1. Introduction
Genetic variation among plant species is believed to limit the explanatory power of abiotic or biotic influential factors on certain plant traits. Several studies have revealed that plant traits, e.g., related to phenology, morphology, physiology, reproduction, and distribution are associated with genetic controls. Neophytou et al. [
1] found a significant variation in the timing of bud burst among different Douglas fir progenies. Likewise, previous studies on poplar hybrids have reported that the patterns of tree biomass distribution above- and below-ground were genetically controlled [
2,
3]. Furthermore, naturally regenerated birch and aspen populations showed a variation between genotypes in the acclimatization to soil moisture conditions by altering biomass, root and leaf morphology, water potential, and gas exchange [
4]. Rousi et al. [
5] documented significant variations in intraspecific reproduction efficiency (anther residuals and seed production) among individuals of
B. pubescens in two neighboring stands in Northern Finland. In addition, information on intraspecific genetic variations plays a crucial role to improve species distribution models [
6]. Under varying environmental conditions, an exposed genotype has the ability to express phenotypic plasticity [
7]. Studies on birch revealed phenotypic plasticity in leaf morphology of transplanted trees related to edaphic conditions [
8] and larger phenotypic plasticity of juvenile above-ground growth traits in response to soil nutrient conditions [
9]. Such findings indicate that traits of plant individuals of the same species growing under similar or different environmental conditions must be understood with the consideration of intraspecific variations.
Pollen are developed in anthers (angiosperms) or in microsporangia (gymnosperms) and their quantity per inflorescence is regarded as pollen production [
10]. Pollen production may be controlled by the genes of taxa, species or varieties. It was suggested that the amount of pollen grains produced per anther and the number of anthers per flowers are genetically fixed and does not vary substantially [
11,
12,
13]. In addition, any further variations could be related to changes in environmental conditions [
12] such as meteorology, primarily air temperature [
14,
15,
16], and edaphic factors [
15,
17], which alter the number of flowers and/or pollen production per flower. However, the role of these and other variables influencing pollen production are poorly known.
Most studies on pollen production of woody plants are limited to genera or species. Yet, a small number of studies have focused on the intraspecific level, for example, related to
Cupressus sempervirens varieties [
18] or
Theobroma cacao clones [
19]. Although Adams and Kunze [
20] studied clonal variations in seed production in spruce, there has been little discussion on pollen production of genetically identical trees.
In general, genetically identical trees are preferentially used for various applications in science because it is assumed that they show the same behavior, e.g., related to phenology [
21,
22]. Long-term phenological observation networks such as the International Phenological Gardens in Europe (IPG) standardized phenological studies by establishing gardens with cloned plant individuals to exclude genetic effects [
21,
23]. Such phenological investigations based on cloned tree species assure that observed variances are due to environmental causes rather than genotypic differences between plants [
24]. There have been attempts to explore the influences or exclusion of genetics on other pollen properties such as allergenicity. Ahlholm et al. [
25] investigated the allergenicity of mountain birch pollen collected from trees of ten half-sib families growing in northern Finland and found that the concentration of the major birch pollen allergen (Bet v 1) is genetically controlled. In addition, concentrations of the allergen Cry j 1 produced by pollen of Japanese cedar were reported to be significantly different between trees of eight clones [
26]. Similarly, Fernández-Caldas et al. [
27] demonstrated considerable variations in pollen allergenicity (Ole e 1) of different varieties of
Olea europaea.
However, studies related to pollen production compared for different clones in birch are lacking and are in general very sparse related to other species of the plant kingdom. Veilleux and Lauer [
28] studied potato (
Solarium phurejas) clones and suggested that plants of the same genotype respond similarly to the environment and produce the same amount of unreduced pollen grains. Panda et al. [
29] observed a wide variation in pollen production per anther, pollen size and pollen viability among selected banana (
Musa spp.) genotypes. Information on the variability of pollen production of genetically identical wind-pollinated plants is, however, largely lacking.
Detailed knowledge on the pollen production of a species is crucial for improving pollen forecasting [
30]. Such forecasts have agronomical importance as seed production and, therefore, harvest outcomes often rely on pollen production [
31]. Pollen production also plays a vital role in allergology. In the past few years, phenological, biometeorological, and aerobiological studies on allergenic plants have become more important due to the high prevalence of allergies around the world. According to the World Allergy Organization (WAO) up to 40% of the global population suffers from allergic sensitization [
32], which could further increase by a parallel increase in pollen production [
33,
34,
35,
36].
Birch has a wide range of distribution in the Northern Hemisphere [
37] and its pollen are highly allergenic [
38,
39] presenting a major source of allergic rhinitis in Europe [
40]. Due to its aesthetic value, silver birch is a frequently used tree species in urban green space planning in Europe [
41,
42]. The abundance of birches, however, is problematic for many people who are allergic to pollen [
43]. Studies on genotypic variations of pollen production of such allergenic tree species could identify clones, which are characterized by a lower pollen production. The breeding of such clones, e.g., for planting in urban green spaces, might also imply a reduction of atmospheric pollen concentration. On the other hand, seed plantations, in which a high pollen production of trees is desirable for a high quantity of seeds, may profit from those clones that are associated with a higher production of pollen. Most important, knowledge on the genetic variability of pollen production will allow for better evaluating the influence of environmental factors/climate change.
In this study, we assessed the pollen production of eleven groups of cloned weeping birch (
Betula pendula Roth) individuals (
n = 28) in three consecutive years (2019–2021). Since natural birch populations show a high grade of hybridization [
44], we sampled inflorescences of genetically identical trees of the same age from a seed plantation (Baden-Württemberg, Germany), assessed the ambient microclimatic conditions and monitored any silvicultural treatments. We especially checked for differences between years as well as between and within clones and considered their synchronicity of pollen production levels. Based on the results, we discussed the implications of selecting clones producing a high/low level of pollen for seed plantations/urban planting.
2. Materials and Methods
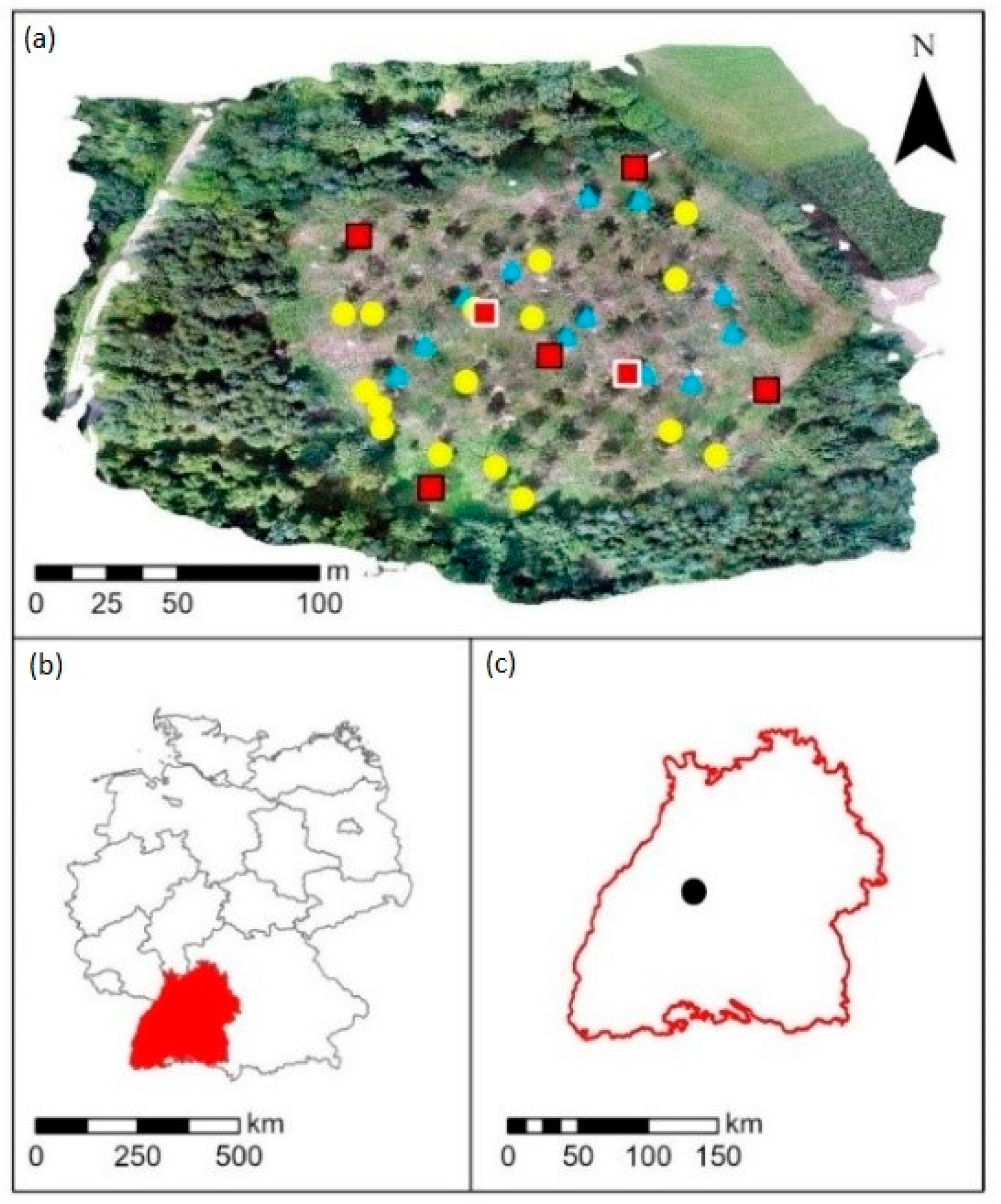
For this study, we selected a birch seed plantation located near Wildberg (48°36′44″ N, 8°42′37″ E, 500 m a.s.l.) in Baden Württemberg, Germany (
Figure 1). The average annual temperature is 8.6 °C and the precipitation sum is 892 mm (German Meteorological Service (DWD) station “Neubulach-Oberhaugstett”, 1991–2020 [
45]). The plantation is located on a west-exposed slope with an inclination of approximately 2°−6° and the soil type is Cambisol [
46]. This 1-hectare sized plantation was established in 2005 and additional birch trees were planted in 2012, resulting in a 7 m × 7 m seedling cluster, which is managed by Forst Baden-Württemberg (Forst BW; territory number 3, Nagoldtal). Initially, 215 trees belonging to 44 different clones were planted in a total of 13 rows and 17 columns. The clones and trees were randomized spatially throughout the site. Until now, almost half of the birch trees were removed as a thinning measure: 113 birch trees from 44 clones (with one to six individuals) are still present in the plantation.
The study was conducted in three successive years (2019–2021). We focussed on 28 trees, all planted in 2005, representing eleven clones from six different geographic origins (
Table 1). These clone origins are, however, located nearby, within approximately 45 km to 130 km from the study site. The trees were selected based on the reachability of twigs and, therefore, inflorescences. The number of studied trees per clone, therefore, varied between one to four.
Male catkins were harvested in March after the beginning of catkin elongation and prior to anthesis. Samples were collected from different branches at 1.5 to 2 m above ground from all cardinal directions. In addition, we measured growth traits: (a) the perimeter at breast height, (b) the height of the tree and crown by use of Suunto PM-5/1520PC Height Meter, and (c) the crown diameter, which was calculated by averaging two perpendicular diameters of the crown at its widest portion.
We counted the number of catkins within a sampling cuboid (50 cm × 50 cm × 50 cm) in the crown, which was considered to characterize the average distribution of catkins in the tree [
18]. We selected an ovoid shape of the crown to estimate pollen production per tree.
In July 2018, tree topping (cutting of the apical parts of the main trunk), which is an intended measure to increase seed production [
47], was carried out in the seed plantation. Therefore, the sampled trees were categorized as topped (
n = 12) and non-topped (
n = 16). Six more sampled trees were topped in July 2020; however, male catkins were already formed in those trees and, therefore, no large effect on pollen production was assumed.
Air temperature and precipitation data were obtained from a 5 km distant DWD climate station “Neubulach-Oberhaugstett” [
45]. In addition, we installed five temperature loggers (HOBO Pro v2 U23-001, Onset, Bourne, MA, USA) from spring 2019 (8 April) until summer 2021 (20 June). One logger was installed in the center and four at the northern, eastern, southern, and western borders of the plantation (red squares with black border in
Figure 1) to determine temperature differences within the site. Each logger was placed in a radiation shield and mounted at a height of 2 m at the northern side of a birch tree. The loggers’ data were retrieved and processed using HOBOware (Version 3.7.23) from Onset, Bourne, MA, USA.
The air quality of the study site was characterized by the measured values of nitrogen dioxide (NO
2), nitrogen oxides (NO
x) and ozone (O
3) concentrations monitored directly at the stem of the birch trees (
n = 2, red squares with white border in
Figure 1). Passive sampling of these pollutants lasted one week in summer 2020 (25 June to 2 July). The passive samplers were supplied and evaluated by Passam AG (Männedorf, Switzerland).
For estimating the potential solar radiation around each tree, the unmanned aerial vehicle Phantom 4 Pro, DJI, Nanshan, Shenzhen, China was used, which features an onboard RGB camera with a sensor resolution of 12 megapixels and a focal length of 24 mm. The flight altitude was 35 m above ground level. During the flight, which took place on 10 August 2019 and lasted approximately 17 min, 712 photos were taken with an overlap of 80%. A digital elevation model was generated using Metashape Professional (Version 1.8.1) from Agisoft LLC, St. Petersburg, Russia. In ArcGIS Pro (version 2.7.0) software from ESRI, Redlands, CA, USA, the spatial analyst tool “Solar radiation (area)” was used to calculate the potential solar radiation (W/m
2) on the surface depending on the time of day and position of the sun as well as the latitude for each pixel of the digital elevation model. We calculated solar radiation for each pixel as a sum for the period 1 May until 31 August as this period is critical for the start and development of the following year’s catkin [
48]. We selected a buffer of two meters around each tree and calculated the mean solar radiation. We assume only minor differences in the canopy of the surrounding forest and, therefore, use the data gained in 2019 for a general site characteristic for the whole study period.
To extract pollen grains, we adapted the method proposed by Damialis et al. [
18]. For each year, one average-sized inflorescence from each cardinal direction and per tree was selected, its length and width were measured (at the widest point), and the number of flowers was counted. Then, each catkin was soaked in a 10% KOH solution [
31,
49] and boiled at 120 °C the following day. Afterwards, the plant material was crushed with a glass rod to break up plant tissues and to allow pollen release. To prevent pollen clumping [
50], we added glycerol (70%), a bipolar solvent, to a volume of 20 mL; safranin was added as a stain. Two aliquot samples (10 µL each) per suspension were obtained using a VITLAB
® micropipette while stirring it vigorously to ensure homogeneity. Subsequently, the extraction was put on microscope slides and covered with slips. Pollen grains on these slides were subsequently counted at 100× magnification (Zeiss AXIO Lab.A1, Germany). In case of a large difference between the pollen counts obtained from these two slides (>30%), the procedure was repeated in order to increase the homogeneity of the suspension.
We estimated pollen production at various scales [
18]: The number of pollen grains per catkin (
Pca) was calculated using Equation (1):
where
and
are the volumes of the suspension (in mL) and the sample taken (in µL), respectively, and
p is the number of pollen grains counted per 10 µL solution.
The number of pollen grains per flower (
) was estimated as follows (Equation (2)):
where
fl is the number of flowers per catkin.
The number of pollen grains per volume unit (m
3) of crown (
) was estimated using Equation (3):
where
is the number of catkins per crown sampling unit (cuboid) and
is the volume of the sampling unit.
The number of pollen grains per individual (
) was estimated using Equation (4):
where
is the number of pollen grains per crown volume unit (see Equation (3)) and
is the total volume (in m
3) of the crown. The volume of an ovoid tree Crown can be calculated as follows (Equation (5)):
where
≈ 3.14,
and
are two perpendicular diameters of the crown, at its widest part, and
is the crown height.
Pollen production per flower, catkin, and volume unit of crown, as well as flowers per catkin and catkins per crown sampling unit, were descriptively analyzed. These reproductive metrics were non-normally distributed according to Shapiro–Wilk test. We checked for differences among sampling years and clones using the Kruskal–Wallis test and post-hoc (Dunn) test. Correlation analyses between the reproduction metrics and between solar radiation and pollen production were conducted using Spearman’s correlation test. The differences between topped and non-topped trees were analyzed using Mann–Whitney U test. The variation within non-topped clones was assessed by comparing the coefficient of variances (CVs). For indicating if one specific clone can be proposed as “good” or “poor” regarding pollen production, we averaged the crown metrics (crown height and crown width) of all non-topped trees and calculated a mean crown volume. We considered that this computed crown dimension would represent an average non-topped birch tree in the seed plantation. Similarly, we calculated the mean Pca and mean Csu obtained from the non-topped trees during the study years. These values allowed us to quantify the total Pin for an average tree (using Equation (5)). Further, we used average Pca and Csu of each clone along with the crown volume of an average tree to calculate the pollen produced by each clone under mean growth parameters to compare the pollen produced by each clone to an average birch tree. All statistical analyses and visualizations were performed in RStudio (version 4.1.2) from RStudio, PBC, Boston, MA, USA, ArcGIS Pro (version 2.7.0) or Microsoft Excel 2016 from Microsoft, Washington, DC, USA.
4. Discussion
Our study investigating pollen production of 28 birch trees in three consecutive years is unique since we examined a large number of male birch inflorescences and assessed the internal variability of pollen production regarding genetic differences and similarities. In addition, this study excludes (major) environmental differences as well as age effects.
We estimated pollen production values at the level of catkins ranging from 48,000 pollen grains to 8.3 million pollen grains (mean 1.66 million). Some studies have already estimated pollen production values for
Betula pendula (syn.
Betula alba,
Betula verrucosa). Erdtman [
51] reported an estimate of 5.5 million pollen grains per inflorescence for
B. verrucose. Jato et al. [
30] estimated values ranging between 8.2 million and 4.8 million pollen grains per inflorescence, sampled from six trees of
B. alba in northwestern Spain in 2002 and 2003, respectively. Piotrowska [
43] estimated a mean value of 10 million pollen grains per inflorescence on the basis of 30 catkins deriving from three individuals. Although these studies have reported higher values compared to the mean
Pca estimated in this study, they were based on either a few trees or estimated only for a single or two study years. Consequently, it is not known if sampling took place in a masting or non-masting years. For this reason, our study can be regarded as important since we have sampled 28 trees for three years and present a robust estimate for the mean pollen production of
Betula pendula.
We found that birch catkins with fewer flowers produce more pollen and
vice versa. This could be considered as an internal compensation since the plant aims at upregulating pollen production when the flower amount is low. Molina et al. [
52] studied ten anemophilous species of aerobiological importance (
Betula ssp. not included) and found a significant decrease in pollen per flower with a higher number of flowers per inflorescence. They suggested that there is a more or less constant amount (within a defined margin) for pollen production in anemophilous tree species. These species tend to compensate for reproductive characteristics (e.g., pollen per anther, flowers per tree, and inflorescences per tree) by increasing some and decreasing others. Our analysis showed that the number of flowers is the most homogenous value since a low coefficient of variance was associated to this measure, e.g., in clonal comparisons.
Our study shows an annual variation in pollen production with the lowest mean values in 2019 and the highest in 2021. Such alterations could be caused by yearly changes in the meteorological conditions of the locality. Some studies examining the relationship between temperature and pollen production suggested that warmer conditions result in higher pollen quantities. For example, experimental studies indicated that an increase in temperature [
16] but also an increase in atmospheric CO
2 concentration [
16,
53,
54] was associated with a higher pollen production of common ragweed (
Ambrosia artemisiifolia). However, it was also found that pollen production of birch (
Betula pendula Roth) along an urban-rural gradient was negatively correlated with temperature [
55]. The authors argue that the physiological performance of birch, which mainly grows at lower temperatures in mid to high latitudes, might be affected by (very) high temperatures and in turn react with a decrease in pollen production, as also suggested by Ziello et al. [
56]. However, any differences in pollen production found in natural environments might also be affected by other factors, which attenuate or diminish the influence of temperature. In addition, the response to temperature might also be species-specific and strongly dependent on the methodologies used.
Although many studies have examined the temporal change in birch pollen concentrations based on pollen trap monitoring, there is no study presenting long-term changes in pollen production assessed using the same birch trees. Detecting the influence of temperature on pollen production based on the data presented in this study is not feasible, since (a) we only cover a period of three years and (b) a small spatial extent (1 ha) with similar temperature conditions, as documented using five installed temperature loggers. Many other environmental factors such as soil type and edaphic conditions as well as air pollutants are regarded to be similar as well. Especially the latter is also supposed to affect pollen production, as documented by Jochner et al. [
55]. In their study, atmospheric NO
2 levels were negatively associated to pollen production.
However, we found differences in solar radiation, which arise mainly from the forested surrounding of the seed plantation. During the study years, the correlations between pollen production and solar radiation did not vary much in magnitude, but they did shift in sign. Therefore, we calculated the correlation coefficient for mean (2019–2021) pollen production, but the association to solar radiation was no longer discernible. Thus, solar radiation, which is known to lead to higher stem and tissue temperatures [
57] might also be inadequate to explain variations of pollen production at a small spatial scale. This was also evident when comparing solar radiation values with the association of birch trees to groups with similar pollen production patterns across the study years.
We did not detect a high synchrony of pollen production levels of birch trees within the birch plantation since we found that six trees exhibited the highest pollen production in 2020, four trees a very high pollen production in 2021 and six trees an almost constant pollen production across the study years. The birch trees allocated to one of these three groups did not necessarily belong to one clone. Thus, a coherence on the level of clones was not evident, except for one clone group.
Masting behavior, the inherent year-to-year variation in pollen production by plant populations [
58,
59], can be observed in several tree species [
60,
61] including birch [
14,
30]. Flowering and annual pollen sums in birch were reported to fluctuate from year to year [
62]. Using aerobiological data gathered from pollen traps that assess the pollen concentration of the ambient outdoor air, a biennial [
63] as well as triennial rhythm [
64] of masting can be observed. Related to
Betula species, Ranta et al. [
59] found that male flowering shows synchronized annual fluctuations among stands at a regional scale; however, stand-specific catkin number during the masting year varies considerably, which in turn might also influence the pollen produced. This is also in accordance with our findings since the numbers of catkins varied (mean
Cs (SD) = 23 (8), 44 (26) and 19 (12) in 2019, 2020, and 2021, respectively,
Table 2) within the plantation.
Asynchronous pollen production levels, which were found in our study might be caused by the resource balance of an individual tree. If the initial resource stock and the resource gained afterwards differ from one individual to the next in the stand, masting synchronization might not occur [
65,
66], even under the same environmental conditions [
65]. In addition, plant-pathogen and plant-mycorrhizosphere interactions may reduce or enhance the impacts of abiotic stress on resource allocation [
67] which could be specific to each tree.
Effects on pollen production and catkin formation were especially obvious two years after topping. Topping and pruning have been considered as adequate tree crown management techniques to enhance seed production, specifically in conifer seed orchards, or to promote the branching of the trees [
47,
68,
69]. Viherä-Aarnio and Ryynänen [
47] studied seed production of silver birch individuals that were topped in the second year in a greenhouse experiment. In the fourth year, a ten times higher amount of seeds per plant (compared to the previous year) was obtained. This was followed by a year with poor flowering and seed production. In our study, we cannot conclude on any effects in upcoming years; therefore, we recommend a longer monitoring of pollen production after topping in further studies.
Birch clones characterized by on average lower pollen production could be an opportunity to reduce the prevalence of allergies. In an experiment, transgenic birch grown in a greenhouse showed the ability to prevent flowering in silver birch trees [
70]. However, such preventions might be associated with adverse side effects such as aberrant branching and growth disturbance. Therefore, we suggest selecting birch clones associated with low pollen production. We estimated
Pca ranging between 1.17 million (clone 30) and 1.97 million (clone 42) pollen. Clones producing less pollen might contribute to lower pollen concentrations in the atmosphere. Therefore, clone 42 could be recommended for urban plantations. Similarly, clone 24 needs 87 trees to produce the same pollen amount as 100 average trees. This clone could be suitable in seed plantations to increase seed production. Since variations within clones were especially obvious when comparing pollen production levels across years (Chapter 3.4), we highly recommend monitoring pollen production for a longer term in order to create robust averages for different clones.