Distribution of Mercury and Methylmercury in Farmland Soils Affected by Manganese Mining and Smelting Activities
Abstract
:1. Introduction
2. Materials and Methods
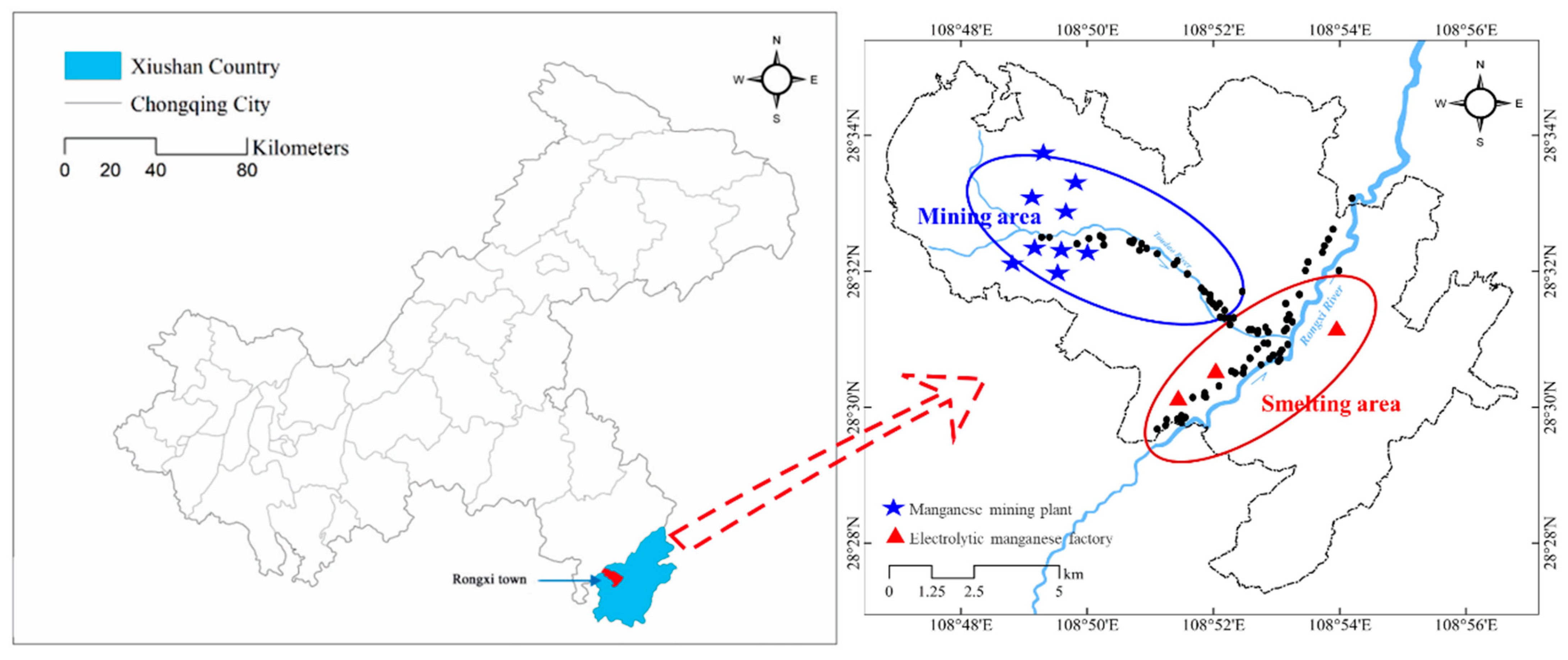
2.1. Studying Area
2.2. Sampling and Preparation
2.3. Chemical Analysis
2.4. Quality Assurance and Quality Control
3. Results and Discussions
3.1. Soil Properties
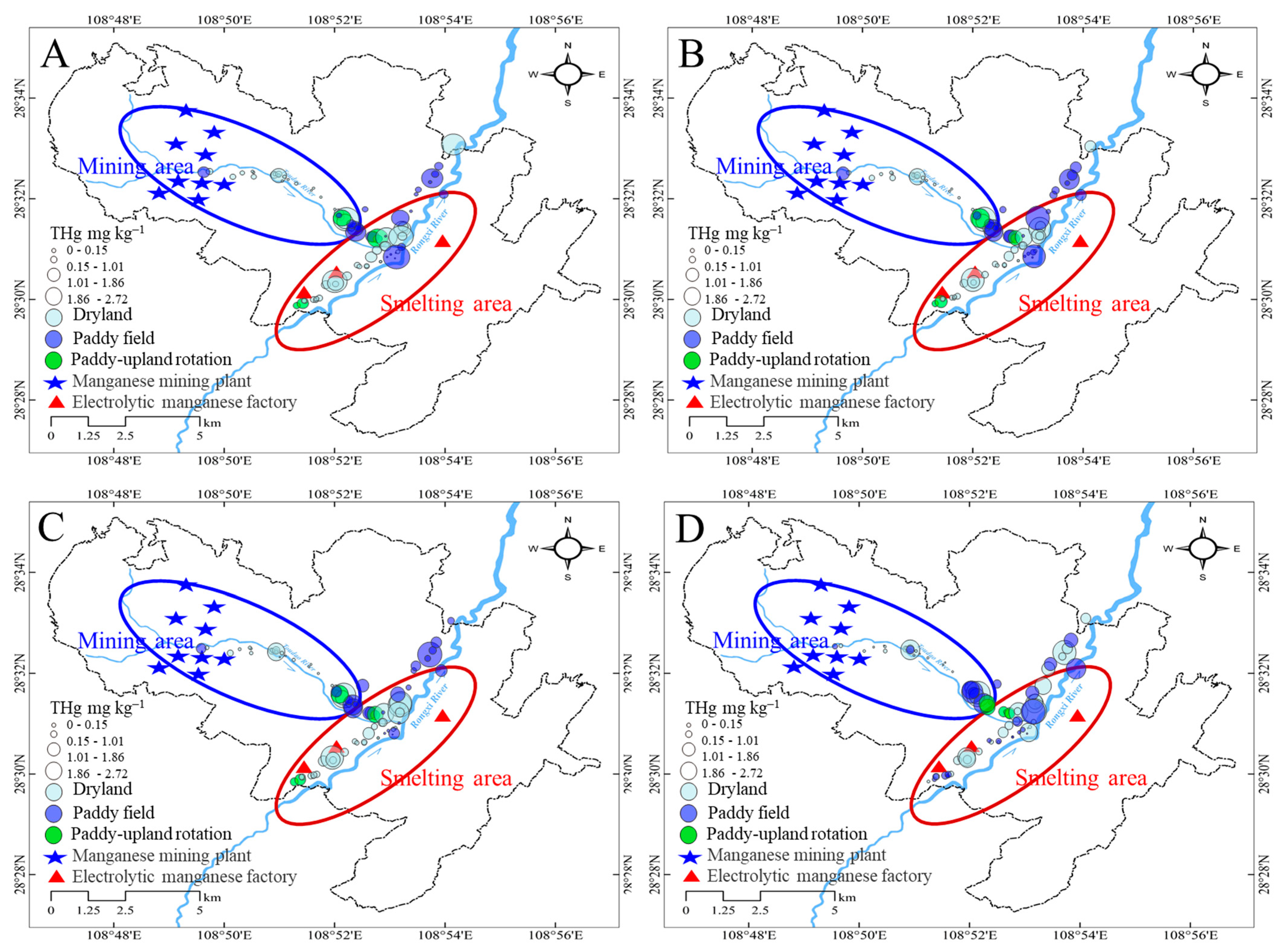
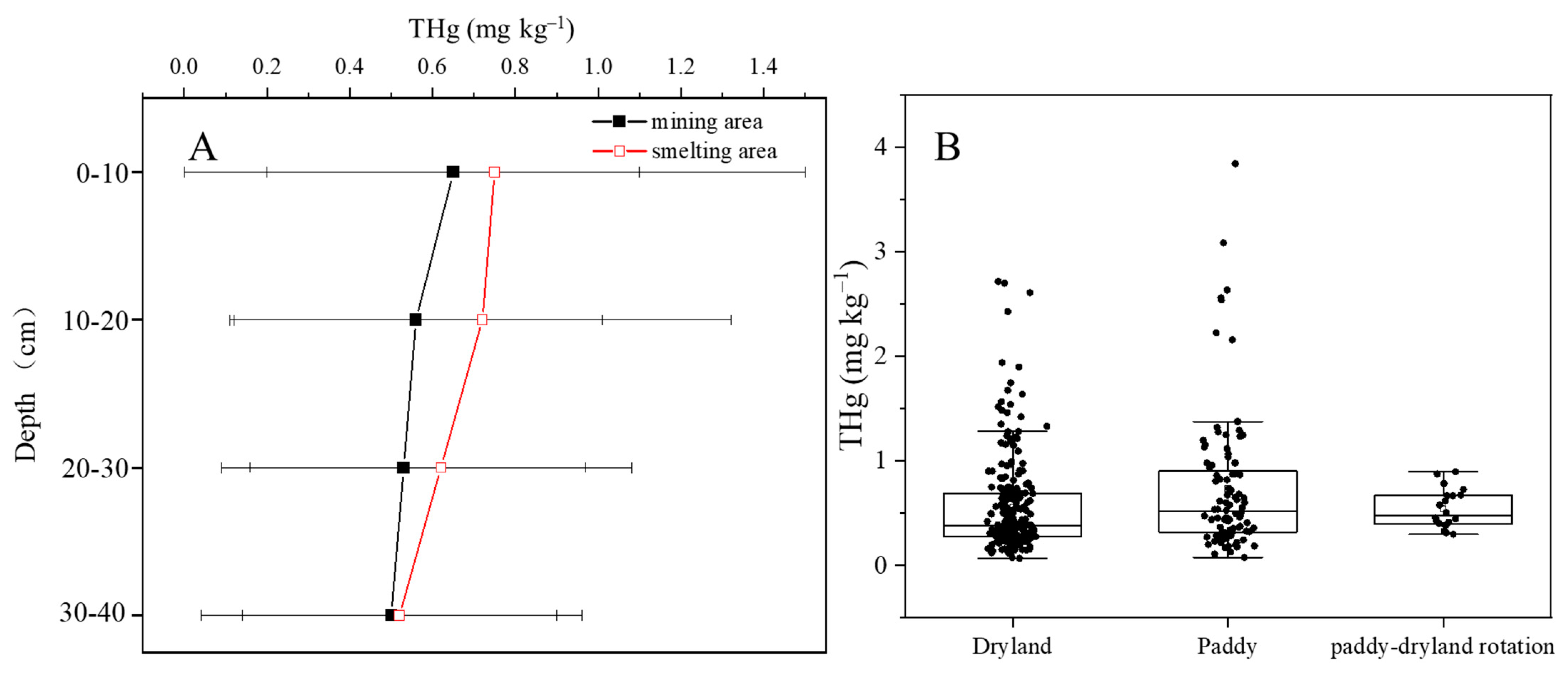
3.2. Distribution of THg in Farmland Soils
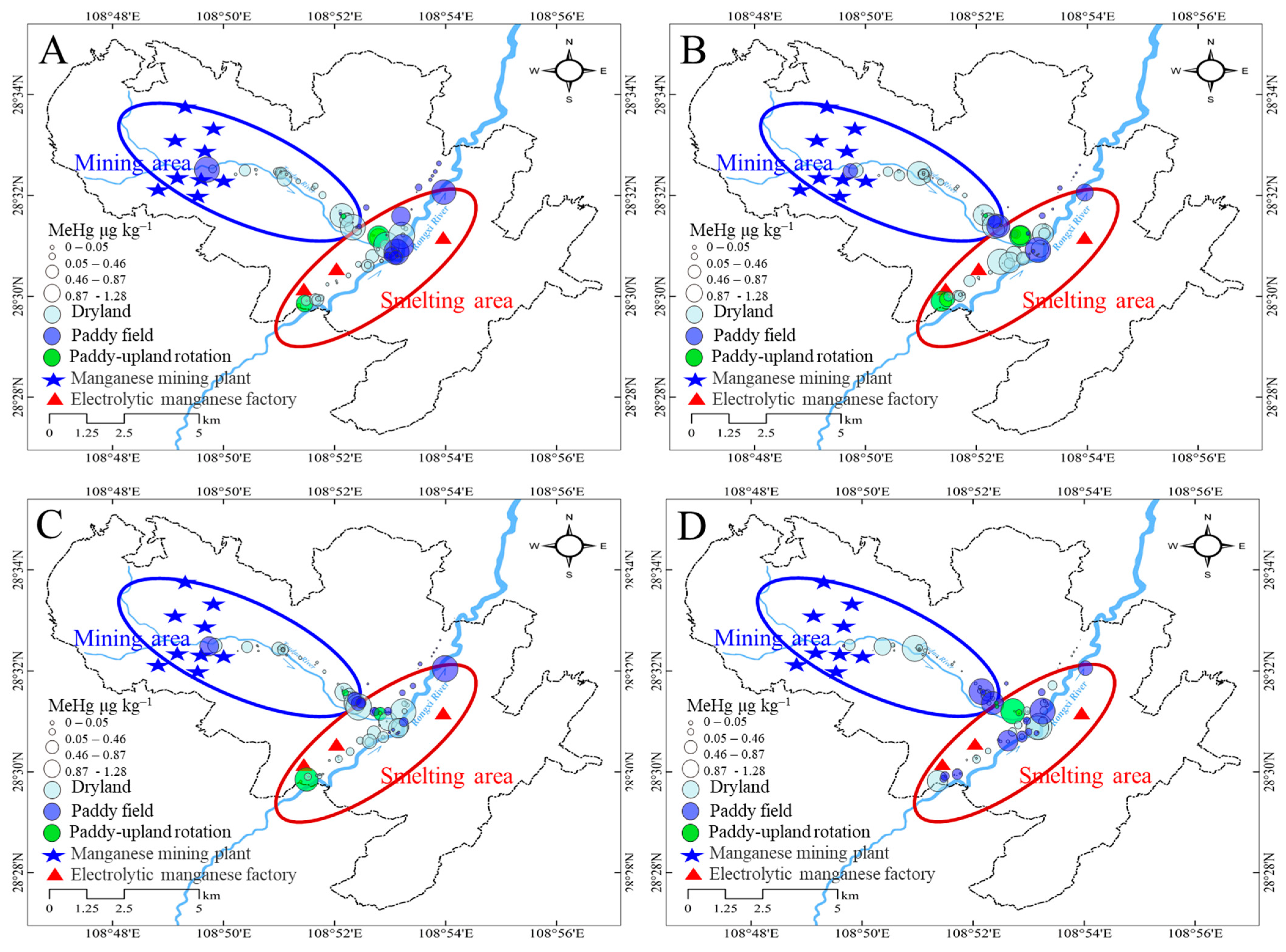
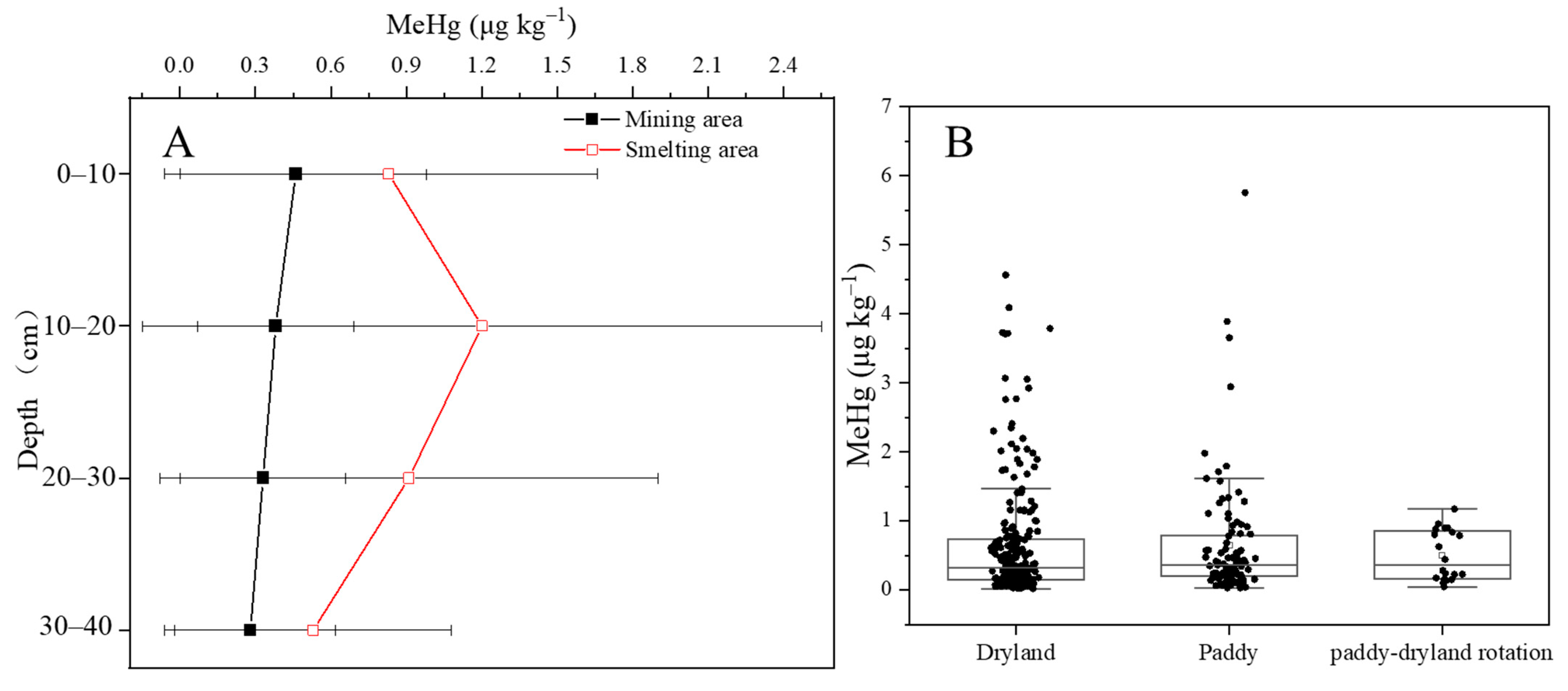
3.3. Distribution of MeHg in Farmland Soils
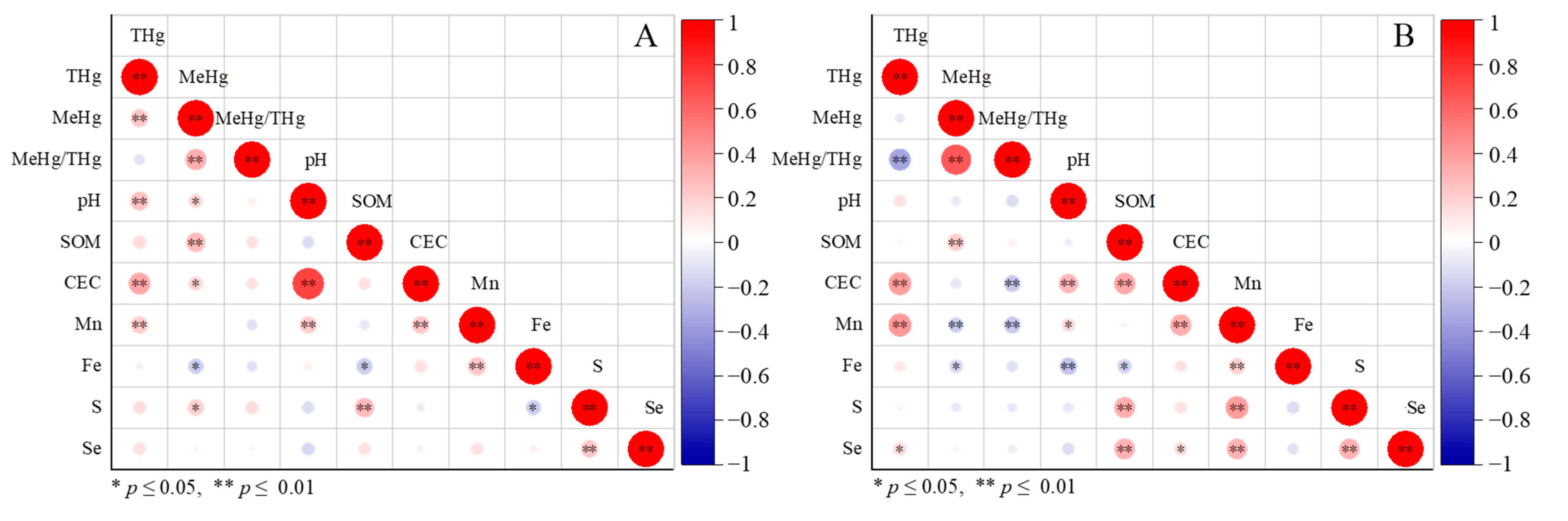
3.4. Effects of Soil Properties on Hg Distribution
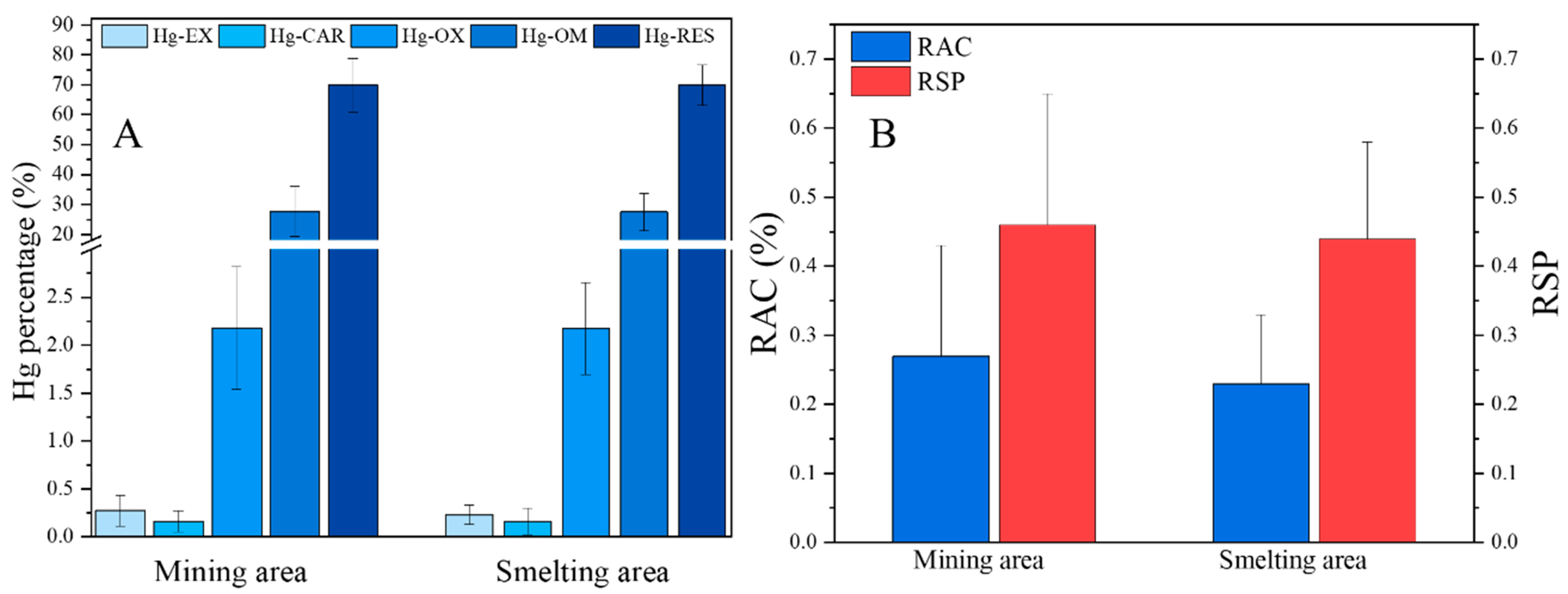
3.5. Potential Hazard Based on Geochemical Fractionation of Hg in Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.B.; Qiu, G.L. Methylmercury exposure and health effects from rice and fish consumption: A review. Int. J. Environ. Res. Public Health 2010, 7, 2666–2691. [Google Scholar] [CrossRef]
- Xia, J.C.; Wang, J.X.; Zhang, L.M.; Anderson, C.W.N.; Wang, X.; Zhang, H.; Dai, Z.H.; Feng, X.B. Screening of native low mercury accumulation crops in a mercury-polluted mining region: Agricultural planning to manage mercury risk in farming communities. J. Clean. Prod. 2020, 262, 121324. [Google Scholar] [CrossRef]
- Feng, X.B.; Li, P.; Qiu, G.L.; Wang, S.F.; Li, G.L.; Shang, L.H.; Meng, B.; Jiang, H.M.; Bai, W.Y.; Li, Z.Z. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ. Sci. Technol. 2008, 42, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.L.; Zhou, X.; He, T.R.; Wu, P.; Ran, S. Remediation of Mercury-Polluted Farmland Soils: A Review. Bull. Environ. Contam. Tox. 2022. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Fan, F.L.; Deng, S.P.; Wang, D.Y. Mercury in rice paddy fields and how does some agricultural activities affect the translocation and transformation of mercury—A critical review. Ecotoxicol. Environ. Saf. 2020, 202, 110950. [Google Scholar] [CrossRef]
- Yin, D.L.; He, T.R.; Yin, R.S.; Zeng, L.X. Effects of soil properties on production and bioaccumulation of methylmercury in rice paddies at a mercury mining area, China. J. Environ. Sci. 2018, 68, 194–205. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, G.; Anderson, C.W.N.; Meng, B.; Wang, D.; Shang, L.; Yan, H.; Feng, X. Mercury methylation in rice paddies and its possible controlling factors in the Hg mining area, Guizhou province, Southwest China. Environ. Pollut. 2016, 215, 1–9. [Google Scholar] [CrossRef]
- Abeysinghe, K.S.; Qiu, G.; Goodale, E.; Cwn, A.; Bishop, K.; Evers, D.C.; Goodale, M.W.; Hintelmann, H.; Liu, S.; Mammides, C. Mercury flow through an Asian rice-based food web. Environ. Pollut. 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, B.; Feng, X.B. Mercury methylation in rice paddy and accumulation in rice plant: A review. Ecotoxicol. Environ. Saf. 2020, 195, 110462. [Google Scholar] [CrossRef]
- Back, S.K.; Sung, J.H.; Moon, Y.H.; Kim, Y.H.; Seok, K.S.; Song, G.J.; Seo, Y.C. Mercury distribution characteristics in primary manganese smelting plants. Environ. Pollut. 2017, 227, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.J.; Jiang, C.S. Heavy metal concentrations in soils and plants in Rongxi Manganese Mine of Chongqing, Southwest of China. Acta Ecol. Sin. 2015, 35, 46–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Ma, M.; Wang, X.; Xie, Q.; Zhang, C.; Wang, Y.; Wang, D. Distribution of mercury and methylmercury in river water and sediment of typical manganese mining area. J. Environ. Sci. 2022, 119, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, H.; Jia, Z.; Li, Y.; Dong, J.; Yan, M.; Zhang, F. Ecological and health risk assessment of heavy metals in farmland soil in northwest Xiushan, Chongqing. Geol. China 2020, 47, 1625–1636. (In Chinese) [Google Scholar]
- Xu, X.; Lin, Y.; Meng, B.; Feng, X.; Xu, Z.; Jiang, Y.; Zhong, W.; Hu, Y.; Qiu, G. The impact of an abandoned mercury mine on the environment in the Xiushan region, Chongqing, southwestern China. Appl. Geochem. 2018, 88, 267–275. [Google Scholar] [CrossRef]
- Ma, M.; Du, H.; Sun, T.; An, S.; Yang, G.; Wang, D. Characteristics of archaea and bacteria in rice rhizosphere along a mercury gradient. Sci. Total Environ. 2019, 650 Pt 1, 1640–1651. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.S.; Hao, Q.J.; Huang, X.J. Investigation and Assessment of Contaminated Soils and Plants by Mn in Manganese Mining Area in Xiushan Autonomous County of Chongqing. Environ. Sci. Technol. 2012, 35, 167–172. (In Chinese) [Google Scholar]
- HJ962-2018; Determination of Soil pH—Potentiometric Method. National Environmental Protection Standards of the People’s Republic of China. Ministry of Environmental Protection: Beijing, China, 2018. (In Chinese)
- NY/T1121.6-2006; Part 6: Determination of Soil Organic Matter. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006. (In Chinese)
- HJ889-2017; Determination of Cation Exchange Capacity in Soil—Extraction of Cobalt Hexachloride—Spectrophotometric Method. Ministry of Environmental Protection: Beijing, China, 2017. (In Chinese)
- Tessier, A.P.; Campbell, P.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- USEPA. Method 1631: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry (Revision E); EPA-821-R-02-019; USEPA: Washington, DC, USA, 2002.
- Bao, Z.; Wang, J.; Feng, X.; Shang, L. Distribution of mercury speciation in polluted soils of Wanshan mercury mining area in Guizhou. Chin. J. Ecol. 2011, 30, 907–913. (In Chinese) [Google Scholar]
- Lin, H.; Zhu, R.; Yu, L.; Cheng, Y.; Zhu, R.; Liu, P.; Ren, Z. Determination of arsenic, mercury, selenium, antimony and bismuth in soil and sediment by water bath Digestion-Atomic Fluorescence Spectrometry. Spectrosc. Spect. Anal. 2020, 40, 1528–1533. (In Chinese) [Google Scholar]
- Wang, G.F.; Wang, W.X.; Zhang, J.W. The Study of Method for detecting Heavy Metal Elements in Soil. Environ. Monit. China 2012, 28, 66–68. (In Chinese) [Google Scholar]
- Zhang, Q.; Li, G.H.; Fan, S.Z.; Pan, Y.S. Study on determination of 42 major, minor and trace elements in soil and stream sediment samples. Chin. J. Anal. Chem. 2008, 27, 51–57. (In Chinese) [Google Scholar]
- GB15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China Environmental Publishing Group: Beijing, China, 2018. (In Chinese)
- Sun, T.; Ma, M.; Du, H.; Wang, X.; Zhang, Y.; Wang, Y.; Wang, D. Effect of different rotation systems on mercury methylation in paddy fields. Ecotoxicol. Environ. Saf. 2019, 182, 109403. [Google Scholar] [CrossRef]
- Frohne, T.; Rinklebe, J.; Langer, U.; Du Laing, G.; Mothes, S.; Wennrich, R. Biogeochemical factors affecting mercury methylation rate in two contaminated floodplain soils. Biogeosciences 2012, 9, 493–507. [Google Scholar] [CrossRef]
- Qiu, G.L.; Feng, X.B.; Li, P.; Wang, S.F.; Li, G.H.; Shang, L.H.; Fu, X.W. Methylmercury Accumulation in Rice (Oryza sativa L.) Grown at Abandoned Mercury Mines in Guizhou, China. J. Agric. Food Chem. 2008, 56, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Tian, L.; Braaten, H.F.V.; Wu, Q.R.; Luo, J.; Cai, L.M.; Meng, J.H.; Lin, Y. Mercury-Organic Matter Interactions in Soils and Sediments: Angel or Devil? B. Environ. Contam. Tox. 2019, 102, 621–627. [Google Scholar] [CrossRef]
- Hang, X.S.; Gan, F.Q.; Wang, J.G.; Chen, X.Q.; Chen, Y.D.; Wang, H.Y.; Zhou, J.M.; Du, C.W. Soil mercury accumulation and transference to different crop grains. Hum. Ecol. Risk Assess. 2016, 22, 1242–1252. [Google Scholar] [CrossRef]
- Chiasson-Gould, S.A.; Blais, J.M.; Poulain, A.J. Dissolved Organic Matter Kinetically Controls Mercury Bioavailability to Bacteria. Environ. Sci. Technol. 2014, 48, 3153–3161. [Google Scholar] [CrossRef]
| Procedure of Modified Tessier Method | Reagent | The Specific Methods |
|---|---|---|
| The water soluble and exchangeable (Hg-EX) | 1mol/L Mg(NO3)2 (HNO3 regulates pH = 7.0) | Accurately weighing 2.0 g soil samples, adding16 mL extractant, shaking at room temperature for1h, centrifugal 20 min (3500 r/min), supernatant through 0.45 μm microporous membrane, analysis. The residue was washed twice with 8ml ultrapure water. |
| The bound to carbonate (Hg-CAR) | 1mol/L NaAc (CH3COOH regulates pH = 5.0) | After the first step, 16 mL extract was added to the residue, shaking at room temperature for 5 h, centrifuged for 20 min, the supernatant was analyzed. The residue was washed twice with 8 mL ultrapure water. |
| The bound to Fe/Mn oxides of low crystallinity (Hg-OX) | 0.4mol/LNH2OH·HCl (soluble in 20%HAc(v/v)) | After treatment in step 2, add 40 mL extract, 96 °C water bath 6 h, centrifugal 20 min, take supernatant analysis. The residue was washed twice with 8 mL ultrapure water. |
| The bound to organic matter (Hg-OM) | H2O2 (HNO3 regulates pH = 2.0) | Residue after step 3 treatment, add 16 mL extract, 80 °C water bath 2 h, add 6 mL extract, 80 °C water bath 3h, centrifugal 20 min, take supernatant analysis. The residue was washed twice with 8 mL ultrapure water. |
| The residual (Hg-RES) | Aqua regia (HNO3:HCl = 1:3) | The residue was treated by step 4, adding 20 mL extractant, 95 °C water bath10min, adding 1.0 mL BrCl and 95 °C water bath 30 min again. |
| Sampling Points | pH | SOM | CEC | Mn | Fe | S | Se | |
|---|---|---|---|---|---|---|---|---|
| (g kg–1) | (cmol+ kg–1) | (mg kg–1) | (mg kg–1) | (mg kg–1) | (mg kg–1) | |||
| Mining area (0–10 cm) | Maximum value | 7.57 | 64.85 | 17.35 | 7535.80 | 42,335.40 | 709.43 | 2.35 |
| Minimum value | 4.53 | 11.72 | 5.40 | 121.40 | 14,357.10 | 100.72 | 0.22 | |
| Average value | 5.97 | 29.69 | 10.50 | 1613.69 | 27,458.69 | 304.83 | 0.85 | |
| Standard deviation | 0.81 | 11.57 | 3.51 | 1879.65 | 6088.50 | 126.72 | 0.50 | |
| Smelting area (0–10 cm) | Maximum value | 7.44 | 47.20 | 25.56 | 10316.60 | 53,781.70 | 933.24 | 1.37 |
| Minimum value | 4.61 | 10.18 | 7.21 | 175.00 | 15,082.00 | 106.89 | 0.34 | |
| Average value | 6.35 | 28.84 | 12.13 | 1609.11 | 28,572.99 | 289.41 | 0.80 | |
| Standard deviation | 0.63 | 9.93 | 3.23 | 2415.33 | 9229.10 | 149.64 | 0.23 | |
| Mining area (10–20 cm) | Maximum value | 7.93 | 43.79 | 16.92 | 8009.39 | 42,148.71 | 786.91 | 2.57 |
| Minimum value | 4.33 | 13.94 | 3.60 | 85.16 | 14,161.04 | 111.64 | 0.26 | |
| Average value | 5.96 | 25.18 | 10.04 | 1682.66 | 27,877.52 | 296.15 | 0.82 | |
| Standard deviation | 0.89 | 6.81 | 3.21 | 1962.86 | 6526.59 | 141.38 | 0.43 | |
| Smelting area (10–20 cm) | Maximum value | 7.67 | 55.94 | 24.30 | 10883.99 | 55,503.22 | 781.91 | 1.95 |
| Minimum value | 4.54 | 10.48 | 6.54 | 193.28 | 14,716.25 | 88.33 | 0.46 | |
| Average value | 6.32 | 27.34 | 11.54 | 1508.11 | 28,694.20 | 275.10 | 0.80 | |
| Standard deviation | 0.72 | 9.70 | 3.26 | 2241.01 | 9486.01 | 134.25 | 0.26 | |
| Mining area (20–30 cm) | Maximum value | 7.88 | 37.60 | 15.96 | 9625.27 | 46,274.67 | 432.42 | 3.02 |
| Minimum value | 4.16 | 4.53 | 4.11 | 135.33 | 14,075.81 | 20.08 | 0.24 | |
| Average value | 5.93 | 20.95 | 9.76 | 1591.87 | 28,911.75 | 257.38 | 0.81 | |
| Standard deviation | 0.96 | 7.65 | 3.04 | 2007.18 | 6698.66 | 79.83 | 0.54 | |
| Smelting area (20–30 cm) | Maximum value | 7.83 | 55.39 | 15.82 | 9259.29 | 63,838.91 | 497.77 | 1.98 |
| Minimum value | 4.15 | 6.10 | 6.32 | 239.19 | 15,324.93 | 31.52 | 0.27 | |
| Average value | 6.56 | 22.14 | 11.08 | 1197.41 | 29,021.45 | 242.43 | 0.74 | |
| Standard deviation | 0.65 | 9.62 | 2.34 | 1572.37 | 9925.21 | 105.46 | 0.34 | |
| Mining area (30–40 cm) | Maximum value | 7.81 | 35.47 | 17.27 | 10858.12 | 49,717.83 | 858.71 | 1.91 |
| Minimum value | 4.18 | 5.53 | 3.37 | 106.05 | 15,132.86 | 31.26 | 0.17 | |
| Average value | 6.17 | 16.49 | 9.59 | 2019.54 | 30,971.28 | 228.46 | 0.64 | |
| Standard deviation | 0.85 | 7.71 | 3.22 | 2620.25 | 7377.17 | 135.62 | 0.35 | |
| Smelting area (30–40 cm) | Maximum value | 7.89 | 55.80 | 29.89 | 7702.29 | 71,869.28 | 353.79 | 1.26 |
| Minimum value | 4.24 | 2.70 | 6.52 | 210.48 | 12,082.49 | 14.04 | 0.33 | |
| Average value | 6.72 | 17.33 | 10.89 | 1115.55 | 31,531.45 | 189.73 | 0.61 | |
| Standard deviation | 0.64 | 10.36 | 3.46 | 1231.52 | 12,662.92 | 96.66 | 0.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, X.; Ma, W.; Yin, D.; Wang, Y.; Zhang, C.; Wang, D. Distribution of Mercury and Methylmercury in Farmland Soils Affected by Manganese Mining and Smelting Activities. Int. J. Environ. Res. Public Health 2022, 19, 10288. https://doi.org/10.3390/ijerph191610288
Zhang Y, Zhou X, Ma W, Yin D, Wang Y, Zhang C, Wang D. Distribution of Mercury and Methylmercury in Farmland Soils Affected by Manganese Mining and Smelting Activities. International Journal of Environmental Research and Public Health. 2022; 19(16):10288. https://doi.org/10.3390/ijerph191610288
Chicago/Turabian StyleZhang, Yongjiang, Xian Zhou, Weibin Ma, Deliang Yin, Yongmin Wang, Cheng Zhang, and Dingyong Wang. 2022. "Distribution of Mercury and Methylmercury in Farmland Soils Affected by Manganese Mining and Smelting Activities" International Journal of Environmental Research and Public Health 19, no. 16: 10288. https://doi.org/10.3390/ijerph191610288
APA StyleZhang, Y., Zhou, X., Ma, W., Yin, D., Wang, Y., Zhang, C., & Wang, D. (2022). Distribution of Mercury and Methylmercury in Farmland Soils Affected by Manganese Mining and Smelting Activities. International Journal of Environmental Research and Public Health, 19(16), 10288. https://doi.org/10.3390/ijerph191610288






