Application of an EMG-Rehabilitation Robot in Patients with Post-Coronavirus Fatigue Syndrome (COVID-19)—A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
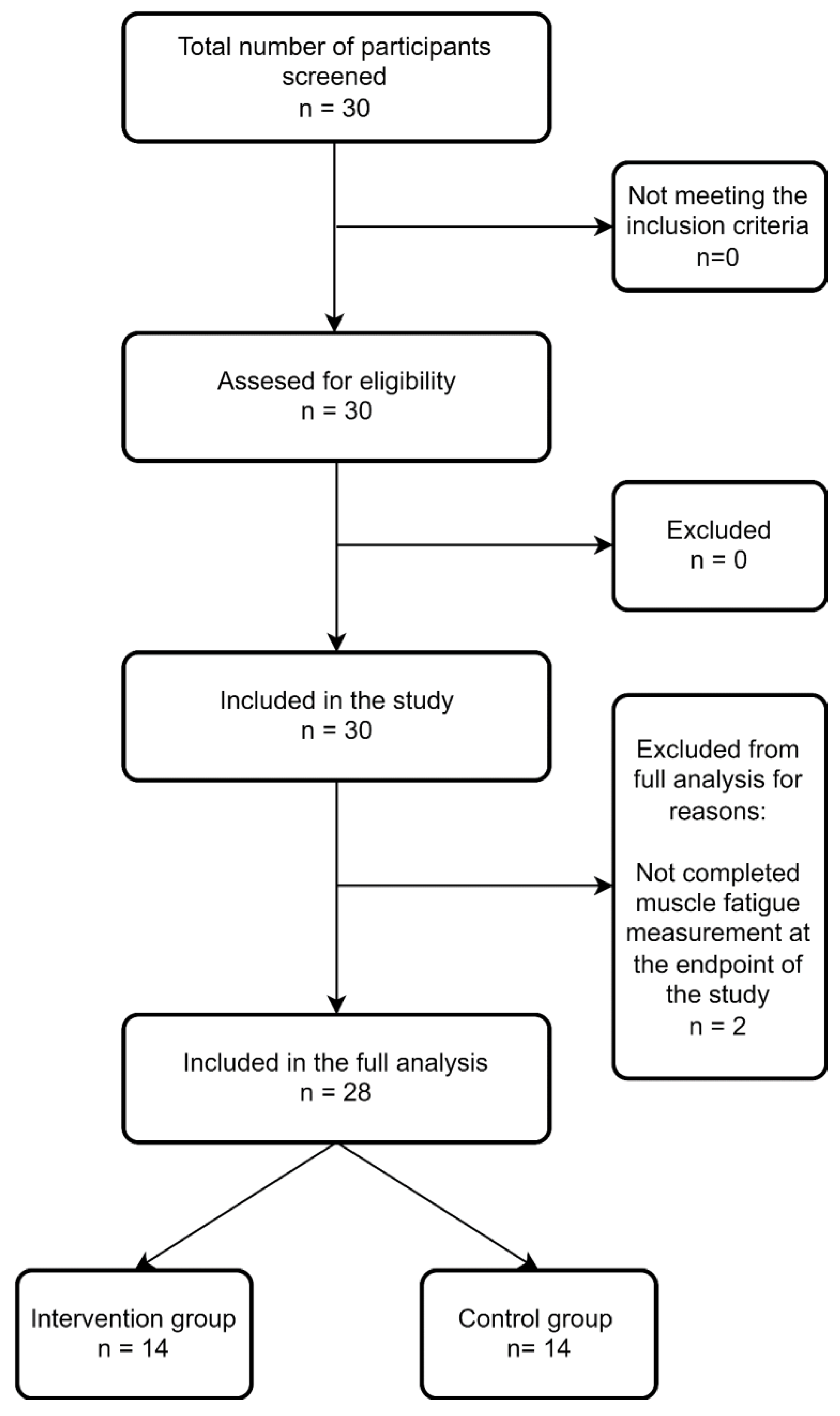
2.1. Study Design
2.2. Study Group
2.3. Study Criteria
2.4. Outcome Measure
2.5. Measurement
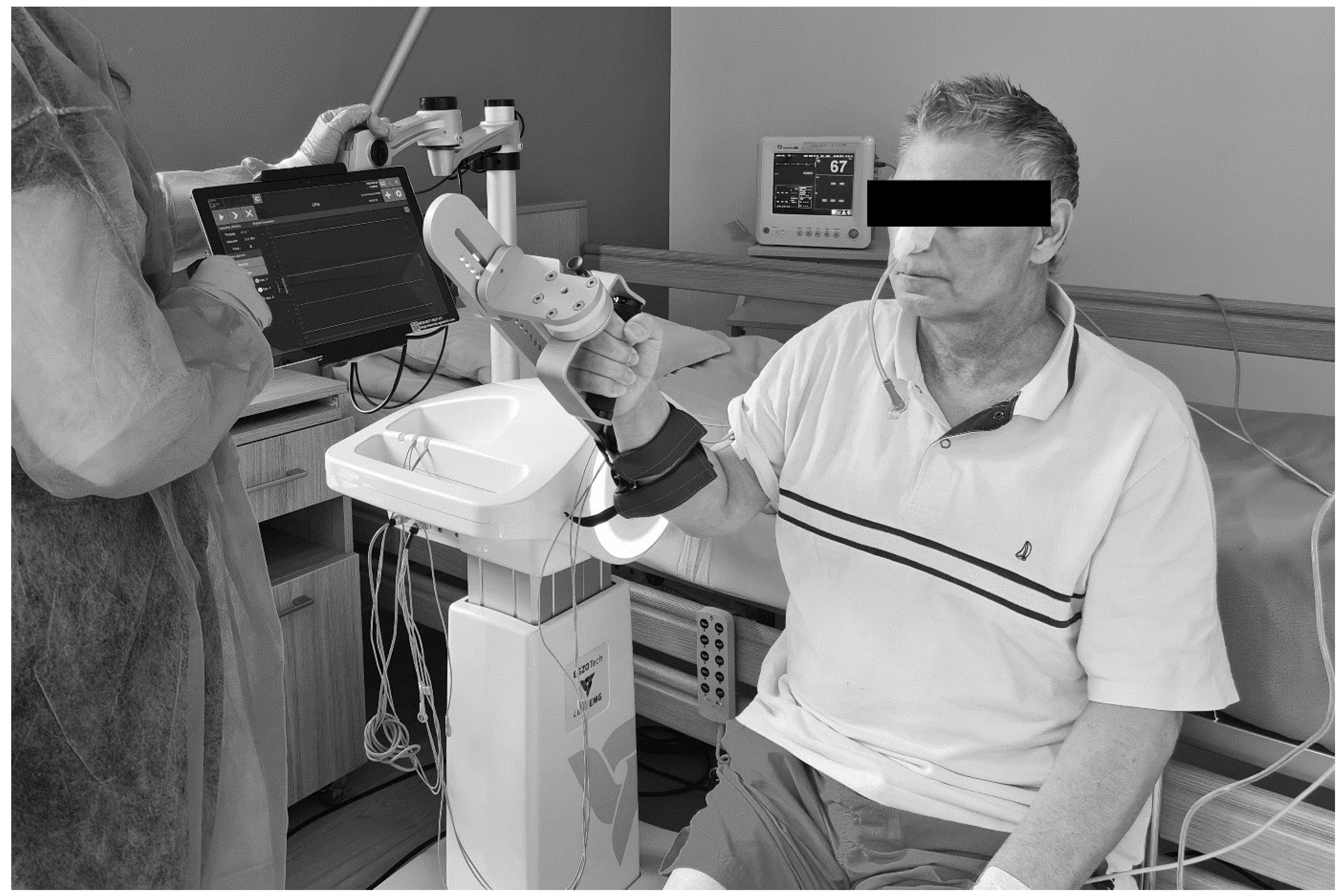
2.6. Rehabilitation
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, R.; Qiu, Y.; He, J.-S.; Tan, J.-Y.; Li, X.-H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Shao, S.-C.; Hsu, C.-K.; Wu, I.-W.; Hung, M.-J.; Chen, Y.-C. Incidence of acute kidney injury in COVID-19 infection: A systematic review and meta-analysis. Crit. Care 2020, 24, 346. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- World Health Organization International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision). Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/569175314 (accessed on 14 March 2022).
- Ostojic, S.M. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef]
- Wostyn, P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med. Hypotheses 2021, 146, 110469. [Google Scholar] [CrossRef] [PubMed]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, M.I. The post-viral syndrome: A review. J. R. Coll. Gen. Pract. 1987, 37, 212–214. [Google Scholar] [PubMed]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashif, A.; Chaudhry, M.; Fayyaz, T.; Abdullah, M.; Malik, A.; Anwer, J.M.A.; Inam, S.H.A.; Fatima, T.; Iqbal, N.; Shoaib, K. Follow-up of COVID-19 recovered patients with mild disease. Sci. Rep. 2021, 11, 13414. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Malani, P.N. COVID-19-New Insights on a Rapidly Changing Epidemic. JAMA 2020, 323, 1339–1340. [Google Scholar] [CrossRef] [Green Version]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef]
- Vink, M.; Vink-Niese, A. Could Cognitive Behavioural Therapy Be an Effective Treatment for Long COVID and Post COVID-19 Fatigue Syndrome? Lessons from the Qure Study for Q-Fever Fatigue Syndrome. Healthcare 2020, 8, 552. [Google Scholar] [CrossRef]
- Pallanti, S.; Grassi, E.; Makris, N.; Gasic, G.P.; Hollander, E. Neurocovid-19: A clinical neuroscience-based approach to reduce SARS-CoV-2 related mental health sequelae. J. Psychiatr. Res. 2020, 130, 215–217. [Google Scholar] [CrossRef]
- Lamprecht, B. Gibt es ein Post-COVID-Syndrom? Pneumologe 2020, 17, 398–405. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Nekhlyudov, L.; Duijts, S.; Hudson, S.V.; Jones, J.M.; Keogh, J.; Love, B.; Lustberg, M.; Smith, K.C.; Tevaarwerk, A.; Yu, X.; et al. Addressing the needs of cancer survivors during the COVID-19 pandemic. J. Cancer Surviv. 2020, 14, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zasadzka, E.; Pieczyńska, A.; Trzmiel, T.; Hojan, K. Virtual Reality as a Promising Tool Supporting Oncological Treatment in Breast Cancer. Int. J. Environ. Res. Public Health 2021, 18, 8768. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Filoni, S.; Billeri, L.; Balletta, T.; Cannavò, A.; Militi, A.; Milardi, D.; Pignolo, L.; Naro, A. Robotic Rehabilitation in Spinal Cord Injury: A Pilot Study on End-Effectors and Neurophysiological Outcomes. Ann. Biomed. Eng. 2021, 49, 732–745. [Google Scholar] [CrossRef]
- Middaugh, S.; Thomas, K.J.; Smith, A.R.; McFall, T.L.; Klingmueller, J. EMG Biofeedback and Exercise for Treatment of Cervical and Shoulder Pain in Individuals with a Spinal Cord Injury: A Pilot Study. Top. Spinal Cord Inj. Rehabil. 2013, 19, 311–323. [Google Scholar] [CrossRef]
- Tamburella, F.; Moreno, J.C.; Herrera Valenzuela, D.S.; Pisotta, I.; Iosa, M.; Cincotti, F.; Mattia, D.; Pons, J.L.; Molinari, M. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: Electromyographic vs joint torque biofeedback. J. Neuroeng. Rehabil. 2019, 16, 95. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Síndrome post-COVID-19: Epidemiología, criterios diagnósticos y mecanismos patogénicos implicados. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Heinemann, A.W.; Linacre, J.M.; Wright, B.D.; Hamilton, B.B.; Granger, C. Relationships between impairment and physical disability as measured by the functional independence measure. Arch. Phys. Med. Rehabil. 1993, 74, 566–573. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- MacDermid, J.C.; Solomon, G.S.; Valdes, K.A. Clinical Assessment Recommendations, 3rd ed.; American Society of Hand Therapists: Mount Laurel, NJ, USA, 2015; ISBN 0692525157. [Google Scholar]
- De Vries, J.; Michielsen, H.; van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Ranzani, R.; Lambercy, O.; Metzger, J.-C.; Califfi, A.; Regazzi, S.; Dinacci, D.; Petrillo, C.; Rossi, P.; Conti, F.M.; Gassert, R. Neurocognitive robot-assisted rehabilitation of hand function: A randomized control trial on motor recovery in subacute stroke. J. Neuroeng. Rehabil. 2020, 17, 115. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Taveggia, G.; Galeri, S.; Bissolotti, L.; Mullè, C.; Imperio, G.; Valdes, K.; Borboni, A.; Negrini, S. Efficacy of Short-Term Robot-Assisted Rehabilitation in Patients With Hand Paralysis After Stroke: A Randomized Clinical Trial. Hand 2018, 13, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Bustamante Valles, K.; Montes, S.; Madrigal, M.d.J.; Burciaga, A.; Martínez, M.E.; Johnson, M.J. Technology-assisted stroke rehabilitation in Mexico: A pilot randomized trial comparing traditional therapy to circuit training in a Robot/technology-assisted therapy gym. J. Neuroeng. Rehabil. 2016, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.-W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Szczegielniak, J.; Bogacz, K.; Majorczyk, E.; Szczegielniak, A.; Łuniewski, J. Post-COVID-19 rehabilitation—A Polish pilot program. Med. Pr. 2021, 72, 611–616. [Google Scholar] [CrossRef]
- Kurtaiş Aytür, Y.; Füsun Köseoglu, B.; Özyemişci Taşkıran, Ö.; Kutay Ordu Gökkaya, N.; Ünsal Delialioğlu, S.; Sonel Tur, B.; Sarıkaya, S.; Şirzai, H.; Tekdemir Tiftik, T.; Alemdaroglu, E.; et al. Pulmonary rehabilitation principles in SARS-CoV-2 infection (COVID-19): The revised guideline for the acute, subacute, and post-COVID-19 rehabilitation. Turk. J. Phys. Med. Rehabil. 2021, 67, 129–145. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Ruiu, P.; Paolucci, T.; Santilli, V.; Bernetti, A. Rehabilitation setting during and after COVID-19: An overview on recommendations. J. Rehabil. Med. 2021, 53, 2737. [Google Scholar] [CrossRef]
- Spielmanns, M.; Pekacka-Egli, A.-M.; Schoendorf, S.; Windisch, W.; Hermann, M. Effects of a Comprehensive Pulmonary Rehabilitation in Severe Post-COVID-19 Patients. Int. J. Environ. Res. Public Health 2021, 18, 2695. [Google Scholar] [CrossRef] [PubMed]
- Udina, C.; Ars, J.; Morandi, A.; Vilaró, J.; Cáceres, C.; Inzitari, M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. J. Frailty Aging 2021, 10, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Daynes, E.; Gerlis, C.; Chaplin, E.; Gardiner, N.; Singh, S.J. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition—A cohort study. Chronic Respir. Dis. 2021, 18, 14799731211015691. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Taub, M.; Creelman, C.; Cahalan, C.; O’Dell, M.W.; Stein, J. Feasibility of an Electromyography-Triggered Hand Robot for People After Chronic Stroke. Am. J. Occup. Ther. 2019, 73, 7304345040p1–7304345040p9. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.J.; Vallence, A.-M.; Cleary, S.; Cooper, I.; Loftus, A.M. The effect of EMG triggered electrical stimulation plus task practice on arm function in chronic stroke patients with moderate-severe arm deficits. Restor. Neurol. Neurosci. 2013, 31, 681–691. [Google Scholar] [CrossRef] [Green Version]
| Intervention Group | Control Group | p-Value | |
|---|---|---|---|
| Sex (%) * | 0.439 | ||
| Female | 40 | 26.67 | |
| Male | 60 | 13.33 | |
| Age (years) + | 69 (43–81) | 66 (39–75) | 0.372 |
| Level of education % * | 0.659 | ||
| primary | 6.67 | 0 | |
| vocational | 26.67 | 40 | |
| secondary | 46.67 | 46.67 | |
| high | 20 | 13.33 | |
| Height (cm) + | 171 (150–188) | 173 (154–192) | 0.442 |
| Weight (kg) + | 77 (50–103) | 80 (49–97) | 0.561 |
| BMI + | 26.33 (20.81–32.32) | 25.25 (19.14–36.07) | 0.130 |
| Time in the ICU (days) | 25 (20–41) | 24 (20–44) | 0.917 |
| Time intubated (days) | 23(15–35) | 21(15–33) | 0.604 |
| Outcome Measure (Median and Range) | Intervention Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p | Pre-Intervention | Post-Intervention | p | |
| FIM | 85 (8–120) | 117 (78–136) | 0.001 | 89 (32–120) | 117 (5–126) | 0.005 |
| HGS | 18 (0–35) | 20 (1–37) | 0.001 | 20 (10–39) | 22 (14–40) | 0.007 |
| BI | 11 (2–14) | 18 (15–20) | 0.001 | 12 (3–14) | 19 (2–20) | 0.001 |
| FAS | 25 (15–42) | 23 (7–38) | 0.001 | 26 (14–42) | 26 (13–48) | 0.041 |
| Fatigue (EMG) | −5.95 (−29.2–5.4) | −6.8 (−17.6–20.9) | 0.778 | −2.2 (−20.1–43.6) | −1.05 (−22.2–10.2) | 0.975 |
| Outcome Measure (Median and Range) | Intervention Group | Control Group | p |
|---|---|---|---|
| FIM | 26 (16–113) | 23 (−27–54) | 0.137 |
| HGS | 3 (0–10) | 4 (−9–10) | 0.367 |
| BI | 8 (4–14) | 6 (−3–11) | 0.233 |
| FAS | −2 (−11–0) | −2 (−7–7) | 0.412 |
| Fatigue (EMG) | 0 (−14.9–34.7) | 2.8 (−55.4–11.3) | 0.909 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasadzka, E.; Tobis, S.; Trzmiel, T.; Marchewka, R.; Kozak, D.; Roksela, A.; Pieczyńska, A.; Hojan, K. Application of an EMG-Rehabilitation Robot in Patients with Post-Coronavirus Fatigue Syndrome (COVID-19)—A Feasibility Study. Int. J. Environ. Res. Public Health 2022, 19, 10398. https://doi.org/10.3390/ijerph191610398
Zasadzka E, Tobis S, Trzmiel T, Marchewka R, Kozak D, Roksela A, Pieczyńska A, Hojan K. Application of an EMG-Rehabilitation Robot in Patients with Post-Coronavirus Fatigue Syndrome (COVID-19)—A Feasibility Study. International Journal of Environmental Research and Public Health. 2022; 19(16):10398. https://doi.org/10.3390/ijerph191610398
Chicago/Turabian StyleZasadzka, Ewa, Sławomir Tobis, Tomasz Trzmiel, Renata Marchewka, Dominika Kozak, Anna Roksela, Anna Pieczyńska, and Katarzyna Hojan. 2022. "Application of an EMG-Rehabilitation Robot in Patients with Post-Coronavirus Fatigue Syndrome (COVID-19)—A Feasibility Study" International Journal of Environmental Research and Public Health 19, no. 16: 10398. https://doi.org/10.3390/ijerph191610398






