Transmission of Antimicrobial Resistant Bacteria at the Hajj: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
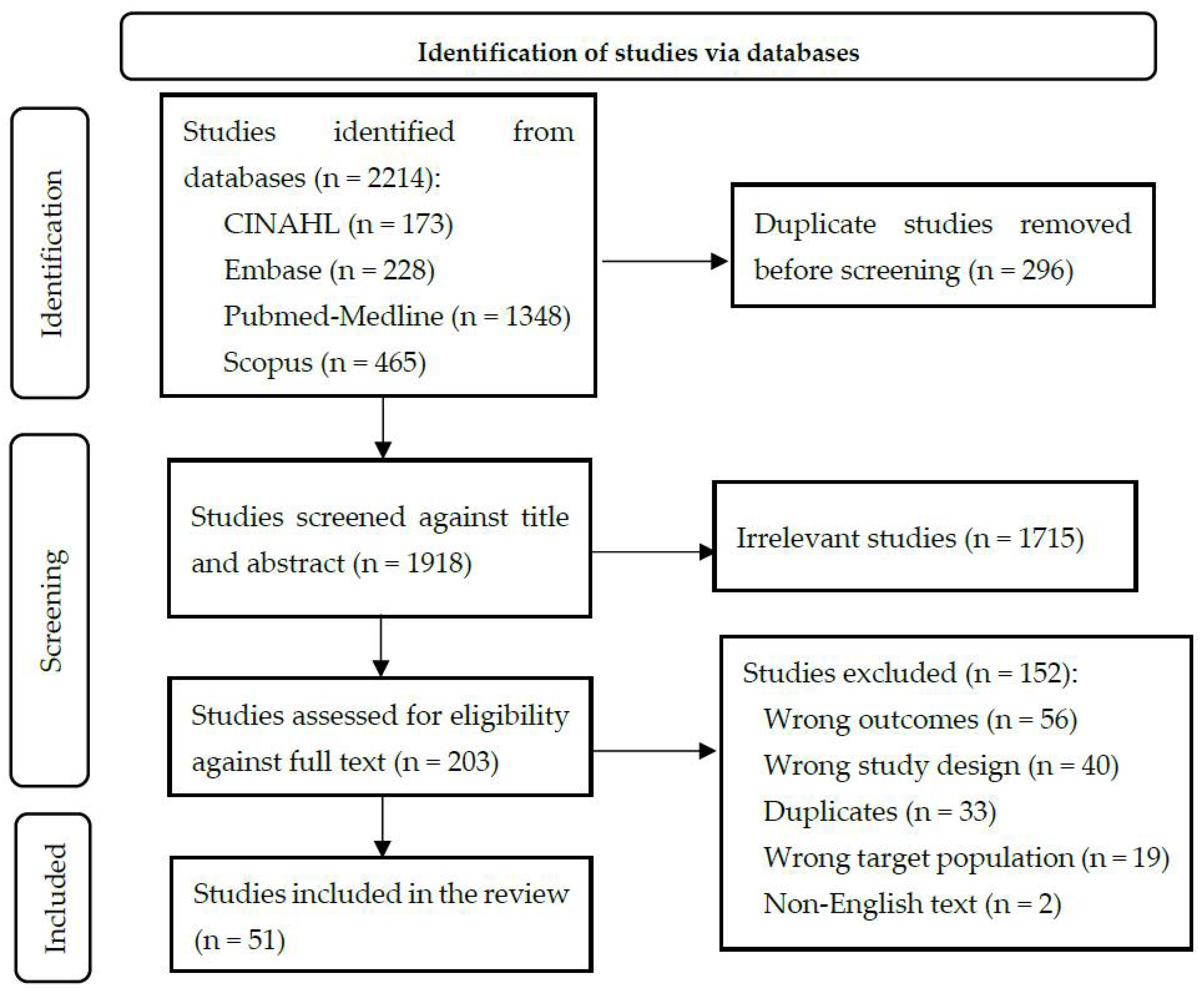
2.1. Article Identification
2.2. Article Screening
2.3. Data Extraction
2.4. Analysis
2.5. Ethics
3. Results
3.1. What AMR Bacteria Are Reported in the Literature?
3.1.1. Enteric Disease-Causing AMR Bacteria
3.1.2. Respiratory Disease-Causing AMR Bacteria
3.1.3. Other AMR Bacteria
| Author [Ref.] | Year | AMR Bacteria | Antibiotic Resistance | Study Type | Summary |
|---|---|---|---|---|---|
| Salih, M. A., et al. [21] | 1990 | Neisseria meningitidis | Sulfadiazine | Outbreak investigation | The outbreak investigation reported 45 AMR-positive Sudanese pilgrims recently returned to Sudan from the Hajj. |
| Ng, P. P. and Taha, M. [53] | 1994 | Vibrio cholerae | Tetracycline, ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole | Case report | The case study reported that tetracycline-resistant Vibrio cholera was found in three pilgrims who recently returned to Malaysia from the Hajj. |
| Yousuf, M. and Nadeem, A. [54] | 1995 | Neisseria meningitidis | Cloxacillin | Case report | the case study reported that cloxacillin-resistant Neisseria meningitidis was found in two American and Indonesian male pilgrims admitted to a hospital in Madinah, Saudi Arabia. |
| Fatani, M. I., et al. [67] | 2002 | Staphylococcus aureus | Penicillin, erythromycin, cephalothin, trimethoprim/sulfamethoxazole, clindamycin, tetracycline, gentamicin, and oxacillin | Cross-sectional study (prospective) | The study reported 47 MRSA isolated from pyodermas patients admitted to a hospital in Makkah during the Hajj season. |
| Asghar, A. H. [27] | 2006 | Escherichia coli, Pseudomonas spp., Acinetobacter spp., Klebsiella spp., Serratia spp., Enterobacter spp., Proteus spp., Salmonella spp., H. influenzae, Citrobacter spp., Bacteroides spp., Burkholderia spp., Brucella spp. | Ampicillin, cefepime, cephalothin, ceftazidime, amoxicillin/clavulanic acid, cefoxitin, piperacillin/tazobactam, piperacillin, gentamicin, imipenem, aztreonam, amikacin, ciprofloxacin, trimethoprim/sulfamethoxazole, penicillin, oxacillin, erythromycin, and clindamycin | Cross-sectional study (prospective) | The study reported 1530 AMR cases (septicaemic patients) from hospitals in Makkah. |
| Asghar, A. H. and Momenah, A. M. [28] | 2006 | Staphylococcus aureus | Methicillin, penicillin, ampicillin, oxacillin, erythromycin, cephalothin, gentamicin, Oxytetracycline, and trimethoprim/sulfamethoxazole | Cross-sectional study (prospective) | The study reported 199 MRSA cases from hospitals in Makkah, of which 157 were found to have MDR. |
| Karima, T. M., et al. [29] | 2006 | Helicobacter pylori | Metronidazole, erythromycin, amoxicillin, tetracycline, and ciprofloxacin | Cross-sectional study (prospective) | The study reported that H. pylori was found in 18 patients (from a general hospital in Makkah) with resistance to at least one antibiotic. |
| Memish, Z. A., et al. [30] | 2006 | Staphylococcus aureus | Methicillin | Cross-sectional study (prospective) | The study reported six MRSA isolates from pilgrims during the Hajj of 2004. |
| Bukhari, S. Z., et al. [31] | 2008 | Ewingella americana | Amikacin, amoxicillin/clavulanic acid, ampicillin/sulbactam, ampicillin, cefazolin, cefepime, cefotaxime, ceftazidime, ceftriaxone, cefuroxime, cephalothin, gentamycin, imipenem, piperacillin/tazobactam, piperacillin, tetracycline, ticarcillin/clavulanic acid, tobramycin, trimethoprim/sulfamethoxazole | Case report | The case report of an AMR E. americana strain, isolated from an Indonesian pilgrim admitted to a hospital in Makkah during the Hajj. |
| Asghar, A. H. and Faidah, H. S. [32] | 2009 | Escherichia coli, Klebsiella. pneumoniae, Klebsiella spp., P. aeruginosa, A. baumannii, Proteus spp., H. influenzae, Enterobacter spp. | Cephalothin, cefoxitin, cefuroxime, ceftazidime, cefotaxime, ceftriaxone, ampicillin, aztreonam, piperacillin, piperacillin/tazobactam, amoxicillin/clavulanic acid, imipenem, meropenem, imipenem/cilastatin, amikacin, gentamycin, tobramycin, ciprofloxacin, levofloxacin, norfloxacin, nalidixic acid, norfloxacin/ciprofloxacin, tetracycline, trimethoprim/sulfamethoxazole, and nitrofurantoin | Cross-sectional study (prospective) | The study found 1046 g-negative bacteria isolated from patients in Makkah hospitals were resistant to at least one antibiotic. |
| Abulreesh, H. H. and Organji, S. R. [69] | 2011 | Staphylococci | Erythromycin, colistin, penicillin, oxacillin, vancomycin | Cross-sectional study (prospective) | The study reported 19 vancomycin-resistance Staphylococci, isolated from food and food handlers in Makkah. |
| Asghar, A. H. [33] | 2011 | Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus viridans, Enterococcus Fecalis, and Enterococcus spp. | Cephalexin, cefazolin, cefoxitin, cefotaxime, ceftriaxone, ceftazidime, cefuroxime, ceftizoxime, penicillin, ampicillin, ampicillin/sulbactam, oxacillin, aztreonam, amoxicillin/clavulanic acid, imipenem/cilastatin sodium, gentamicin, neomycin, amikacin, ciprofloxacin, ciprofloxacin/norfloxacin, nalidixic acid, gemifloxacin, levofloxacin, vancomycin, erythromycin, clindamycin, quinupristin/dalfopristin, linezolid, tetracycline, chloramphenicol, trimethoprim/sulfamethoxazole, rifampicin, nitrofurantoin, and polymyxin B | Cross-sectional study (prospective) | The study of patients admitted to hospitals in Makkah found that the most common resistance reported was against beta-lactams. |
| El-Amin, N. M. and Faidah, H. S. [34] | 2011 | Enterococci | Vancomycin | Cross-sectional study (retrospective) | The retrospective study reported vancomycin-resistant Enterococci infections in seven patients from hospitals in Makkah. |
| Asghar, A. H. [35] | 2012 | Pseudomonas aeruginosa | Amikacin, amoxicillin/clavulanic acid, ampicillin, aztreonam, cefepime, cefotaxime, gentamycin, ceftriaxone, cefoxitin, ceftazidime, cefuroxime, cephalothin, ciprofloxacin, imipenem, meropenem, piperacillin, piperacillin/tazobactam, tetracycline, and trimethoprim/sulfamethoxazole | Cross-sectional study (prospective) | The study reported that metallo-beta-lactamase (MBL) -producing P. aeruginosa were identified in 76 patients (from 30 nationalities) admitted to hospitals in Makkah. |
| Asghar, A. H. [36] | 2014 | Staphylococcus aureus | Methicillin | Cross-sectional study (prospective) | The study reported that MRSA was identified in 114 patients admitted to hospitals in Makkah, of which 100 carried the mecA gene. |
| Khan, M. M., et al. [37] | 2014 | Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis-homin, Staphylococcus hominis-novo, Staphylococcus warneri, Staphylococcus hominis, Staphylococcus capitis, Staphylococcus lugdunensis, and Staphylococcus auricularis | Ampicillin, amoxicillin/clavulanic acid, azithromycin, clindamycin, daptomycin, erythromycin, gentamicin, oxacillin, penicillin, and quinupristin/dalfopristin | Cross-sectional study (prospective) | The study reported that 189 coagulase negative staphylococci (CoNS) isolates (from neonates admitted to a hospital in Makkah) were resistant to at least one of the tested antibiotics. |
| Abdel-Haleem, A. M., et al. [38] | 2015 | Stenotrophomonas maltophilia | Beta-lactams, cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, tetracyclines, polymyxin, trimethoprim, gentamicin, and tigecycline | Case report | The case study reported MDR S. maltophilia strain was isolated from a patient with reoccurring urinary tract infection admitted to a tertiary hospital in Makkah. |
| Alyamani, E. J., et al. [39] | 2015 | Acinetobacter baumannii | Cefepime, ceftazidime, and multidrug resistance | Cross-sectional study (prospective) | The study reported 100 MDR A. baumannii isolates collected from patients admitted to hospitals in Makkah. |
| Memish, Z. A., et al. [70] | 2015 | Streptococcus pneumoniae | Penicillin, amoxicillin, cefotaxime, chloramphenicol, clindamycin, erythromycin, levofloxacin, moxifloxacin, tetracycline, or sulfamethoxazole/trimethoprim | Cross-sectional study (prospective) | The study reported 137 AMR positive pilgrims returning from the Hajj to their home countries (Algeria, Chad, Comoros, Egypt, Ethiopia, Guinea, India, Indonesia, Libya, Mauritania, Nigeria, and Sudan). |
| Olaitan, A. O., et al. [66] | 2015 | Salmonella enterica | Colistin, amoxicillin, amoxicillin/clavulanic acid, ceftriaxone, aztreonam, ceftazidime, imipenem, and gentamicin | Cross-sectional study (prospective) | The study reported MDR Salmonella in five French pilgrims who recently returned to Marseille, France after the Hajj. |
| Algowaihi, R., et al. [40] | 2016 | Klebsiella pneumoniae | Amikacin, amoxicillin/clavulanic acid, ampicillin, cefazolin, cefepime, cefotaxime, cefoxitin, ceftazidime, cefuroxime, ciprofloxacin, ertapenem, fosfomycin, gentamicin, imipenem, levofloxacin, meropenem, mezlocillin, moxifloxacin, nitrofurantoin, norfloxacin, piperacillin/tazobactam, tetracycline, tigecycline, tobramycin, trimethoprim, and sulfamethoxazole | Case report | The study reported MDR K. pneumoniae strain isolated from a female patient with a urinary tract infection, admitted to a tertiary hospital in Makkah. |
| Alyamani, E. J., et al. [41] | 2016 | Escherichia coli | Penicillin, carbapenems, cephamycin, tetracycline, chloramphenicol, acriflavine, and polymyxin | Case report | The case study reported MDR uropathogenic Escherichia coli O25b:H4 strain isolated from a male patient admitted to a hospital in Makkah. |
| Haseeb, A., et al. [42] | 2016 | Acinetobacter baumannii, Escherichia coli, Enterobacter cloacae, Enterococcus spp., Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Salmonellae, Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus spp. | Amoxicillin/clavulanic acid, ampicillin, aztreonam, cefazolin, cefepime, cefoxitin, cefuroxime, ceftazidime, cefotaxime, ceftriaxone, cephalothin, ertapenem, imipenem, meropenem, oxacillin, penicillin, piperacillin/tazobactam, ticarcillin, mezlocillin, amikacin, gentamicin, tobramycin, ciprofloxacin, levofloxacin, moxifloxacin, nalidixic acid, and norfloxacin | Cross-sectional study (retrospective) | The retrospective study reported 214 AMR bacterial isolates collected from pilgrims who visited emergency care departments of Makkah hospitals. |
| Johargy, A. K. [43] | 2016 | Enterococcus faecium, Escherichia coli, Staphylococcu aureus and P. aeruginosa | Amoxicillin/clavulanic acid, amikacin, ceftazidime, cephalothin, erythromycin, gentamycin, chloramphenicol, oxacillin, clindamycin, ciprofloxacin, penicillin, vancomycin, piperacillin, cefotaxime, nalidixic acid, nitrofurantoin, oxacillin, and sulfamethoxazole | Cross-sectional study (prospective) | The study reported 129 AMR bacterial isolates collected from diabetic patients from hospitals in Makkah. |
| Khan, M. A. and Faiz, A. [44] | 2016 | Pseudomonas aeruginosa | Amikacin, aztreonam, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, levofloxacin, meropenem, piperacillin, piperacillin/tazobactam, and ticarcillin | Cross-sectional study (prospective) | The study reported 27 AMR and 8 MDR P. aeruginosa isolates collected from patients admitted to hospitals in Makkah. |
| Leangapichart, T., et al. [58] | 2016 | Escherichia coli and Klebsiella pneumoniae | Ticarcillin/clavulanic acid, ceftriaxone, and gentamicin | Cross-sectional study (prospective) | The study reported 28 AMR Escherichia coli or K. pneumoniae isolates collected from French pilgrims before and after the Hajj. |
| Leangapichart, T., et al. [59] | 2016 | Escherichia coli and Klebsiella pneumoniae | Amoxicillin, amoxicillin/clavulanic acid, ceftriaxone, ciprofloxacin, fosfomycin, gentamicin, nalidixic acid, and sulfamethoxazole/trimethoprim | Cross-sectional study (prospective) | A letter to editor reporting the presence of colistin-resistance gene, mcr-1 among 23 French pilgrims (before and after the Hajj of 2013 and 2014), ten of which were Escherichia. coli, and one was K. pneumoniae. |
| Leangapichart, T., et al. [60] | 2016 | Acinetobacter baumannii and Escherichia coli | Aztreonam, cefoxitin, ceftriaxone, cefotaxime, amoxicillin/clavulanic acid, ticarcillin/clavulanic acid, amoxicillin, tobramycin, gentamicin, ciprofloxacin, ofloxacin, imipenem, and sulfamethoxazole/trimethoprim | Cross-sectional study (prospective) | A study reporting MDR A. baumannii and Escherichia coli among 43 French pilgrims (before and after returning from the Hajj of 2014) |
| Marglani, O. A., et al. [61] | 2016 | Staphylococcus aureus, Klebsiella pneumoniae, Klebsiella oxytoca, Escherichia coli, Enterobacter spp., and Citrobacter spp. | Amoxicillin/clavulanic acid, ampicillin, cefoxitin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, levofloxacin, gentamicin, imipenem, piperacillin/tazobactam, sulfamethoxazole/trimethoprim, clindamycin, azithromycin, erythromycin, tetracycline, and vancomycin | Cross-sectional study (prospective) | A study reporting 57 AMR isolates collected from pilgrims with acute rhinosinusitis) during the Hajj of 2014. |
| Memish, Z. A., et al. [71] | 2016 | Streptococcus pneumoniae | Erythromycin, clindamycin, tetracycline, penicillin, amoxicillin, cefotaxime, levofloxacin, moxifloxacin, chloramphenicol, and sulfamethoxazole/trimethoprim | Cross-sectional study (prospective) | The study reported 94 AMR S. pneumoniae isolates collected from pilgrims (before and during the Hajj of 2013) from 12 countries in Africa, Asia, USA, and Europe. |
| Abd El Ghany, M., et al. [62] | 2017 | Salmonella spp., Shigella spp., and Escherichia coli, Yersinia enterocolitica | Beta-lactams | Cross-sectional study (prospective) | The study reported 70 AMR bacterial isolates collected from pilgrims (from 40 different countries) who acquired enteric infections during the Hajj of 2011 to 2013. |
| Abulreesh, Hussein H., et al. [68] | 2017 | Staphylococcus aureus | Amoxicillin/clavulanic acid, ampicillin, azithromycin, cefoxitin, clindamycin, erythromycin, fusidic acid, gentamicin; imipenem, oxacillin, penicillin, and tetracycline | Cross-sectional study (prospective) | The study reported 50 AMR S. aureus isolates collected from clinical laboratories in Makkah. |
| Al-Gethamy, M. M., et al. [45] | 2017 | Acinetobacter baumannii | Ceftazidime, ciprofloxacin, imipenem, trimethoprim, amikacin, gentamicin. | Casecontrol study | The case–control study reported MDR A. baumannii isolates (collected from patients admitted to a hospital in Makkah), that mainly resistance to imipenem and gentamycin with 83% and 73%, respectively. |
| Alyamani, E. J., et al. [46] | 2017 | Escherichia coli | Ampicillin, cefoxitin, ciprofloxacin, cefepime, aztreonam, cefotaxime, and ceftazidime | Cross-sectional study (prospective) | The study reported 58 AMR Escherichia coli isolates, collected from pilgrims admitted hospitals in Makkah, during the Hajj of 2014 and 2015. |
| Ahmed Khan, T., et al. [55] | 2018 | Staphylococcus aureus and Proteus spp. | Ampicillin, ciprofloxacin, fusidic acid, penicillin, cefuroxime, ceftriaxone, cefixime, erythromycin, cefoxitin, and tetracycline | Case report | The case study reported MDR S. aureus and proteus spp. isolates, collected from a burn aggravated infected foot wart in a pilgrim who came from Pakistan to perform the Hajj of 2017. |
| Ganaie, F, et al. [72] | 2018 | Streptococcus pneumoniae | Penicillin, cefotaxime, levofloxacin, erythromycin, tetracycline, and sulfamethoxazole/trimethoprim | Cross-sectional study (prospective) | The study reported 145 AMR S. pneumoniae isolates, collected from Indian pilgrims before and after returning from the Hajj of 2016, with higher AMR rates within the post-Hajj samples. |
| Khan, M. A., et al. [47] | 2019 | Klebsiella pneumoniae, Escherichia coli, E. cloacae and Proteus mirabilis | Cephalosporins (ceftazidime, cefotaxime, ceftriaxone, cefepime) and carbapenems | Cross-sectional study (prospective) | The study reported 27 carbapenemase Enterobacteriaceae isolates (collected from patients admitted to hospitals in Makkah), of which 21 were carbapenemase producing K. pneumoniae. |
| Mater, M. E., et al. [48] | 2020 | Staphylococcus aureus | methicillin | Cross-sectional study (retrospective) | The retrospective study reported 92 MRSA isolates, collected from burn and paediatric patients admitted to a hospital in Makkah, from January 2016 to January 2017. |
| Sambas, MFMK, et al. [74] | 2020 | Mycobacterium tuberculosis | Streptomycin, isoniazid, ethambutol, and rifampicin, | Cross-sectional study (prospective) | The study reported 27 AMR Tuberculosis (TB) isolates and 8 MDR-TB isolates, collected from TB patients admitted to a hospital in Makkah. |
| Willerton, L., et al. [22] | 2020 | Neisseria meningitidis | Penicillin and ciprofloxacin | Cross-sectional study (retrospective) | The retrospective study reported penicillin and ciprofloxacin N. meningitidis isolates, from N. meningitidis patients returning from Makkah, after preforming umrah pilgrimage to England. |
| Ahmed, O. B., et al. [49] | 2021 | Klebsiella pneumoniae | Amoxicillin/clavulanic acid, ciprofloxacin, cefotaxime, ampicillin, aztreonam, cefuroxime, cefepime, and imipenem | Cross-sectional study (retrospective) | The retrospective study reported 51 aminoglycoside-resistant K. pneumonia isolates, collected from hospital admitted patients in Makkah. |
| Ahmed, Omar B., et al. [50] | 2021 | Klebsiella pneumoniae, Escherichia coli, P. aeruginosa, A. baumannii, K. oxytoca, P. mirabilis, and Enterobacter spp. | Tobramycin, kanamycin, gentamicin, neomycin, amikacin, streptomycin, cefotaxime, amoxicillin/clavulanic acid, and ciprofloxacin | Cross-sectional study (retrospective) | The retrospective study reported 69 g-negative bacterial isolates with aminoglycoside-resistant gene(s), collected from hospital admitted patients in Makkah. |
| Al-Hayani, A. M, et al. [75] | 2021 | Mycobacterium tuberculosis | isoniazid, streptomycin, ethambutol, rifampicin, and pyrazinamide | Cross-sectional study (retrospective) | The retrospective study reported 93 TB patients with AMR, where data collected from the registry of the Central TB Laboratory in Makkah. |
| Al-Zahrani, I. A. and Al-Ahmadi, B. M. [51] | 2021 | Pseudomonas aeruginosa | Beta-lactams, amikacin, and colistin | Cross-sectional study (prospective) | The study reported 35 carbapenem-resistant P. aeruginosa isolates, collected from 26 hospital admitted patients in Makkah. |
| Alghamdi, S. [63] | 2021 | Acinetobacter species, Klebsiella pneumonia, Escherichia coli, Staph. aureus species and Pseudomonas aeruginosa. | Cefoxitin, penicillin, gentamicin, ampicillin, methicillin, clindamycin, sulfamethoxazole/trimethoprim, vancomycin, Trimoxazole, linezolid, ciprofloxacin, levofloxacin, cefuroxime, amikacin, ceftazidime, cefepime, and cefoperazone/sulbactam | Cross-sectional study (retrospective) | The retrospective study reported 123 AMR bacterial isolates, 15 were MRSA, and 6 MDR Acinetobacter spp. isolates, collected from cancer patients who were admitted to Makkah hospitals. |
| Harimurti, K., et al. [73] | 2021 | Streptococcus pneumoniae | Erythromycin, clindamycin, chloramphenicol, penicillin, sulfamethoxazole/trimethoprim, and tetracycline | Cross-sectional study (prospective) | The study reported 85 AMR S. pneumoniae isolates, collected from Indonesian pilgrims before and after the Hajj of 2015. |
| Hoang, V. T., et al. [64] | 2021 | Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Staphylococcus aureus, Acinetobacter baumannii | Imipenem, doripenem, piperacillin/tazobactam, fosfomycin, sulfamethoxazole/trimethoprim, ciprofloxacin, ticarcillin, ticarcillin/clavulanic acid, tobramycin, fusidic acid, erythromycin, methicillin, amoxicillin/clavulanic acid, cefepime, ceftriaxone, and colistin | Cross-sectional study (prospective) | The study reported 81 MDR isolates, collected from pilgrims from Marseille, France, during the Hajj of 2017 and 2018, of which 23 were isolated from pre-Hajj, and 52 from post-Hajj. |
| Leangapichart, T., et al. [56] | 2021 | Shewanella xiamenensis | Amoxicillin, amoxicillin/clavulanic acid, and ticarcillin/clavulanic acid | Case report | The case study reported two beta-lactam resistant S. xiamenensis strains, isolated from a Moroccan pilgrim from France, one before and one during travels to the Hajj of 2013. |
| Mohd Baharin, I. E., et al. [65] | 2021 | Streptococcus pneumoniae and Klebsiella pneumoniae | Beta-lactams and macrolide | Cross-sectional study (prospective) | The study reported 14 AMR K. pneumoniae and S. pneumoniae isolates, collected from Malaysian pilgrims returning to Kelantan, Malaysia from the Hajj. |
| Turkstani, M. A., et al. [57] | 2021 | Staphylococcus spp., Micrococcus spp., Bacillus spp., Microbacterium spp., Geobacillus spp., Brachybacterium spp. | Penicillin, erythromycin, ampicillin, chloramphenicol, clindamycin, gentamicin, sulfamethoxazole/trimethoprim, fusidic acid, oxacillin, and cefepime | Environmental study | The environmental research study reported 40 AMR bacterial isolates, collected from surface swab samples from two membership-based gyms in Makkah. |
| Haseeb, A., et al. [52] | 2022 | Staphylococcus aureus, non-fermenter Gram-negative bacilli, Enterobacteriaceae, Enterococci. | Methicillin, beta-lactams, carbapenems, third generation cephalosporins, and vancomycin. | Cross-sectional study (prospective) | The study reported 106 AMR isolates and 46 MRSA isolates, collected from patients admitted to Makkah hospitals. |
3.2. The Methods of Detecting AMR Bacteria
3.3. The Geospatial Distribution of AMR Cases Reported
3.3.1. Geospatial Distribution of Enteric Disease-Causing AMR Bacteria
3.3.2. Geospatial Distribution of Respiratory Illness-Causing AMR Bacteria
3.4. The Resistance Profile for AMR Bacteria
3.4.1. Resistance Profile of Enteric Disease-Causing AMR Bacteria
3.4.2. Resistance Profile of Respiratory Illness-Causing AMR Bacteria
4. Discussion
4.1. Enteric Disease-Causing Beta-Lactam Resistance Bacteria
4.2. Respiratory Disease-Causing Beta-Lactam Resistance Bacteria
4.2.1. AMR Isolated from Hospital Settings
4.2.2. Surveillance of AMR Bacteria
4.2.3. Preventive Measures for AMR Bacteria
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Division: National Action Plans and Monitoring and Evaluation. In Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; p. 45.
- World Health Organization. Fact Sheets: Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 16 March 2022).
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- The Review on Antimicrobial Resistance. In Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; O’Neill, J. (Ed.) Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef]
- Sridhar, S.; Turbett, S.E.; Harris, J.B.; LaRocque, R.C. Antimicrobial-resistant bacteria in international travelers. Curr. Opin. Infect. Dis. 2021, 34, 423–431. [Google Scholar] [CrossRef]
- Ahmed, Q.A.; Arabi, Y.M.; Memish, Z.A. Health risks at the Hajj. Lancet 2006, 367, 1008–1015. [Google Scholar] [CrossRef]
- World Health Organization. Public Health for Mass Gatherings: Key Considerations; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Shafi, S.; Booy, R.; Haworth, E.; Rashid, H.; Memish, Z.A. Hajj: Health lessons for mass gatherings. J. Infect. Public Health 2008, 1, 27–32. [Google Scholar] [CrossRef]
- Zumla, A.; McCloskey, B.; Endericks, T.; Azhar, E.I.; Petersen, E. The challenges of cholera at the 2017 Hajj pilgrimage. Lancet Infect. Dis. 2017, 17, 895–897. [Google Scholar] [CrossRef]
- Yezli, S.; Assiri, A.M.; Alhakeem, R.F.; Turkistani, A.M.; Alotaibi, B. Meningococcal disease during the Hajj and Umrah mass gatherings. Int. J. Infect. Dis. 2016, 47, 60–64. [Google Scholar] [CrossRef]
- Novelli, V.M.; Lewis, R.G.; Dawood, S.T. Epidemic group A meningococcal disease in Haj pilgrims. Lancet 1987, 2, 863. [Google Scholar] [CrossRef]
- Taha, M.K.; Achtman, M.; Alonso, J.M.; Greenwood, B.; Ramsay, M.; Fox, A.; Gray, S.; Kaczmarski, E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000, 356, 2159. [Google Scholar] [CrossRef]
- Bokhary, H.; Rashid, H.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Rise of Antimicrobial Resistance in Mass Gatherings. In Handbook of Healthcare in the Arab World; Laher, I., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar] [CrossRef] [Green Version]
- Leangapichart, T.; Rolain, J.M.; Memish, Z.A.; Al-Tawfiq, J.A.; Gautret, P. Emergence of drug resistant bacteria at the Hajj: A systematic review. Travel Med. Infect. Dis. 2017, 18, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, S.J.; Meyer, D.; Cameron, E.; Nalabandian, M.; Pervaiz, B.; Nuzzo, J.B. Establishing a theoretical foundation for measuring global health security: A scoping review. BMC Public Health 2019, 19, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salih, M.A.; Danielsson, D.; Backman, A.; Caugant, D.A.; Achtman, M.; Olcen, P. Characterization of epidemic and nonepidemic Neisseria meningitidis serogroup A strains from Sudan and Sweden. J. Clin. Microbiol. 1990, 28, 1711–1719. [Google Scholar] [CrossRef] [Green Version]
- Willerton, L.; Lucidarme, J.; Campbell, H.; Caugant, D.A.; Claus, H.; Jacobsson, S.; Ladhani, S.N.; Molling, P.; Neri, A.; Stefanelli, P.; et al. Geographically widespread invasive meningococcal disease caused by a ciprofloxacin resistant non-groupable strain of the ST-175 clonal complex. J. Infect. 2020, 81, 575–584. [Google Scholar] [CrossRef]
- Hazra, A. Using the confidence interval confidently. J. Thorac. Dis. 2017, 9, 4125–4130. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.A.; Gardner, M.J. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br. Med. J. 1988, 296, 1313–1316. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S. A dirty dozen: Twelve p-value misconceptions. Semin. Hematol. 2008, 45, 135–140. [Google Scholar] [CrossRef]
- PRISMA Statement. PRISMA Flow Diagram. Available online: http://www.prisma-statement.org/PRISMAStatement/FlowDiagram (accessed on 15 March 2022).
- Asghar, A.H. Frequency and antimicrobial susceptibility patterns of bacterial pathogens isolated from septicemic patients in Makkah hospitals. Saudi Med. J. 2006, 27, 361–367. [Google Scholar]
- Asghar, A.H.; Momenah, A.M. Methicillin resistance among Staphylococcus aureus isolates from Saudi hospitals. Med. Princ. Pract. 2006, 15, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Karima, T.M.; Bukhari, S.Z.; Ghais, M.A.; Fatani, M.I.; Hussain, W.M. Prevalence of Helicobacter pylori infection in patients with peptic ulcer diseases. Saudi Med. J. 2006, 27, 621–626. [Google Scholar] [PubMed]
- Memish, Z.A.; Balkhy, H.H.; Almuneef, M.A.; Al-Haj-Hussein, B.T.; Bukhari, A.I.; Osoba, A.O. Carriage of Staphylococcus aureus among Hajj pilgrims. Saudi Med. J. 2006, 27, 1367–1372. [Google Scholar] [PubMed]
- Bukhari, S.Z.; Hussain, W.M.; Fatani, M.I.; Ashshi, A.M. Multi-drug resistant Ewingella americana. Saudi Med. J. 2008, 29, 1051–1053. [Google Scholar]
- Asghar, A.H.; Faidah, H.S. Frequency and antimicrobial susceptibility of gram-negative bacteria isolated from 2 hospitals in Makkah, Saudi Arabia. Saudi Med. J. 2009, 30, 1017–1023. [Google Scholar]
- Asghar, A.H. Frequency and antibiotic susceptibility of gram-positive bacteria in Makkah hospitals. Ann. Saudi Med. 2011, 31, 462–468. [Google Scholar] [CrossRef]
- El-Amin, N.M.; Faidah, H.S. Vancomycin-resistant Enterococci. Prevalence and risk factors for fecal carriage in patients at tertiary care hospitals. Saudi Med. J. 2011, 32, 966–967. [Google Scholar]
- Asghar, A.H. Antimicrobial susceptibility and metallo-beta-lactamase production among Pseudomonas aeruginosa isolated from Makkah hospitals. Pak. J. Med. Sci. 2012, 28, 7. [Google Scholar]
- Asghar, A.H. Molecular characterization of methicillin-resistant Staphylococcus aureus isolated from tertiary care hospitals. Pak. J. Med. Sci. 2014, 30, 698–702. [Google Scholar] [CrossRef]
- Khan, M.M.; Faiz, A.; Ashshi, A.M. Clinically significant Coagulase Negative Staphylococci and their antibiotic resistance pattern in a tertiary care hospital. J. Pak. Med. Assoc. 2014, 64, 1171–1174. [Google Scholar]
- Abdel-Haleem, A.M.; Rchiad, Z.; Khan, B.K.; Abdallah, A.M.; Naeem, R.; Nikhat Sheerin, S.; Solovyev, V.; Ahmed, A.; Pain, A. Genome Sequence of a Multidrug-Resistant Strain of Stenotrophomonas maltophilia with Carbapenem Resistance, Isolated from King Abdullah Medical City, Makkah, Saudi Arabia. Genome Announc. 2015, 3, e01166-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyamani, E.J.; Khiyami, M.A.; Booq, R.Y.; Alnafjan, B.M.; Altammami, M.A.; Bahwerth, F.S. Molecular characterization of extended-spectrum beta-lactamases (ESBLs) produced by clinical isolates of Acinetobacter baumannii in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algowaihi, R.; Ashgar, S.; Sirag, B.; Shalam, S.; Nassir, A.; Ahmed, A. Draft Genome Sequence of a Multidrug-Resistant Klebsiella pneumoniae Strain Isolated from King Abdullah Medical City, Makkah, Saudi Arabia. Genome Announc. 2016, 4, e00375-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Bahwerth, F.S.; Vaisvil, B.; Schmitt, D.P.; Kapatral, V. Genome sequence and comparative pathogenic determinants of multidrug resistant uropathogenic Escherichia coli o25b:h4, a clinical isolate from Saudi Arabia. J. Pure Appl. Microbiol. 2016, 10, 2475–2484. [Google Scholar] [CrossRef]
- Haseeb, A.; Faidah, H.S.; Bakhsh, A.R.; Malki, W.H.; Elrggal, M.E.; Saleem, F.; Rahman, S.U.; Khan, T.M.; Hassali, M.A. Antimicrobial resistance among pilgrims: A retrospective study from two hospitals in Makkah, Saudi Arabia. Int. J. Infect. Dis. 2016, 47, 92–94. [Google Scholar] [CrossRef]
- Johargy, A.K. Antimicrobial susceptibility of bacterial and fungal infections among infected diabetic patients. J. Pak. Med. Assoc. 2016, 66, 1291–1295. [Google Scholar] [PubMed]
- Khan, M.A.; Faiz, A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann. Saudi Med. 2016, 36, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Al-Gethamy, M.M.; Faidah, H.S.; Adetunji, H.A.; Haseeb, A.; Ashgar, S.S.; Mohanned, T.K.; Mohammed, A.H.; Khurram, M.; Hassali, M.A. Risk factors associated with multi-drug-resistant Acinetobacter baumannii nosocomial infections at a tertiary care hospital in Makkah, Saudi Arabia—A matched case-control study. J. Int Med. Res. 2017, 45, 1181–1189. [Google Scholar] [CrossRef]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Mohamed, A.M.; Faiz, A.; Ahmad, J. Enterobacterial infection in Saudi Arabia: First record of Klebsiella pneumoniae with triple carbapenemase genes resistance. J. Infect. Dev. Ctries 2019, 13, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Mater, M.E.; Yamani, A.E.; Aljuffri, A.A.; Binladen, S.A. Epidemiology of burn-related infections in the largest burn unit in Saudi Arabia. Saudi Med. J. 2020, 41, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.B.; Asghar, A.H.; Bahwerth, F.S. Increasing frequency of Aminoglycoside-Resistant Klebsiella pneumoniae during the era of pandemic COVID-19. Mater. Today Proc. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.B.; Asghar, A.H.; Bahwerth, F.S.; Assaggaf, H.M.; Bamaga, M.A. The prevalence of aminoglycoside-resistant genes in Gram-negative bacteria in tertiary hospitals. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Al-Zahrani, I.A.; Al-Ahmadi, B.M. Dissemination of VIM-producing Pseudomonas aeruginosa associated with high-risk clone ST654 in a tertiary and quaternary hospital in Makkah, Saudi Arabia. J. Chemother. 2021, 33, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Faidah, H.S.; Algethamy, M.; Alghamdi, S.; Alhazmi, G.A.; Alshomrani, A.O.; Alqethami, B.R.; Alotibi, H.S.; Almutiri, M.Z.; Almuqati, K.S.; et al. Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health 2022, 19, 254. [Google Scholar] [CrossRef]
- Ng, P.P.; Taha, M. Tetracycline resistant Vibrio cholerae in pilgrims returning from Mecca. Med. J. Malays. 1994, 49, 195. [Google Scholar]
- Yousuf, M.; Nadeem, A. Fatal meningococcaemia due to group W135 amongst Haj pilgrims: Implications for future vaccination policy. Ann. Trop. Med. Parasitol. 1995, 89, 321–322. [Google Scholar] [CrossRef]
- Ahmed Khan, T.; Sheikh, M.; Azher, I.; Sheikh, A.K. Burn aggravated infected wart in a patient with type 2 diabetes: A medical challenge. BMJ Case Rep. 2018, 2018, bcr-2017-222897. [Google Scholar] [CrossRef]
- Leangapichart, T.; Hadjadj, L.; Gautret, P.; Rolain, J.M. Comparative genomics of two Shewanella xiamenensis strains isolated from a pilgrim before and during travels to the Hajj. Gut Pathog. 2021, 13, 9. [Google Scholar] [CrossRef]
- Turkstani, M.A.; Sultan, R.M.S.; Al-Hindi, R.R.; Ahmed, M.M.M. Molecular identification of microbial contaminations in the fitness center in Makkah region. Biosci. J. 2021, 37, e37020. [Google Scholar] [CrossRef]
- Leangapichart, T.; Dia, N.M.; Olaitan, A.O.; Gautret, P.G.; Brouqui, P.; Rolain, J.M. Acquisition of Extended-Spectrum beta-Lactamases by Escherichia coli and Klebsiella pneumoniae in Gut Microbiota of Pilgrims during the Hajj Pilgrimage of 2013. Antimicrob. Agents Chemother. 2016, 60, 3222–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leangapichart, T.; Gautret, P.; Brouqui, P.; Mimish, Z.; Raoult, D.; Rolain, J.M. Acquisition of mcr-1 plasmid-mediated colistin resistance in Escherichia coli and Klebsiella pneumoniae during Hajj 2013 and 2014. Antimicrob. Agents Chemother. 2016, 60, 6998–6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leangapichart, T.; Gautret, P.; Griffiths, K.; Belhouchat, K.; Memish, Z.; Raoult, D.; Rolain, J.M. Acquisition of a High Diversity of Bacteria during the Hajj Pilgrimage, Including Acinetobacter baumannii with blaOXA-72 and Escherichia coli with blaNDM-5 Carbapenemase Genes. Antimicrob. Agents Chemother. 2016, 60, 5942–5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marglani, O.A.; Alherabi, A.Z.; Herzallah, I.R.; Saati, F.A.; Tantawy, E.A.; Alandejani, T.A.; Faidah, H.S.; Bawazeer, N.A.; Marghalani, A.A.; Madani, T.A. Acute rhinosinusitis during Hajj season 2014: Prevalence of bacterial infection and patterns of antimicrobial susceptibility. Travel Med. Infect. Dis. 2016, 14, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Abd El Ghany, M.; Alsomali, M.; Almasri, M.; Padron Regalado, E.; Naeem, R.; Tukestani, A.; Asiri, A.; Hill-Cawthorne, G.A.; Pain, A.; Memish, Z.A. Enteric Infections Ci.irculating during Hajj Seasons, 2011-2013. Emerg. Infect. Dis. 2017, 23, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, S. Microbiological profile and antibiotic vulnerability of bacterial isolates from cancer patients. Cell Mol. Biol. 2021, 67, 190–194. [Google Scholar] [CrossRef]
- Hoang, V.T.; Dao, T.L.; Ly, T.D.A.; Gouriet, F.; Hadjadj, L.; Belhouchat, K.; Chaht, K.L.; Yezli, S.; Alotaibi, B.; Raoult, D.; et al. Acquisition of multidrug-resistant bacteria and encoding genes among French pilgrims during the 2017 and 2018 Hajj. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1199–1207. [Google Scholar] [CrossRef]
- Mohd Baharin, I.E.; Hasan, H.; Nik Mohd Noor, N.Z.; Mohamed, M. Molecular detection of selected zoonotic respiratory pathogens and the presence of virulence and antibiotic resistance genes via PCR among Kelantan Hajj pilgrims. Malays. J. Microbiol. 2021, 17, 254–265. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Dia, N.M.; Gautret, P.; Benkouiten, S.; Belhouchat, K.; Drali, T.; Parola, P.; Brouqui, P.; Memish, Z.; Raoult, D.; et al. Acquisition of extended-spectrum cephalosporin- and colistin-resistant Salmonella enterica subsp. enterica serotype Newport by pilgrims during Hajj. Int. J. Antimicrob. Agents 2015, 45, 600–604. [Google Scholar] [CrossRef]
- Fatani, M.I.; Bukhari, S.Z.; Al-Afif, K.A.; Karima, T.M.; Abdulghani, M.R.; Al-Kaltham, M.I. Pyoderma among Hajj Pilgrims in Makkah. Saudi Med. J. 2002, 23, 782–785. [Google Scholar]
- Abulreesh, H.H.; Organji, S.R.; Osman, G.E.H.; Elbanna, K.; Almalki, M.H.K.; Ahmad, I. Prevalence of antibiotic resistance and virulence factors encoding genes in clinical Staphylococcus aureus isolates in Saudi Arabia. Clin. Epidemiol. Glob. Health 2017, 5, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Abulreesh, H.H.; Organji, S.R. The Prevalence of Multidrug-resistant Staphylococci in Food and the Environment of Makkah, Saudi Arabia. Res. J. Microbiol. 2011, 6, 510–523. [Google Scholar] [CrossRef] [Green Version]
- Memish, Z.A.; Assiri, A.; Almasri, M.; Alhakeem, R.F.; Turkestani, A.; Al Rabeeah, A.A.; Akkad, N.; Yezli, S.; Klugman, K.P.; O’Brien, K.L.; et al. Impact of the Hajj on pneumococcal transmission. Clin. Microbiol Infect. 2015, 21, 77.e11–77.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memish, Z.A.; Al-Tawfiq, J.A.; Almasri, M.; Akkad, N.; Yezli, S.; Turkestani, A.; van der Linden, M.; Assiri, A. A cohort study of the impact and acquisition of naspharyngeal carriage of Streptococcus pneumoniae during the Hajj. Travel Med. Infect. Dis. 2016, 14, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.; Nagaraj, G.; Govindan, V.; Basha, R.R.; Hussain, M.; Ashraf, N.; Ahmed, S.; Ravi Kumar, K.L. Impact of Hajj on the S. pneumoniae carriage among Indian pilgrims during 2016- a longitudinal molecular surveillance study. Travel Med. Infect. Dis. 2018, 23, 64–71. [Google Scholar] [CrossRef]
- Harimurti, K.; Saldi, S.R.F.; Dewiasty, E.; Alfarizi, T.; Dharmayuli, M.; Khoeri, M.M.; Paramaiswari, W.T.; Salsabila, K.; Tafroji, W.; Halim, C.; et al. Streptococcus pneumoniae carriage and antibiotic susceptibility among Indonesian pilgrims during the Hajj pilgrimage in 2015. PLoS ONE 2021, 16, e0246122. [Google Scholar] [CrossRef]
- Sambas, M.F.M.K.; Rabbani, U.; Al-Gethamy, M.M.M.; Surbaya, S.H.; Alharbi, F.F.I.; Ahmad, R.G.A.; Qul, H.K.H.; Nassar, S.M.S.; Maddah, A.K.M.A.; Darweesh, B.A.K. Prevalence and Determinants of Multidrug-Resistant Tuberculosis in Makkah, Saudi Arabia. Infect. Drug Resist. 2020, 13, 4031–4038. [Google Scholar] [CrossRef]
- Al-Hayani, A.M.; Kamel, S.A.; Almudarra, S.S.; Alhayani, M.; Abu-Zaid, A. Drug Resistance to Anti-Tuberculosis Drugs: A Cross-Sectional Study From Makkah, Saudi Arabia. Cureus 2021, 13, e17069. [Google Scholar] [CrossRef]
- Santos, A.L.; Dos Santos, A.P.; Ito, C.R.M.; Queiroz, P.H.P.; de Almeida, J.A.; de Carvalho Junior, M.A.B.; de Oliveira, C.Z.; Avelino, M.A.G.; Wastowski, I.J.; Gomes, G.; et al. Profile of Enterobacteria Resistant to Beta-Lactams. Antibiotics 2020, 9, 410. [Google Scholar] [CrossRef]
- Rupp, M.E.; Fey, P.D. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: Considerations for diagnosis, prevention and drug treatment. Drugs 2003, 63, 353–365. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 60, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, V.T.; Ali-Salem, S.; Belhouchat, K.; Meftah, M.; Sow, D.; Dao, T.L.; Ly, T.D.A.; Drali, T.; Ninove, L.; Yezli, S.; et al. Respiratory tract infections among French Hajj pilgrims from 2014 to 2017. Sci. Rep. 2019, 9, 17771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, V.T.; Nguyen, T.T.; Belhouchat, K.; Meftah, M.; Sow, D.; Benkouiten, S.; Dao, T.L.; Anh Ly, T.D.; Drali, T.; Yezli, S.; et al. Antibiotic use for respiratory infections among Hajj pilgrims: A cohort survey and review of the literature. Travel Med. Infect. Dis 2019, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bokhary, H.; Research Team, H.; Barasheed, O.; Othman, H.B.; Saha, B.; Rashid, H.; Hill-Cawthorne, G.A.; Abd El Ghany, M. Evaluation of the rate, pattern and appropriateness of antibiotic prescription in a cohort of pilgrims suffering from upper respiratory tract infection during the 2018 Hajj season. Access. Microbiol. 2022, 4, 000338. [Google Scholar] [CrossRef] [PubMed]
- Bokhary, H.; Barasheed, O. Hajj Specific Appropriate Medication and Antibiotic Prescription: A Call for Development. Saudi J. Health Syst. Res. 2021, 1, 147–149. [Google Scholar] [CrossRef]
- Stein, G.E. Antimicrobial resistance in the hospital setting: Impact, trends, and infection control measures. Pharmacotherapy 2005, 25, 44S–54S. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Mohandhas, T.X. Prevalence of antimicrobial resistance in Acinetobacter calcoaceticus-baumannii complex in a Saudi Arabian hospital. Infect. Control. Hosp. Epidemiol. 2007, 28, 870–872. [Google Scholar] [CrossRef]
- Sunenshine, R.H.; Wright, M.O.; Maragakis, L.L.; Harris, A.D.; Song, X.; Hebden, J.; Cosgrove, S.E.; Anderson, A.; Carnell, J.; Jernigan, D.B.; et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect. Dis. 2007, 13, 97–103. [Google Scholar] [CrossRef]
- Leung, E.; Weil, D.E.; Raviglione, M.; Nakatani, H.; World Health Organization World Health Day Antimicrobial Resistance Technical Working Group. The WHO policy package to combat antimicrobial resistance. Bull. World Health Organ. 2011, 89, 390–392. [Google Scholar] [CrossRef]
- Yezli, S.; Yassin, Y.; Mushi, A.; Balkhi, B.; Stergachis, A.; Khan, A. Medication Handling and Storage among Pilgrims during the Hajj Mass Gathering. Healthcare 2021, 9, 626. [Google Scholar] [CrossRef]
| Organism(s) Name | Aminoglycoside | Beta-Lactams | Macrolides | Quinolones | Sulphonamides | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Reported AMR Isolates | Reported in (n) Studies | Number of Reported AMR Isolates | Reported in (n) Studies | Number of Reported AMR Isolates | Reported in (n) Studies | Number of Reported AMR Isolates | Reported in (n) Studies | Number of Reported AMR Isolates | Reported in (n) Studies | |
| Enteric bacteria | ||||||||||
| Acinetobacter spp. | 393 | 8 | 491 | 9 | 0 | 0 | 316 | 7 | 323 | 5 |
| Bacillus spp. | 0 | 0 | 3 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| Bacteroides spp. | 3 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Brachybacterium spp. | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Burkholderia spp. | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Citrobacter spp. | 4 | 2 | 8 | 2 | 0 | 0 | 3 | 1 | 2 | 1 |
| Enterobacter spp. | 25 | 4 | 69 | 6 | 0 | 0 | 18 | 3 | 32 | 3 |
| Enterococcus spp. | 68 | 2 | 176 | 2 | 56 | 2 | 39 | 1 | 21 | 1 |
| Escherichia coli | 489 | 9 | 726 | 13 | 18 | 1 | 407 | 9 | 426 | 5 |
| Klebsiella spp. | 339 | 5 | 475 | 8 | 0 | 0 | 264 | 6 | 242 | 4 |
| Proteus spp. | 51 | 2 | 74 | 4 | 0 | 0 | 45 | 2 | 29 | 2 |
| Pseudomonas spp. | 736 | 5 | 800 | 5 | 0 | 0 | 397 | 4 | 240 | 2 |
| Salmonella spp. | 3 | 2 | 34 | 3 | 0 | 0 | 0 | 0 | 7 | 1 |
| Serratia spp. | 4 | 1 | 21 | 1 | 0 | 0 | 1 | 1 | 7 | 1 |
| Shigella spp. | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vibrio cholerae | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 1 |
| Yersinia enterocolitica | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Respiratory bacteria | ||||||||||
| Haemophilus influenzae | 38 | 2 | 47 | 2 | 0 | 0 | 15 | 1 | 38 | 2 |
| Neisseria meningitidis | 0 | 0 | 5 | 2 | 0 | 0 | 3 | 1 | 45 | 1 |
| Staphylococcus spp. | 621 | 9 | 1748 | 16 | 818 | 10 | 57 | 4 | 696 | 6 |
| Stenotrophomonas maltophilia | 1 | 100 | 1 | 100 | 1 | 100 | 1 | 100 | 1 | 100 |
| Streptococcus spp. | 113 | 1 | 146 | 1 | 266 | 1 | 43 | 1 | 380 | 1 |
| Other bacteria | ||||||||||
| Brucella spp. | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Ewingella americana | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Helicobacter pylori | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 1 |
| Microbacterium spp. | 0 | 0 | 6 | 1 | 1 | 1 | 0 | 0 | 3 | 1 |
| Micrococcus spp. | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shewanella xiamenensis | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| AMR Rate among Respiratory Disease–Causing Bacteria Isolated In Hospital Settings (n = 1824) | AMR Rate among Enteric Disease–Causing Bacteria Isolated in Hospital Settings (n = 3632) | |||||
|---|---|---|---|---|---|---|
| Antibiotic Class | n | (%) | 95% CI (%) | n | (%) | 95% CI (%) |
| Aminoglycoside | 561 | 30.76 | [28.64–32.87] | 1996 | 54.96 | [53.34–56.57] |
| Beta–lactams | 1424 | 78.07 | [76.17–79.97] | 2730 | 75.17 | [73.76–76.57] |
| Lincosamides | 359 | 19.68 | [17.86–21.51] | 67 | 1.84 | [1.41–2.28] |
| Macrolides | 693 | 37.99 | [35.77–40.22] | 89 | 2.45 | [1.95–2.95] |
| Quinolones | 22 | 1.21 | [0.71–1.71] | 1415 | 38.96 | [37.37–40.55] |
| Sulphonamides | 767 | 42.05 | [39.79–44.32] | 1291 | 35.55 | [33.99–37.10] |
| Tetracyclines | 237 | 12.99 | [11.45–1454] | 486 | 13.38 | [12.27–14.49] |
| Resistance in TB Isolates | |||
|---|---|---|---|
| n | Rate (%) | 95% CI (%) | |
| AMR to anti-TB drug | |||
| Ethambutol | 33 | 6.61 | [4.43–8.79] |
| Isoniazid | 30 | 6.01 | [3.93–8.10] |
| pyrazinamide | 25 | 5.01 | [3.10–6.92] |
| Rifampicin | 25 | 5.01 | [3.10–6.92] |
| Streptomycin | 46 | 9.22 | [6.68–11.76] |
| Number of anti-TB drugs with AMR | |||
| Monoresistance (one drug only) | 71 | 14.23 | [11.16–17.29] |
| Two drugs only | 25 | 5.01 | [3.10–6.92] |
| Three or more | 14 | 2.81 | [1.36–4.25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alreeme, S.; Bokhary, H.; Craig, A.T. Transmission of Antimicrobial Resistant Bacteria at the Hajj: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 14134. https://doi.org/10.3390/ijerph192114134
Alreeme S, Bokhary H, Craig AT. Transmission of Antimicrobial Resistant Bacteria at the Hajj: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(21):14134. https://doi.org/10.3390/ijerph192114134
Chicago/Turabian StyleAlreeme, Sara, Hamid Bokhary, and Adam T. Craig. 2022. "Transmission of Antimicrobial Resistant Bacteria at the Hajj: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 21: 14134. https://doi.org/10.3390/ijerph192114134
APA StyleAlreeme, S., Bokhary, H., & Craig, A. T. (2022). Transmission of Antimicrobial Resistant Bacteria at the Hajj: A Scoping Review. International Journal of Environmental Research and Public Health, 19(21), 14134. https://doi.org/10.3390/ijerph192114134







