Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Cells and Growth Condition
2.2. Pomegranate Peel Extract
2.3. Fungal Growth and Biofilm Formation Assays
2.4. Mass Spectrometry Analysis of PomeGr Extract
2.5. Mass Spectrometry Analysis of Fungal Autoinducers
2.6. Statistical Analysis
3. Results
3.1. Anti-Candida Activity of PomeGr
3.2. Biochemical Profile of Pomegr Exposed or Not to Candida
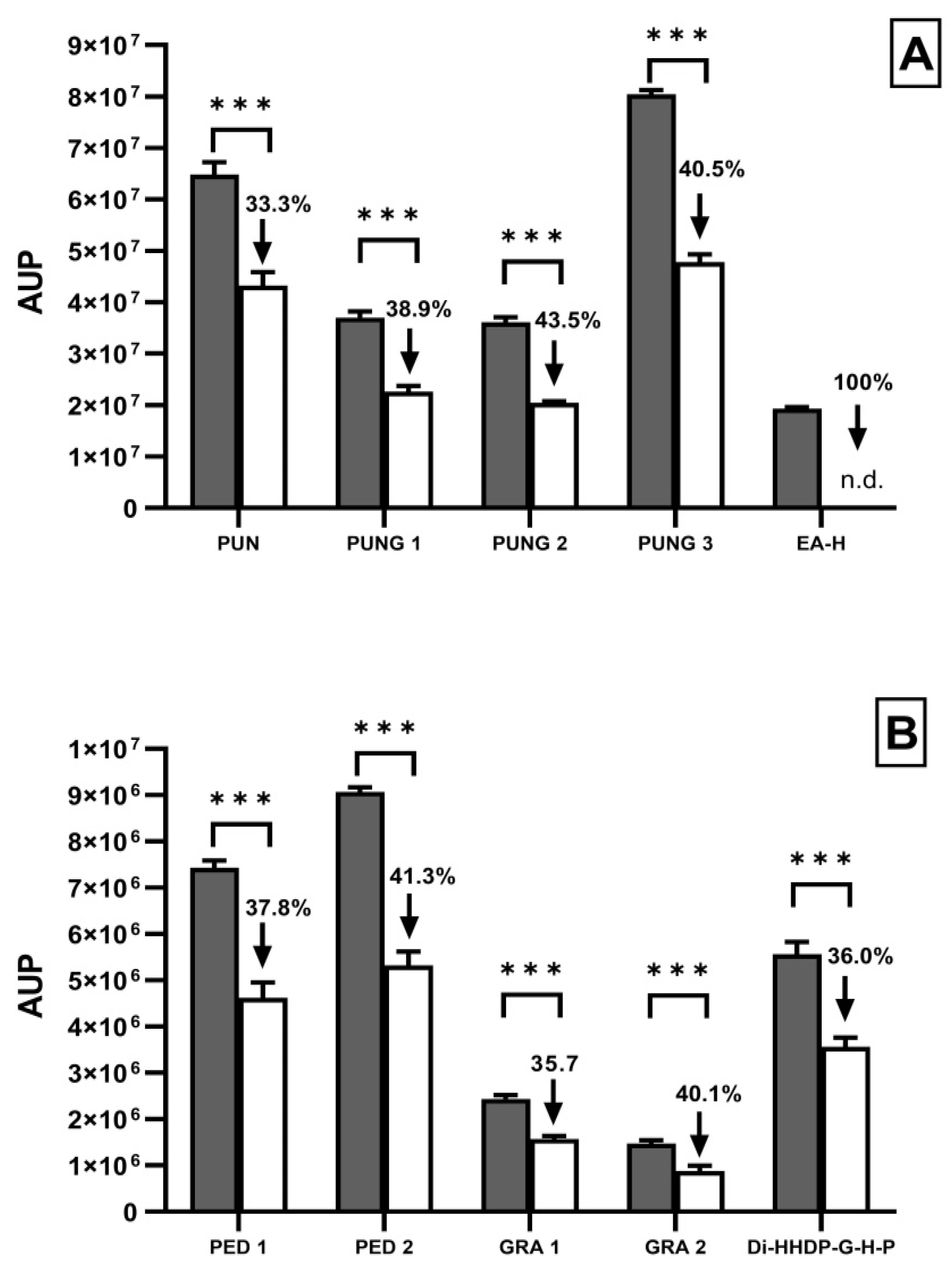
3.3. Alteration in the Release of AI by Candida upon Treatment with PomeGr
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Joo, H.S.; Otto, M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Hostacká, A.; Ciznár, I.; Stefkovicová, M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol. 2010, 55, 75–78. [Google Scholar] [CrossRef]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial Shelters Against Antibiotics. Microb. Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Majoros, L. Fungal Quorum-Sensing Molecules: A Review of Their Antifungal Effect against Candida Biofilms. J. Fungi 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Wongsuk, T.; Pumeesat, P.; Luplertlop, N. Fungal quorum sensing molecules: Role in fungal morphogenesis and pathogenicity. J. Basic Microbiol. 2016, 56, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Thomas, D.P.; López-Ribot, J.L. Candida biofilms: An update. Eukaryot. Cell 2005, 4, 633–638. [Google Scholar] [CrossRef]
- Henriques, M.; Martins, M.; Azeredo, J.; Oliveira, R. Effect of farnesol on Candida dubliniensis morphogenesis. Lett. Appl. Microbiol. 2007, 44, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Morehead, M.S.; Scarbrough, C. Emergence of Global Antibiotic Resistance. Prim. Care 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Colombari, B.; Meto, A.; Boaretto, G.; Pinetti, D.; Marchetti, L.; Benvenuti, S.; Pellati, F.; Blasi, E. Propolis Affects Pseudomonas aeruginosa Growth, Biofilm Formation, eDNA Release and Phenazine Production: Potential Involvement of Polyphenols. Microorganisms 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Marina, M.L.; García, M.C. Extraction and identification by high resolution mass spectrometry of bioactive substances in different extracts obtained from pomegranate peel. J. Chromatogr. A. 2019, 1594, 82–92. [Google Scholar] [CrossRef]
- Li, Y.; Ye, T.; Yang, F.; Hu, M.; Liang, L.; He, H.; Li, Z.; Zeng, A.; Li, Y.; Yao, Y.; et al. Punica granatum (pomegranate) peel extract exerts potent antitumor and anti-metastasis activity in thyroid cancer. RSC Adv. 2016, 6, 84523–84535. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Baz, A.F.; Salem, M.F.; El-Hadary, M.H. Potential applications of pomegranate peel extract for the control of citrus green mould. J. Plant Dis. Prot. 2009, 116, 252–256. [Google Scholar] [CrossRef]
- Brighenti, V.; Iseppi, R.; Pinzi, L.; Mincuzzi, A.; Ippolito, A.; Messi, P.; Sanzani, S.M.; Rastelli, G.; Pellati, F. Antifungal Activity and DNA Topoisomerase Inhibition of Hydrolysable Tannins from Punica granatum L. Int. J. Mol. Sci. 2021, 22, 4175. [Google Scholar] [CrossRef] [PubMed]
- Odorici, A.; Colombari, B.; Bellini, P.; Meto, A.; Venturelli, I.; Blasi, E. Novel Options to Counteract Oral Biofilm Formation: In Vitro Evidence. Int. J. Environ. Res. Public Health. 2022, 19, 8056. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.P.; Bertram, G.; Egerton, M.; Gow, N.A.R.; Falkow, S.; Brown, A.J.P. Yeast-enhanced green fluorescent protein (yEGFP): A reporter of gene expression in Candida albicans. Microbiology 1997, 143, 303–311. [Google Scholar] [CrossRef]
- Colombari, B.; Alfano, G.; Gamberini, C.; Cappelli, G.; Blasi, E. EDTA and Taurolidine Affect Pseudomonas aeruginosa Virulence In Vitro-Impairment of Secretory Profile and Biofilm Production onto Peritoneal Dialysis Catheters. Microbiol. Spectr. 2021, 9, e0104721. [Google Scholar] [CrossRef]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas aeruginosa Biofilm. Front Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Ayşe Erdoğan Eliuz, E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar]
- Fidalgo, T.K.; Barcelos, R.; Portela, M.B.; Soares, R.M.; Gleiser, R.; Silva-Filho, F.C. Inhibitory activity of root canal irrigants against Candida albicans, Enterococcus faecalis and Staphylococcus aureus. Braz. Oral Res. 2010, 24, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, D.; Comic, L.; Stefanovic, O.; Solujic-Sukdolak, S. Antimicrobial effects of sodium benzoate, sodium nitrite and potassium sorbate and their synergistic action in vitro. Bulg. J. Agric. Sci. 2009, 15, 307–311. [Google Scholar]
- Ardizzoni, A.; Boaretto, G.; Pericolini, E.; Pinetti, D.; Capezzone de Joannon, A.; Durando, L.; Ragni, L.; Blasi, E. Effects of benzydamine and mouthwashes containing benzydamine on Candida albicans adhesion, biofilm formation, regrowth, and persistence. Clin. Oral Investig. 2022, 26, 3613–3625. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, L.; Sala, A.; Ardizzoni, A.; De Seta, F.; Singh, D.K.; Gacser, A.; Blasi, E.; Pericolini, E. Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro. Microbiol. Spectr. 2022, 10, e0269621. [Google Scholar] [CrossRef] [PubMed]
- Kupnik, K.; Primožič, M.; Vasić, K.; Knez, Ž.; Leitgeb, M. A Comprehensive Study of the Antibacterial Activity of Bioactive Juice and Extracts from Pomegranate (Punica granatum L.) Peels and Seeds. Plants 2021, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, S.H.; Mashouf, R.; Mortazavi, H.; Moghaddam, M.; Roozbahani, N.; Vahedi, M. Antibacterial and antifungal activities of punica granatum peel extracts against oral pathogens. J. Dent. (Tehran, Iran) 2011, 8, 1–6. [Google Scholar]
- Endo, E.H.; Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef]
- Moreira, D.; Gullón, B.; Gullón, P.; Gomes, A.; Tavaria, F. Bioactive packaging using antioxidant extracts for the prevention of microbial food-spoilage. Food Funct. 2016, 7, 3273–3282. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Linus Opara, U. Functional properties of pomegranate fruit parts: Influence of packaging systems and storage time. J. Food Meas. Charact. 2017, 11, 2233–2246. [Google Scholar] [CrossRef]
- Nejatbakhsh’s, S.; Ilkhanizadeh-Qomi’s, M.; Razzaghi-Abyaneh, M.; Jahanshiri, Z. The Effects of Ellagic Acid on Growth and Biofilm Formation of Candida albicans. J. Med. Microbiol. Infect. Dis. 2020, 8, 14–18. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, M.; Arora, N.; Pruthi, V.; Poluri, K.M. Chemistry and Biology of Farnesol and its Derivatives: Quorum Sensing Molecules with Immense Therapeutic Potential. Curr. Top. Med. Chem. 2018, 18, 1937–1954. [Google Scholar] [CrossRef] [PubMed]
- Egbe, N.E.; Dornelles, T.O.; Paget, C.M.; Castelli, L.M.; Ashe, M.P. Farnesol inhibits translation to limit growth and filamentation in C. albicans and S. cerevisiae. Microb. Cell 2017, 4, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Singkum, P.; Muangkaew, W.; Suwanmanee, S.; Pumeesat, P.; Wongsuk, T.; Luplertlop, N. Suppression of the pathogenicity of Candida albicans by the quorum-sensing molecules farnesol and tryptophol. J. Gen. Appl. Microbiol. 2020, 65, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fujita, M.; Feng, Q.; Clardy, J.; Fink, G.R. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 5048–5052. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; López-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
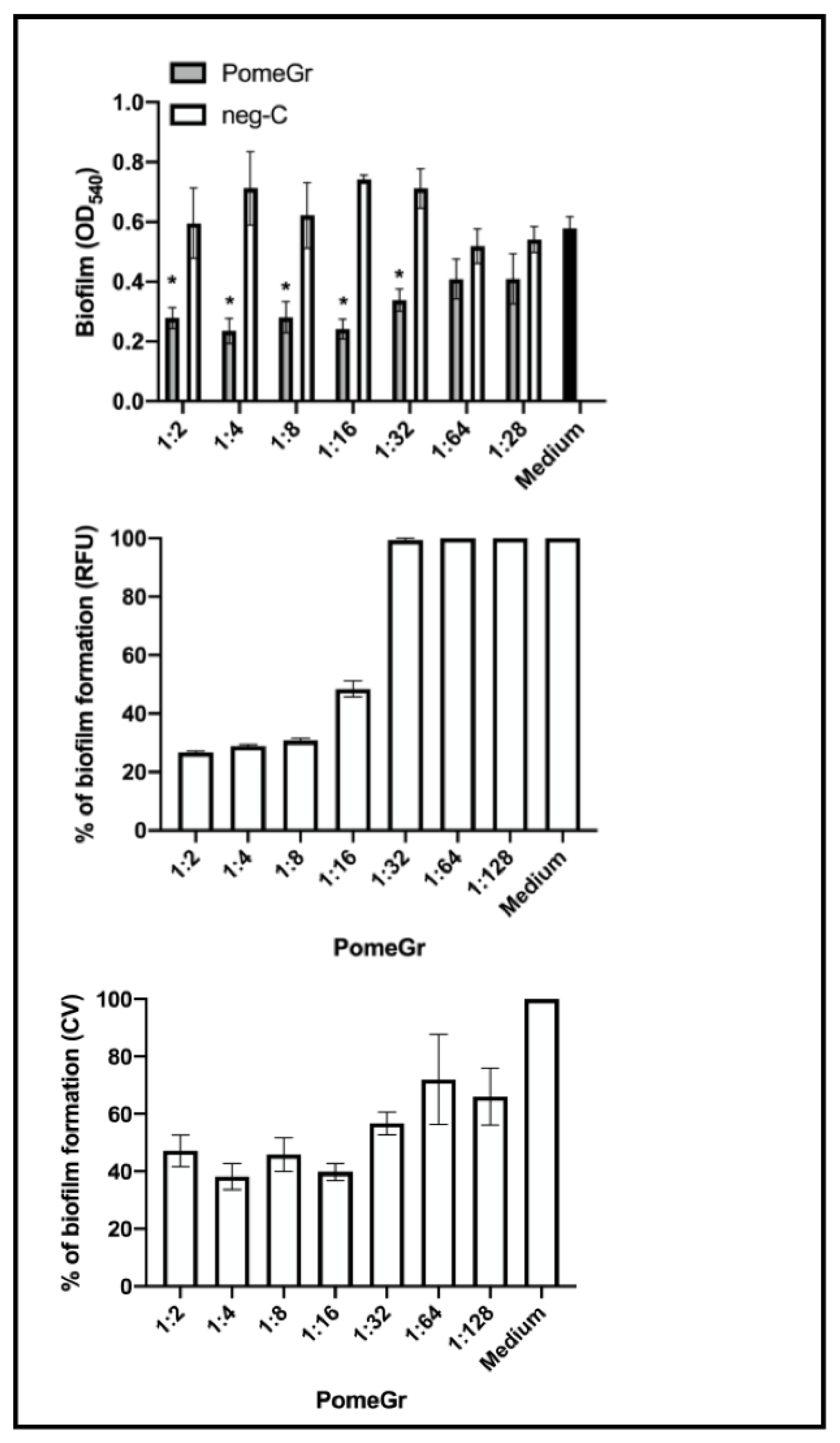

| Dilution | RFU ± SEM | % Decrease | CFU/mL ± SEM | % Decrease |
|---|---|---|---|---|
| 1:2 | 0.45 ± 0.027 * | 77.2 | 2.4 × 106 ± 1.8 × 105 | 94 |
| 1:4 | 0.49 ± 0.007 * | 75.4 | 3.4 × 106 ± 3.0 × 105 | 92 |
| 1:8 | 0.51 ± 0.013 * | 74.3 | 4.0 × 106 ± 3.2 × 105 | 90 |
| 1:16 | 0.57 ± 0.020 * | 71.4 | 5.4 × 106 ± 5.0 × 105 | 87 |
| 1:32 | 1.15 ± 0.082 | 42.2 | 2.0 × 107 ± 2.0 × 106 | 51 |
| 1:64 | 1.42 ± 0.016 | 28.7 | 2.7 × 107 ± 3.3 × 106 | 35 |
| 1:128 | 2.19 ± 0.066 | 0 | 4.6 × 107 ± 5.2 × 106 | 0 |
| Medium | 1.995 ± 0.025 | - | 4.1 × 107 ± 6.8 × 105 | - |
| Dilution | Levels of OD595 ± SEM | CFU/mL × 106 ± SEM | ||
| PomeGr | neg-C | PomeGr | neg-C | |
| 1:2 | 0.21 ± 0.021 * | 1.17 ± 0.084 | 13.06 ± 2.14 | 109.06 ± 8.39 |
| 1:4 | 0.20 ± 0.022 * | 1.18 ± 0.084 | 12.38 ± 2.24 | 110.88 ± 8.36 |
| 1:8 | 0.19 ± 0.022 * | 1.19 ± 0.088 | 11.36 ± 2.19 | 111.46 ± 8.80 |
| 1:16 | 0.23 ± 0.037 * | 1.20 ± 0.091 | 15.22 ± 3.68 | 112.39 ± 9.13 |
| 1:32 | 0.68 ± 0.072 * | 1.20 ± 0.082 | 60.37 ± 7.23 | 112.43 ± 8.25 |
| 1:64 | 0.87 ± 0.060 | 1.07 ± 0.056 | 79.18 ± 6.02 | 99.05 ± 5.58 |
| 1:128 | 0.97 ± 0.056 | 1.11 ± 0.086 | 89.56 ± 5.63 | 103.79 ± 8.60 |
| Medium | 0.94 ± 0.02 | 86.44 ± 2.05 | ||
| Levels of AI | ||||
|---|---|---|---|---|
| Treatment | Tyrosol | % Decrease | Farnesol | % Decrease |
| (AUP) | (AUP) | |||
| Medium | 8.08 × 107 | - | n.d. | - |
| PomeGr | 3.16 × 107 | 60.89 | 2.15 × 107 | n.m. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombari, B.; Tagliazucchi, D.; Odorici, A.; Pericolini, E.; Foltran, I.; Pinetti, D.; Meto, A.; Peppoloni, S.; Blasi, E. Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release. Int. J. Environ. Res. Public Health 2022, 19, 14146. https://doi.org/10.3390/ijerph192114146
Colombari B, Tagliazucchi D, Odorici A, Pericolini E, Foltran I, Pinetti D, Meto A, Peppoloni S, Blasi E. Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release. International Journal of Environmental Research and Public Health. 2022; 19(21):14146. https://doi.org/10.3390/ijerph192114146
Chicago/Turabian StyleColombari, Bruna, Davide Tagliazucchi, Alessandra Odorici, Eva Pericolini, Ismaela Foltran, Diego Pinetti, Aida Meto, Samuele Peppoloni, and Elisabetta Blasi. 2022. "Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release" International Journal of Environmental Research and Public Health 19, no. 21: 14146. https://doi.org/10.3390/ijerph192114146
APA StyleColombari, B., Tagliazucchi, D., Odorici, A., Pericolini, E., Foltran, I., Pinetti, D., Meto, A., Peppoloni, S., & Blasi, E. (2022). Pomegranate Extract Affects Fungal Biofilm Production: Consumption of Phenolic Compounds and Alteration of Fungal Autoinducers Release. International Journal of Environmental Research and Public Health, 19(21), 14146. https://doi.org/10.3390/ijerph192114146










