Prevalence of Congenital Anomaly and Its Relationship with Maternal Education and Age According to Local Development in the Extreme South of Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Data Analysis
2.3. Ethical Aspects
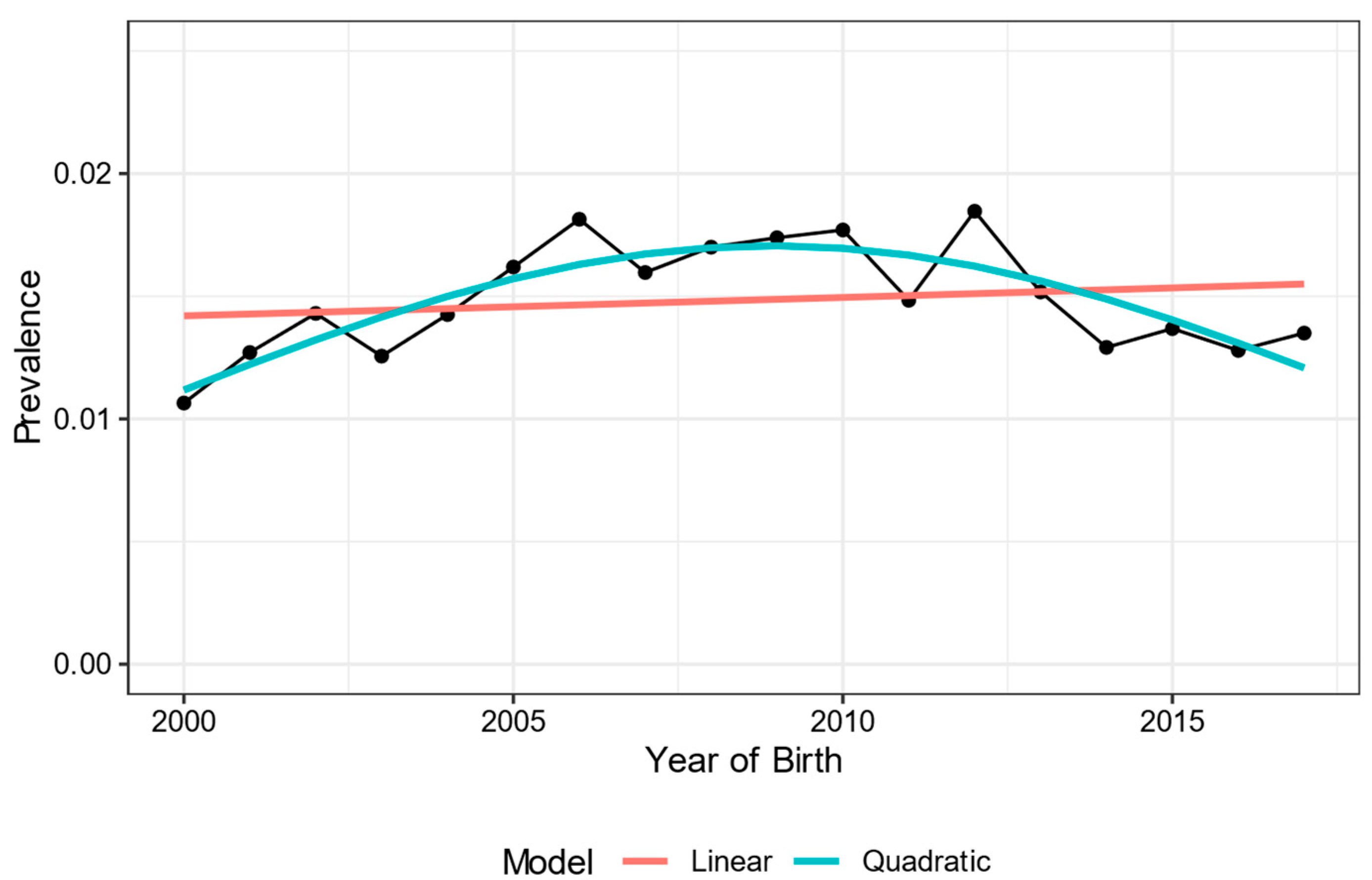
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Birth Defects Surveillance Training: Facilitator’s Guide; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Boyle, B.; Addor, M.C.; Arriola, L.; Barisic, I.; Bianchi, F.; Csáky-Szunyogh, M.; de Walle, H.E.K.; Dias, C.M.; Draper, E.; Gatt, M.; et al. Estimating Global Burden of Disease due to congenital anomaly: An analysis of European data. Arch. Dis. Child.-Fetal Neonatal Ed. 2018, 103, F22–F28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Choi, Y.J.; Cho, J.; Lee, H.; Park, S.J.; Park, J.S.; Hong, Y.C. Environmental and Genetic Risk Factors of Congenital Anomalies: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Korean Med. Sci. 2021, 36, e183. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.C.; Jesuino, R.S.A.; Pinheiro, D.D.S.; Rebelo, A.C.S. Anomalias congênitas e suas principais causas evitáveis: Uma revisão. Rev. Méd. Minas Gerais 2018, 28, 1–6. [Google Scholar]
- Tsehay, B.; Shitie, D.; Lake, A.; Abebaw, E.; Taye, A.; Essa, E. Determinants and seasonality of major structural birth defects among newborns delivered at primary and referral hospital of East and West Gojjam zones, Northwest Ethiopia 2017–2018: Case-control study. BMC Res. Notes 2019, 12, 495. [Google Scholar] [CrossRef]
- Jones, A.M.; Isenburg, J.; Salemi, J.L.; Arnold, K.E.; Mai, C.T.; Aggarwal, D.; Arias, W.; Carrino, G.E.; Ferrell, E.; Folorunso, O.; et al. Increasing Prevalence of Gastroschisis–14 States, 1995–2012. Morb. Mortal. Wkly. Rep. 2016, 65, 23–26. [Google Scholar] [CrossRef]
- Stallings, E.B.; Isenburg, J.L.; Short, T.D.; Heinke, D.; Kirby, R.S.; Romitti, P.A.; Canfield, M.A.; O’Leary, L.A.; Liberman, R.F.; Forestieri, N.E.; et al. Population-based birth defects data in the United States, 2012–2016: A focus on abdominal wall defects. Birth Defects Res. 2019, 111, 1436–1447. [Google Scholar] [CrossRef]
- Moorthie, S.; Blencowe, H.; Darlison, M.W.; Lawn, J.; Morris, J.K.; Modell, B.; Bittles, A.H.; Christianson, A.; Cousens, S.; Gibbons, S.; et al. Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. J. Community Genet. 2018, 9, 387–396. [Google Scholar] [CrossRef]
- Ministério da Saúde. Boletim Epidemiológico, Anomalias Congênitas no Brasil, 2010 a 2019: Análise de um Grupo Prioritário para a Vigilância ao Nascimento; Ministério da Saúde: Brasilia, Brazil, 2021; Volume 52.
- Ajao, A.E.; Adeoye, I.A. Prevalence, risk factors and outcome of congenital anomalies among neonatal admissions in OGBOMOSO, Nigeria. BMC Pediatr. 2019, 19, 88. [Google Scholar] [CrossRef]
- Ratowiecki, J.; Santos, M.R.; Poletta, F.; Heisecke, S.; Elias, D.; Gili, J.; Gimenez, L.; Pawluk, M.; Uranga, R.; Cosentino, V.; et al. Social inequities in teenage mothers and the relationship to adverse perinatal outcomes in South American populations. Cad. Saúde Pública 2021, 36, e00247719. [Google Scholar] [CrossRef]
- Monteiro, D.L.M.; Monteiro, I.P.; Machado, M.S.C.; Bruno, Z.V.; Silveira, F.A.D.; Rehme, M.F.B.; Takiuti, A.D.; Rodrigues, N.C.P. Trends in teenage pregnancy in Brazil in the last 20 years (2000–2019). Rev. Assoc. Med. Bras. 2021, 67, 759–765. [Google Scholar] [CrossRef]
- Koffi, A.K.; Maina, A.; Yaroh, A.G.; Habi, O.; Bensaïd, K.; Kalter, H.D. Social determinants of child mortality in Niger: Results from the 2012 National Verbal and Social Autopsy Study. J. Glob. Health 2016, 6, 010603. [Google Scholar] [CrossRef] [PubMed]
- Mensch, B.S.; Chuang, E.K.; Melnikas, A.J.; Psaki, R.S. Evidence for causal links between education and maternal and child health: Systematic review. Trop. Med. Int. Health 2019, 24, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Geib, L.T.; Fréu, C.M.; Brandão, M.; Nunes, M.L. Social and biological determinants of infant mortality in population cohort in the city of Passo Fundo, Rio Grande do Sul State. Ciência Saúde Coletiva 2010, 15, 363–370. [Google Scholar] [CrossRef] [PubMed]
- França, E.B.; Lansky, S.; Rego, M.A.S.; Malta, D.C.; França, J.S.; Teixeira, R.; Porto, D.; Almeida, M.F.; Souza, M.F.M.; Szwarcwald, C.L.; et al. Leading causes of child mortality in Brazil, in 1990 and 2015: Estimates from the Global Burden of Disease study. Rev. Bras. Epidemiol. 2017, 20 (Suppl. S1), 46–60. [Google Scholar] [CrossRef]
- Mathews, T.; MacDorman, M.F.; Thoma, M.E. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl. Vital Stat. Rep. 2015, 64, 9. [Google Scholar]
- Sitkin, N.A.; Ozgediz, D.; Donkor, P.; Farmer, D.L. Congenital anomalies in low- and middle-income countries: The unborn child of global surgery. World J. Surg. 2015, 39, 36–40. [Google Scholar] [CrossRef]
- Adegbosin, A.E.; Zhou, H.; Wang, S.; Stantic, B.; Sun, J. Systematic review and meta-analysis of the association between dimensions of inequality and a selection of indicators of Reproductive, Maternal, Newborn and Child Health (RMNCH). J. Glob. Health 2019, 9, 010429. [Google Scholar] [CrossRef]
- Observapoa. Indicadores: Porto Alegre em Análise. 2019. Available online: http://www.observapoa.com.br/default.php?p_secao=4 (accessed on 20 October 2019).
- PNUD; IPEA; FJP. Atlas do Desenvolvimento Humano nas Regiões Metropolitanas Brasileiras; IPEA; Fundação João Pinheiro; PNUD: Brasilia, Brasil, 2014.
- Ministério da Saúde. A Experiência Brasileira em Sistemas de Informação em Saúde; Editora do Ministério da Saúde: Brasilia, Brasil, 2009; Volume 2. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/experiencia_brasileira_sistemas_saude_volume2.pdf (accessed on 15 March 2022).
- World Health Organization. Orientation Programme on Adolescent Health for Health Care Providers; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Women’s Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Wit, E.; Heuvel, E.V.D.; Romeijn, J.W. ‘All models are wrong…’: An introduction to model uncertainty. Stat. Neerl. 2012, 66, 217–236. [Google Scholar]
- Morris, J.K.; Springett, A.L.; Greenlees, R.; Loane, M.; Addor, M.C.; Arriola, L.; Barisic, I.; Bergman, J.E.H.; Csaky-Szunyogh, M.; Dias, C.; et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS ONE 2018, 13, e0194986. [Google Scholar] [CrossRef]
- Mai, C.T.; Isenburg, J.L.; Canfield, M.A.; Meyer, R.E.; Correa, A.; Alverson, C.J.; Lupo, P.J.; Riehle-Colarusso, T.; Cho, S.J.; Aggarwal, D.; et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019, 111, 1420–1435. [Google Scholar] [CrossRef]
- Bronberg, R.; Groisman, B.; Bidondo, M.P.; Barbero, P.; Liascovich, R. Birth prevalence of congenital anomalies in Argentina, according to socioeconomic level. J. Community Genet. 2021, 12, 345–355. [Google Scholar] [CrossRef]
- Ministério da Saúde. Portaria n° 1.793, de 11 de agosto de 2009. Institui a Comissão Interinstitucional para Implementação, Acompanhamento e Monitoramento das Ações de Fortificação de Farinhas de Trigo, de Milho e de seus Subprodutos. Diário Oficial da União, 2009. [Google Scholar]
- Ministério da Saúde. Alguns Documentos Introdutórios Sobre a Rede Cegonha. Distribuição na Oficina Sobre Rede Cegonha no Seminário do CONASEMS; Ministério da Saúde: Brasilia, Brasil, 2011.
- Raupp, L.; Adorno, R.D.C.F. Psychotropic territories in the center of Porto Alegre city, Rio Grande do Sul, Brazil. Saúde Soc. 2015, 24, 803–815. [Google Scholar]
- Bastos, F.I.B.; Bertoni, N. Pesquisa Nacional sobre o uso de crack: Quem são os usuários de crack e/ou similares do Brasil? Quantos são nas capitais brasileiras? In Pesquisa Nacional sobre o Uso de Crack: Quem são os Usuários de Crack e/ou Similares do Brasil? Quantos são nas Capitais Brasileiras? ICICT; FIOCRUZ: Rio de Janeiro, Brasil, 2014; p. 221. [Google Scholar]
- Zaganjor, I.; Sekkarie, A.; Tsang, B.L.; Williams, J.; Razzaghi, H.; Mulinare, J.; Sniezek, J.E.; Cannon, M.J.; Rosenthal, J. Describing the Prevalence of Neural Tube Defects Worldwide: A Systematic Literature Review. PLoS ONE 2016, 11, e0151586. [Google Scholar] [CrossRef]
- Toobaie, A.; Yousef, Y.; Balvardi, S.; St-Louis, E.; Baird, R.; Guadagno, E.; Poenaru, D. Incidence and prevalence of congenital anomalies in low- and middle-income countries: A systematic review. J. Pediatr. Surg. 2019, 54, 1089–1093. [Google Scholar] [CrossRef]
- Cunha, J.; Gomes, A.L.M.; Finkler, A.; Belló, I.L.D.S.; Silva, K.C.D.; Oliveira, T.C.M.D. SINASC Sistema de Informação sobre Nascidos Vivos: Relatório 2015 Geral: Informações Referentes ao Número de Nascidos Vivos em Porto Alegre, Variáveis Maternas, do Parto e do Recém Nascido; Prefeitura Municipal de Porto Alegre–PMPA: Porto Alegre, Brasil, 2017. [Google Scholar]
- Moraes, C.L.; Mendonça, C.R.; Melo, N.C.E.; Amaral, W.N.D. Prevalence and Association of Congenital Anomalies According to the Maternal Body Mass Index: Cross-Sectional Study. Rev. Bras. Ginecol. Obs. 2019, 41, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Vanassi, B.M.; Parma, G.C.; Magalhaes, V.S.; Santos, A.C.C.D.; Iser, B.P.M. Congenital anomalies in Santa Catarina: Case distribution and trends in 2010–2018. Rev. Paul. Pediatr. 2021, 40, e2020331. [Google Scholar] [CrossRef]
- Kishimba, R.S.; Mpembeni, R.; Mghamba, J. Factors associated with major structural birth defects among newborns delivered at Muhimbili National Hospital and Municipal Hospitals in Dar Es Salaam, Tanzania 2011–2012. Pan Afr. Med. J. 2015, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Machin, R.; Mendosa, D.; Augusto, M.H.O.; Monteleone, P.A.A. Assisted Reproductive Technologies in Brazil: Characterization of centers and profiles from patients treated. JBRA Assist. Reprod. 2020, 24, 235–240. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, L.V.; Araujo Júnior, E.; Guazzelli, C.A.; Cernach, M.C.; Torloni, M.R.; Moron, A.F. Congenital Anomalies Detected at Birth in Newborns of Adolescent Women. Acta Med. Port. 2015, 28, 708–714. [Google Scholar] [PubMed]
- Cosme, H.W.; Lima, L.S.; Barbosa, L.G. Prevalence of congenital anomalies and their associated factors in newborns in the city of São Paulo from 2010 to 2014. Rev. Paul. Pediatr. 2017, 35, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Anele, C.R.; Hirakata, V.N.; Goldani, M.Z.; da Silva, C.H. The influence of the municipal human development index and maternal education on infant mortality: An investigation in a retrospective cohort study in the extreme south of Brazil. BMC Public Health 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, G.A.; Meentken, M.G.; Utens, E.M.W.J. Mental Health Problems in Parents of Children with Congenital Heart Disease. Front. Pediatr. 2017, 5, 102. [Google Scholar] [CrossRef]
- Kasparian, N.A.; Kan, J.M.; Sood, E.; Wray, J.; Pincus, H.A.; Newburger, J.W. Mental health care for parents of babies with congenital heart disease during intensive care unit admission: Systematic review and statement of best practice. Early Hum. Dev. 2019, 139, 104837. [Google Scholar] [CrossRef]
- Benny, C.; Yamamoto, S.; McDonald, S.; Chari, R.; Pabayo, R. Modelling Maternal Depression: An Agent-Based Model to Examine the Complex Relationship between Relative Income and Depression. Int. J. Environ. Res. Public Health 2022, 19, 4208. [Google Scholar] [CrossRef]
- Paes, N.A.; Santos, C.S.A.D. Birth statistics and maternal and infant risk factors in the micro-regions of Northeast Brazil: A factor analysis study. Cad. Saúde Pública 2010, 26, 311–322. [Google Scholar] [CrossRef][Green Version]
- Pedraza, D.F. Quality of the Information System on Live Births /SINASC: A critical analysis of published studies. Ciênc. Saúde Coletiva 2012, 17, 2729–2737. [Google Scholar] [CrossRef]

| MHDI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEDIUM | HIGH | VERY HIGH | ||||||||||
| TOTAL n (%) | CA n (%) | RR[CI95%] | p | TOTAL n (%) | CA n (%) | RR[CI95%] | p | TOTAL n (%) | CA n (%) | RR[CI95%] | p | |
| Maternal Age (Years) | ||||||||||||
| 10 to 19 | 12,003 (21.4) | 202 (1.7) | 1.22[1.04–1.43] | 0.016 | 34,632 (17.6) | 599 (1.7) | 1.19[1.09–1.31] | <0.001 | 8296 (9.1) | 136 (1.6) | 1.34[1.12–1.61] | 0.001 |
| ≥36 | 5399 (9.6) | 122 (2.3) | 1.64[1.35–1.99] | 22,593 (11.5) | <0.001 | 460 (2.0) | 1.40[1.27–1.55] | <0.001 | 18,971 (20.8) | 253 (1.3) | 1.09[0.95–1.26] | 0.215 |
| 20 to 35 | 38,727 (69.0) | 535 (1.4) | 1 | - | 139,765 (70.9) | 2024 (1.4) | 1 | - | 64,099 (70.2) | 782 (1.2) | 1 | - |
| 56,129 (100) | 859 (1.5) | - | - | 196,990 (100) | 3083 (1.6) | - | - | 91,366 (100) | 1171 (1.3) | - | - | |
| Maternal Education (Years) | ||||||||||||
| <8 | 21,378 (38.3) | 354 (1.7) | 1.27[1.01–1.59] | 0.042 | 62,183 (31.7) | 1096 (1.8) | 1.48[1.33–1.64] | <0.001 | 14,145 (15.5) | 276 (2.0) | 2.08[1.80–2.42] | <0.001 |
| ≥8 to 11 | 27,483 (49.2) | 401 (1.5) | 1.12[0.89–1.40] | 0.336 | 91,556 (46.6) | 1466 (1.6) | 1.34[1.21–1.48] | <0.001 | 27,855 (30.6) | 423 (1.5) | 1.62[1.42–1.85] | <0.001 |
| ≥12 | 6969 (12.5) | 91 (1.3) | 1 | - | 42,548 (21.7) | 508 (1.2) | 1 | - | 49,100 (53.9) | 460 (0.9) | 1 | - |
| 55,830 (100) | 846 (1.5) | - | - | 196,287 (100) | 3070 (1.6) | - | - | 91,100 (100) | 1159 (1.3) | - | - | |
| Marital Status | ||||||||||||
| Single/Separated/Widowed | 39,733 (71.0) | 630 (1.6) | 1.15[0.99–1.34] | 0.072 | 131,945 (67.1) | 2212 (1.7) | 1.26[1.17–1.36] | <0.001 | 46,605 (51.1) | 682 (1.5) | 1.35[1.20–1.51] | <0.001 |
| Married/Stable Union | 16,230 (29.0) | 224 (1.4) | 1 | 64,615 (32.9) | 860 (1.3) | 1 | - | 44,576 (48.9) | 484 (1.1) | 1 | - | |
| 55,963 (100) | 854 (1.5) | - | - | 196,560 (100) | 3072 (1.6) | - | - | 91,181 (100) | 1166 (1.3) | - | - | |
| Parity | ||||||||||||
| Multipara | 34,259 (61.2) | 504 (1.5) | 0.91[0.79–1.04] | 0.161 | 112,149 (57) | 1830 (1.6) | 1.10[1.031–1.19] | 0.005 | 43,846 (48.1) | 624 (1.4) | 1.25[1.12–1.40] | <0.001 |
| Primipara | 21,724 (38.8) | 352 (1.6) | 1 | - | 84,510 (43.0) | 1245 (1.5) | 1 | - | 47,367 (51.9) | 539 (1.1) | 1 | 1 |
| 55,983 (100) | 856 (1.5) | - | - | 196,659 (100) | 3075 (1.6) | - | - | 91,213 (100) | 1163 (1.3) | - | - | |
| Prenatal Care (Number of Consultations) | ||||||||||||
| None | 1955 (3.5) | 24 (1.2) | 0.84[0.56–1.26] | 0.405 | 6344 (3.2) | 128 (2.0) | 1.38[1.15–1.64] | <0.001 | 1875 (2.1) | 30 (1.6) | 1.38[0.96–1.98] | 0.079 |
| 1 to 3 | 5823 (10.4) | 101 (1.7) | 1.19[0.96–1.47] | 0.111 | 16,917 (8.6) | 294 (1.7) | 1.19[1.05–1.34] | 0.006 | 4539 (5.0) | 85 (1.9) | 1.62[1.30–2.02] | <0.001 |
| 4 to 6 | 14,406 (25.8) | 239 (1.7) | 1.14[0.98–1.33] | 0.101 | 43,538 (22.2) | 747 (1.7) | 1.17[1.08–1.27] | <0.001 | 12,947 (14.2) | 220 (1.7) | 1.47[1.27–1.70] | <0.001 |
| ≥7 | 33,647 (60.3) | 491 (1.5) | 1 | - | 129,607 (66.0) | 1899 (1.5) | 1 | - | 71,807 (78.8) | 831 (1.2) | 1 | - |
| 55,831 (100) | 855 (1.5) | - | - | 196,406 (100) | 3068 (1.6) | - | - | 91,168 (100) | 1166 (1.3) | - | - | |
| Birth | ||||||||||||
| Cesarean | 20,613 (36.7) | 386 (1.9) | 1.40[1.23–1.60] | <0.001 | 86,773 (44.1) | 1524 (1.8) | 1.24[1.16–1.33] | <0.001 | 56,237 (61.6) | 707 (1.3) | 0.95[0.85–1.07] | 0.405 |
| Vaginal | 35,518 (63.3) | 473 (1.3) | 1 | - | 110,210 (55.9) | 1558 (1.4) | 1 | - | 35,127 (38.4) | 464 (1.3) | 1 | - |
| 56,131 (100) | 859 (1.5) | - | - | 196,983 (100) | 3082 (1.6) | 91,364 (100) | 1171 (1.3) | - | - | |||
| Gestational Age (Weeks) | ||||||||||||
| <27 | 276 (0.5) | 11 (4.0) | 3.01[1.68–5.40] | <0.001 | 1001 (0.5) | 55 (5.5) | 3.95[3.05–5.13] | <0.001 | 420 (0.5) | 19 (4.5) | 4.03[2.59–6.29] | <0.001 |
| 28 to 31 | 575 (1.0) | 19 (3.3) | 2.50[1.59–3.91] | <0.001 | 1897 (1.0) | 79 (4.2) | 3.00[2.40–3.73] | <0.001 | 915 (1.0) | 33 (3.6) | 3.22[2.29–4.52] | <0.001 |
| 32 to 36 | 5122 (9.1) | 162 (3.2) | 2.39[2.02–2.83] | <0.001 | 18,022 (9.2) | 488 (2.7) | 1.95[1.77–2.14] | <0.001 | 8943 (9.8) | 206 (2.3) | 2.05[1.77–2.39] | <0.001 |
| ≥37 | 50,027 (89.3) | 662 (1.3) | 1 | - | 175,665 (89.3) | 2442 (1.4) | 1 | - | 80,936 (88.7) | 908 (1.1) | 1 | - |
| 56,000 (100) | 854 (1.5) | - | - | 196,585 (100) | 3064 (1.6) | - | - | 91,214 (100) | 1166 (1.3) | - | - | |
| Apgar (5th Minute) | ||||||||||||
| <7 | 746 (1.3) | 72 (9.7) | 6.79[5.40–8.55] | <0.001 | 2458 (1.3) | 220 (9.0) | 6.15[5.39–7.01] | <0.001 | 765 (0.8) | 81 (10.6) | 8.85[7.14–10.96] | <0.001 |
| ≥7 | 54,894 (98.7) | 780 (1.4) | 1 | - | 193,441 (98.7) | 2816 (1.5) | 1 | - | 90,323 (99.2) | 1081 (1.2) | 1 | - |
| 55,640 (100) | 852 (1.5) | - | - | 195,899 (100) | 3036 (1.5) | - | - | 91,088 (100) | 1162 (1.3) | - | - | |
| Low Birth Weight | ||||||||||||
| Yes (<2500 g) | 5693 (10.1) | 201 (3.5) | 2.70[2.32–3.16] | <0.001 | 19,438 (9.9) | 647 (3.3) | 2.43[2.23–2.64] | <0.001 | 8743 (9.6) | 278 (3.2) | 2.94[2.58–3.36] | <0.001 |
| No (≥2500 g) | 50,438 (89.9) | 658 (1.3) | 1 | - | 177,554 (90.1) | 2436 (1.4) | 1 | - | 82,625 (90.4) | 893 (1.1) | 1 | - |
| 56,131 (100) | 859 (1.5) | - | - | 196,992 (100) | 3083 (1.6) | - | - | 91,368 (100) | 1171 (1.3) | - | - | |
| Newborn Sex | ||||||||||||
| Male | 28,892 (51.5) | 515 (1.8) | 1.45[1.26–1.66] | <0.001 | 100,771 (51.2) | 1747 (1.7) | 1.27[1.18–1.36] | <0.001 | 46,646 (51.1) | 665 (1.4) | 1.28[1.14–1.44] | <0.001 |
| Female | 27,231 (48.5) | 336 (1.2) | 1 | - | 96,202 (48.8) | 1317 (1.4) | 1 | - | 44,714 (48.9) | 498 (1.1) | 1 | |
| 56,123 (100) | 851 (1.5) | - | - | 196,973 (100) | 3064 (1.6) | - | - | 91,360 (100) | 1163 (1.3) | - | - | |
| Type of Hospital | ||||||||||||
| Public | 26,783 (48.1) | 499 (1.9) | 2.42[1.81–3.23] | <0.001 | 100,926 (51.7) | 1976 (2.0) | 2.50[2.22–2.82] | <0.001 | 30,538 (33.7) | 578 (1.9) | 2.78[2.43–3.20] | <0.001 |
| Mixed | 22,358 (40.2) | 293 (1.3) | 1.70[1.26–2.29] | <0.001 | 54,987 (28.1) | 718 (1.3) | 1.67[1.46–1.90] | <0.001 | 14,228 (15.7) | 225 (1.6) | 2.32[1.96–2.76] | <0.001 |
| Private | 6487 (11.7) | 50 (0.8) | 1 | - | 39,418 (20.2) | 309 (0.8) | 1 | - | 45,856 (50.6) | 312 (0.7) | 1 | - |
| 55,628 (100) | 842 (1.5) | 195,331 (100) | 3003 (1.5) | 90,622 (100) | 1115 (1.2) | - | - | |||||
| Pregnancy | ||||||||||||
| Multiple | 1152 (2.1) | 25 (2.2) | 1.43[0.97–2.12] | 0.075 | 4579 (2.3) | 86 (1.9) | 1.21[0.98–1.49] | 0.084 | 2859 (3.1) | 31 (1.1) | 0.84[0.59–1.20] | 0.341 |
| Single | 54,977 (97.9) | 834 (1.5) | 1 | - | 192,406 (97.7) | 2997 (1.6) | 1 | - | 88,503 (96.9) | 1140 (1.3) | 1 | - |
| 56,129 (100) | 859 (1.5) | - | - | 196,985 (100) | 3083 (1.6) | - | - | 91,362 (100) | 1171 (1.3) | - | - | |
| Medium MHDI | High MHDI | Very High MHDI | ||||
|---|---|---|---|---|---|---|
| RR[CI95%] | p | RR[CI95%] | p | RR[CI95%] | p | |
| Maternal Age (Years) | ||||||
| 10 to 19 | 1.06[0.88–1.27] | 0.552 | 1.07[0.97–1.20] | 0.175 | 0.99[0.80–1.21] | 0.890 |
| ≥36 | 1.60[1.30–1.97] | <0.001 | 1.40[1.26–1.55] | <0.001 | 1.28[1.10–1.50] | 0.002 |
| 20 to 35 | 1 | - | 1 | - | 1 | - |
| Maternal Education (Years) | ||||||
| <8 | 1.10[0.85–1.44] | 0.460 | 1.04[0.92–1.18] | 0.577 | 1.27[1.03–1.54] | 0.027 |
| ≥8 to 11 | 0.99[0.77–1.27] | 0.928 | 1.00[0.90–1.12] | 0.975 | 1.08[0.91–1.28] | 0.349 |
| ≥12 | 1 | - | 1 | - | 1 | - |
| Maternal Status | ||||||
| Single/Separated/Widowed | 1.12[0.96–1.32] | 0.162 | 1.17[1.08–1.27] | <0.001 | 1.01[0.88–1.15] | 0.927 |
| Married/Stable Union | 1 | - | 1 | - | 1 | - |
| Prenatal Care (Number of Consultations) | ||||||
| None | 0.69[0.46–1.06] | 0.091 | 0.93[0.76–1.13] | 0.463 | 0.57[0.38–0.86] | 0.008 |
| from 1 to 3 | 0.88[0.70–1.13] | 0.328 | 0.85[0.74–0.97] | 0.018 | 0.79[0.61–1.01] | 0.064 |
| from 4 to 6 | 0.96[0.82–1.13] | 0.617 | 0.93[0.84–1.01] | 0.082 | 0.82[0.69–0.97] | 0.018 |
| ≥7 | 1 | - | 1 | - | 1 | - |
| Birth | ||||||
| Cesarean | 1.32[1.14–1.52] | <0.001 | 1.36[1.26–1.47] | <0.001 | 1.27[1.12–1.44] | <0.001 |
| Vaginal | 1 | - | 1 | - | 1 | - |
| Gestational Age (Weeks) | ||||||
| <27 weeks | 0.80[0.40–1.60] | 0.520 | 0.88[0.62–1.25] | 0.479 | 0.77[0.43–1.39] | 0.390 |
| 28 to 31 weeks | 0.86[0.51–1.46] | 0.580 | 1.11[0.85–1.45] | 0.440 | 1.17[0.77–1.79] | 0.463 |
| 32 to 36 weeks | 1.54[1.20–1.98] | 0.001 | 1.29[1.13–1.48] | <0.001 | 1.29[1.03–1.62] | 0.026 |
| ≥37 | 1 | - | 1 | - | 1 | - |
| Apgar (5th Minute) | ||||||
| <7 | 4.74[3.56–6.30] | <0.001 | 4.29[3.66–5.04] | <0.001 | 4.99[3.84–6.51] | <0.001 |
| ≥7 | 1 | - | 1 | - | 1 | - |
| Low Weight | ||||||
| Yes (<2500 g) | 1.87[1.46–2.4] | <0.001 | 1.76[1.54–2.00] | <0.001 | 2.49[1.99–3.10] | <0.001 |
| No (≥2500 g) | 1 | - | 1 | - | 1 | - |
| Newborn Sex | ||||||
| Male | 1.43[1.24–1.64] | <0.001 | 1.29[1.20–1.38] | <0.001 | 1.28[1.14–1.44] | <0.001 |
| Female | 1 | - | 1 | - | 1 | - |
| Type of Hospital | ||||||
| Public | 2.48[1.80–3.43] | <0.001 | 2.70[2.34–3.12] | <0.001 | 2.94[2.43–3.56] | <0.001 |
| Mixed | 1.69[1.20–2.36] | 0.002 | 1.73[1.49–2.02] | <0.001 | 2.22[1.83–2.70] | <0.001 |
| Private | 1 | - | 1 | - | 1 | - |
| Pregnancy | ||||||
| Multiple | 0.75[0.49–1.13] | 0.171 | 0.63[0.50–0.80] | <0.001 | 0.41[0.28–0.61] | <0.001 |
| Single | 1 | - | 1 | - | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anele, C.R.; Goldani, M.Z.; Schüler-Faccini, L.; da Silva, C.H. Prevalence of Congenital Anomaly and Its Relationship with Maternal Education and Age According to Local Development in the Extreme South of Brazil. Int. J. Environ. Res. Public Health 2022, 19, 8079. https://doi.org/10.3390/ijerph19138079
Anele CR, Goldani MZ, Schüler-Faccini L, da Silva CH. Prevalence of Congenital Anomaly and Its Relationship with Maternal Education and Age According to Local Development in the Extreme South of Brazil. International Journal of Environmental Research and Public Health. 2022; 19(13):8079. https://doi.org/10.3390/ijerph19138079
Chicago/Turabian StyleAnele, Carolina Ribeiro, Marcelo Zubaran Goldani, Lavínia Schüler-Faccini, and Clécio Homrich da Silva. 2022. "Prevalence of Congenital Anomaly and Its Relationship with Maternal Education and Age According to Local Development in the Extreme South of Brazil" International Journal of Environmental Research and Public Health 19, no. 13: 8079. https://doi.org/10.3390/ijerph19138079
APA StyleAnele, C. R., Goldani, M. Z., Schüler-Faccini, L., & da Silva, C. H. (2022). Prevalence of Congenital Anomaly and Its Relationship with Maternal Education and Age According to Local Development in the Extreme South of Brazil. International Journal of Environmental Research and Public Health, 19(13), 8079. https://doi.org/10.3390/ijerph19138079







