South Africa. Mortality and Causes of Death in South Africa: Findings from Death Notification; Statistics South Africa: Pretoria, South Africa, 2017. [Google Scholar]
- Morakinyo, O.M.; Mukhola, M.S.; Mokgobu, M.I. Ambient gaseous pollutants in an urban area in South Africa: Levels and potential human health risk. Atmosphere 2020, 11, 751. [Google Scholar] [CrossRef]
- Arowosegbe, O.O.; Röösli, M.; Adebayo-Ojo, T.C.; Dalvie, M.A.; de Hoogh, K. Spatial and Temporal Variations in PM(10) Concentrations between 2010–2017 in South Africa. Int. J. Environ. Res. Public Health 2021, 18, 13348. [Google Scholar] [CrossRef] [PubMed]
- Jury, M.R. Statistics and Meteorology of Air Pollution Episodes over the South African Highveld Based on Satellite–Model Datasets. J. Appl. Meteorol. Climatol. 2017, 56, 1583–1594. [Google Scholar] [CrossRef]
- Costa, A.F.; Hoek, G.; Brunekreef, B.; Ponce de Leon, A.C. Air Pollution and Deaths among Elderly Residents of São Paulo, Brazil: An Analysis of Mortality Displacement. Environ. Health Perspect. 2017, 125, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Geng, H.; Dong, C.; Bai, T. The short-term harvesting effects of ambient particulate matter on mortality in Taiyuan elderly residents: A time-series analysis with a generalized additive distributed lag model. Ecotoxicol. Environ. Saf. 2021, 207, 111235. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. Mortality displacement in the association of ozone with mortality: An analysis of 48 cities in the United States. Am. J. Respir. Crit. Care Med. 2008, 177, 184–189. [Google Scholar] [CrossRef]
- Zanobetti, A.; Wand, M.P.; Schwartz, J.; Ryan, L.M. Generalized additive distributed lag models: Quantifying mortality displacement. Biostatistics 2000, 1, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J.; Samoli, E.; Gryparis, A.; Touloumi, G.; Atkinson, R.; Le Tertre, A.; Bobros, J.; Celko, M.; Goren, A.; et al. The temporal pattern of mortality responses to air pollution: A multicity assessment of mortality displacement. Epidemiology 2002, 13, 87–93. [Google Scholar] [CrossRef]
- Qiu, H.; Pun, V.C.; Tian, L. Short-term effects of fine and coarse particles on deaths in Hong Kong elderly population: An analysis of mortality displacement. Environ. Pollut. 2018, 241, 148–154. [Google Scholar] [CrossRef]
- Schwartz, J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am. J. Epidemiol. 2000, 151, 440–448. [Google Scholar] [CrossRef]
- Zeger, S.L.; Dominici, F.; Samet, J. Harvesting-resistant estimates of air pollution effects on mortality. Epidemiology 1999, 10, 171–175. [Google Scholar] [CrossRef] [Green Version]


| Percentiles | By Season-Mean (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Min | Max | IQR | 25th | 50th | 75th | Warm/Dry | Cold/Wet |
| Cardiovascular disease | n = 33,427 | n = 20,929 | ||||||||
| All ages and sex | 14.88 | 4.49 | 2 | 33 | 6 | 12 | 15 | 18 | 13.80 (4.01) | 17.01 (4.61) |
| n = 54,356 | ||||||||||
| 15–64 years | 5.52 | 2.50 | 0 | 18 | 3 | 4 | 5 | 7 | 5.25 (2.37) | 6.05 (2.67) |
| n = 20,145 | ||||||||||
| >65 years | 9.35 | 3.49 | 0 | 26 | 5 | 7 | 9 | 12 | 8.54 (3.12) | 11 (3.61) |
| n = 34,164 | ||||||||||
| Female | 7.70 | 3.01 | 0 | 20 | 4 | 6 | 7 | 10 | 7.14 (2.78) | 8.81 (3.13) |
| n = 28,133 | ||||||||||
| Male | 7.17 | 2.90 | 0 | 23 | 4 | 5 | 7 | 9 | 6.65 (2.68) | 8.19 (3.04) |
| n = 26,167 | ||||||||||
| Respiratory disease | n = 11,625 | n = 8751 | ||||||||
| All ages and sex | 5.58 | 2.85 | 0 | 75 | 3 | 4 | 5 | 7 | 4.80 (2.36) | 7.11 (3.09) |
| n = 20,376 | ||||||||||
| 15–64 years | 2.67 | 1.78 | 0 | 32 | 3 | 1 | 2 | 4 | 2.31 (1.55) | 3.36 (1.98) |
| n = 9735 | ||||||||||
| >65 years | 2.90 | 1.93 | 0 | 17 | 3 | 1 | 3 | 4 | 2.47 (1.67) | 3.74 (2.14) |
| n = 10,588 | ||||||||||
| Female | 2.48 | 1.74 | 0 | 44 | 2 | 1 | 2 | 3 | 2.12 (1.53) | 3.19 (1.91) |
| n = 11,270 | ||||||||||
| Male | 3.09 | 1.94 | 0 | 40 | 2 | 2 | 3 | 4 | 2.67 (1.68) | 3.90 (2.18) |
| n = 9063 | ||||||||||
| Air pollutants | ||||||||||
| PM10 (µg/m3) | 30.35 | 13.6 | 6.56 | 98.63 | 16.35 | 20.44 | 27.32 | 36.8 | 29.5 (12.33) | 31.53 (15.73) |
| n = 3647 | ||||||||||
| NO2 (µg/m3) | 16.63 | 8.81 | 2.63 | 59.24 | 10.66 | 10.47 | 14.77 | 21.13 | 14.57 (7.61) | 20.33 (9.58) |
| n = 3603 | ||||||||||
| SO2 (µg/m3) | 10.54 | 5.46 | 2.31 | 49 | 6 | 6.88 | 9.20 | 12.87 | 9.81 (4.78) | 12 (6.35) |
| n = 2769 | ||||||||||
| O3 (µg/m3) | 33.06 | 12.28 | 2.38 | 89.08 | 15.60 | 24.87 | 33.06 | 40.46 | 32.35 (12) | 34.55 (12.7) |
| n = 2672 | ||||||||||
| Meteorological data | ||||||||||
| Temperature (°C) | 17.39 | 11.02 | 30.67 | 100 | 15.67 | 62 | 70.3 | 77 | 19.3 (3.49) | 13.6 (2.33) |
| Relative humidity (%) | 69.8 | 4.2 | 7.5 | 30 | 6.8 | 14 | 17.3 | 20.7 | 67 (10) | 76 (10.8) |
| PM10 | NO2 | SO2 | O3 | Temperature | Humidity | |
|---|---|---|---|---|---|---|
| PM10 | 1 | 0.38 | 0.27 | 0.12 | 0.17 | −0.25 |
| NO2 | 1 | 0.40 | 0.06 | −0.35 | 0.07 | |
| SO2 | 1 | −0.11 | −0.12 | −0.01 | ||
| O3 | 1 | −0.13 | 0.01 | |||
| Temperature | 1 | −0.41 | ||||
| Humidity | 1 |
| Cardiovascular Disease Deaths by Pollutants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per IQR µg/m3 | Per 16 µg/m3 PM10 | Per 11 µg/m3 NO2 | Per 6 µg/m3 SO2 | Per 16 µg/m3 O3 | ||||||||
| Groups | RR | 95% Confidence Interval | RR | 95% Confidence Interval | RR | 95% Confidence Interval | RR | 95% Confidence Interval | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||
| All | 1.024 | 1.009 | 1.039 | 1.022 | 1.004 | 1.041 | 1.014 | 1 | 1.028 | 1.025 | 1.002 | 1.048 |
| Age 15–64 | 1.011 | 0.986 | 1.036 | 1.01 | 0.981 | 1.041 | 1.01 | 0.988 | 1.032 | 1.019 | 0.982 | 1.057 |
| Age ≥65 | 1.033 | 1.014 | 1.052 | 1.031 | 1.008 | 1.054 | 1.017 | 0.999 | 1.034 | 1.029 | 1 | 1.059 |
| Female | 1.032 | 1.012 | 1.053 | 1.026 | 1.001 | 1.051 | 1.007 | 0.989 | 1.026 | 1.018 | 0.987 | 1.05 |
| Male | 1.015 | 0.994 | 1.037 | 1.019 | 0.993 | 1.045 | 1.022 | 1.003 | 1.041 | 1.035 | 1.002 | 1.069 |
| Respiratory disease deaths by Pollutants | ||||||||||||
| Per IQR µg/m3 | Per 16 µg/m3 PM10 | Per 11 µg/m3 NO2 | Per 6 µg/m3 SO2 | Per 16 µg/m3 O3 | ||||||||
| Groups | RR | 95% Confidence Interval | RR | 95% Confidence Interval | RR | 95% Confidence Interval | RR | 95% Confidence Interval | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||
| All | 1.003 | 0.978 | 1.029 | 1.045 | 1.014 | 1.076 | 1.013 | 0.99 | 1.036 | 1.006 | 0.965 | 1.049 |
| Age 15–64 | 1.003 | 0.969 | 1.039 | 1.041 | 0.997 | 1.085 | 1.01 | 0.979 | 1.042 | 1.007 | 0.951 | 1.067 |
| Age ≥65 | 1.003 | 0.969 | 1.038 | 1.049 | 1.007 | 1.093 | 1.016 | 0.985 | 1.047 | 1.004 | 0.948 | 1.063 |
| Female | 1.009 | 0.973 | 1.047 | 1.049 | 1.004 | 1.096 | 1.021 | 0.988 | 1.055 | 0.949 | 0.894 | 1.008 |
| Male | 1 | 0.967 | 1.033 | 1.042 | 1.002 | 1.083 | 1.005 | 0.976 | 1.035 | 1.054 | 0.998 | 1.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adebayo-Ojo, T.C.; Wichmann, J.; Arowosegbe, O.O.; Probst-Hensch, N.; Schindler, C.; Künzli, N. Short-Term Effects of PM10, NO2, SO2 and O3 on Cardio-Respiratory Mortality in Cape Town, South Africa, 2006–2015. Int. J. Environ. Res. Public Health 2022, 19, 8078. https://doi.org/10.3390/ijerph19138078
Adebayo-Ojo TC, Wichmann J, Arowosegbe OO, Probst-Hensch N, Schindler C, Künzli N. Short-Term Effects of PM10, NO2, SO2 and O3 on Cardio-Respiratory Mortality in Cape Town, South Africa, 2006–2015. International Journal of Environmental Research and Public Health. 2022; 19(13):8078. https://doi.org/10.3390/ijerph19138078
Chicago/Turabian StyleAdebayo-Ojo, Temitope Christina, Janine Wichmann, Oluwaseyi Olalekan Arowosegbe, Nicole Probst-Hensch, Christian Schindler, and Nino Künzli. 2022. "Short-Term Effects of PM10, NO2, SO2 and O3 on Cardio-Respiratory Mortality in Cape Town, South Africa, 2006–2015" International Journal of Environmental Research and Public Health 19, no. 13: 8078. https://doi.org/10.3390/ijerph19138078
APA StyleAdebayo-Ojo, T. C., Wichmann, J., Arowosegbe, O. O., Probst-Hensch, N., Schindler, C., & Künzli, N. (2022). Short-Term Effects of PM10, NO2, SO2 and O3 on Cardio-Respiratory Mortality in Cape Town, South Africa, 2006–2015. International Journal of Environmental Research and Public Health, 19(13), 8078. https://doi.org/10.3390/ijerph19138078







