Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage 1: Identifying the Research Question
2.2. Stage 2: Identifying Relevant Studies
2.3. Stage 3: Selecting Studies
2.4. Stage 4: Charting the Data
2.5. Stage 5: Collating, Summarizing, and Reporting the Results
3. Results
3.1. Characteristics and Participants of the Selected Studies
3.2. Association of Vitamin D with Mild Cognitive Impairment, Frailty, and Cognitive Frailty
| Author, Year, Country | Study Design | Participant Characteristics | Micronutrients in Blood Profiles’ Outcomes and Methods of Measurement | Findings |
|---|---|---|---|---|
| Chei et al., 2014 [33] China | Cross-sectional study | Subjects: 2004 older adults from the Chinese Longitudinal Healthy Longevity Survey (936 males and 1068 females) Age: 60 years old and older | Fasting venous blood was collected and then plasma was stored at −80 °C until analysis. Plasma 25(OH)D3 levels were measured using an enzyme-linked immunosorbent assay. | There was a reverse association between plasma 25(OH)D3 levels and cognitive impairment (OR = 2.15, 95% CI: 1.05–4.41, p < 0.05). |
| Chhetri et al., 2018 [34] France | Cross-sectional study | Subjects: 1680 older adults from the Multi-domain Alzheimer Disease Preventive Trial (MAPT) Age: 70 years old and older | Blood samples were taken during enrollment and total plasma 25- hydroxyvitamin (D3 and D2 forms) were measured using a commercially available electro-chemiluminescence competitive binding assay. | High vitamin D was associated with a reduced likelihood of physical limitation and cognitive impairment (OR = 0.97, 95% CI: 0.95–0.99, p = 0.011). |
| Hooshmand et al., 2014 [35] Sweden | Cross-sectional study | Subjects: 75 patients (29 with subjective cognitive impairment, 28 with mild cognitive impairment, 18 with AD) referred to the Memory Clinic Mean age: 61.6 years old | Plasma samples were obtained during the diagnostic workup. Plasma levels of 25(OH)D were determined using the DiaSorin immunoassay method. | Elevated plasma 25(OH)D was significantly associated with better cognitive status (OR = 0.969, 95% CI: 0.948–0.990 per increase of 1 nmol/L of 25(OH)D). |
| Lee et al., 2017 [36] Korea | Cross-sectional study | Subjects: 2940 older adults from the Korean Urban Rural Elderly cohort study Age: 65 years old and older | Blood samples were collected after an overnight fast and stored at −80 °C until the time of analysis. Serum 25-hydroxyvitamin D (25[OH]D) levels were measured using a chemiluminescence immunoassay. | Lower 25(OH)D levels were significantly associated with cognitive impairment (OR = 1.81, 95% CI: 1.11–2.94, p = 0.017). |
| Llewellyn et al., 2011 [37] United States | Cross-sectional study | Subjects: 3396 older adults from the Third National Health and Nutrition Examination Survey Age: 65 years old and older | Blood samples were collected, and serum 25(OH)D concentration was measured by radioimmunoassay. | Participants who were severely 25(OH)D deficient were more likely to suffer from cognitive impairment (OR = 3.9, 95% CI: 1.5–10.4, p = 0.02). |
| Pavlovic et al., 2018 [41] United States | Cross-sectional study | Subjects: 4358 patients from the Cooper Clinic in Dallas Age: 55–65 years old | Serum 25-hydroxyvitamin D (25(OH)D) concentration was measured by a DiaSorin liasion chemiluminescence analyzer. | Low vitamin D was shown to be significantly associated with cognitive impairment (OR = 1.24, 95% CI: 1.01–1.51, P = 0.038). |
| Rosa et al., 2019 [39] Brazil | Cross-sectional study | Subjects: 165 older adults Age: 80 years old and older | All blood samples were collected after a 12 h fast, and then the serum was stored at −20 °C until analysis. Serum levels of vitamin D (25-hydroxyvitamin D) were measured using chemiluminescent microparticle immunoassay on the BitLab system. | Low vitamin D (≤18 ng mL−1) was significantly associated with a high risk of cognitive decline. Older adults with vitamin D levels > 19 ng mL−1 showed a lower prevalence of cognitive decline (Prevalence ratio = 0.59, 95% CI: 0.39–0.87). |
| Sakuma et al., 2018 [40] Japan | Cross-sectional study | Subjects: 740 patients (527 males and 385 females from the Project in Sado for Total Health (PROST)) Age: 65 years old and older | Blood samples were collected at the time of enrollment. Serum 25(OH)D levels were measured by a double-antibody radioimmunoassay (RIA2). | Low serum 25(OH)D levels were independently associated with a higher prevalence of cognitive impairment (OR = 2.70, 95% CI: 1.38–5.28, p = 0.0110). |
| Vedak et al., 2015 [41] India | Cross-sectional study | Subjects: 86 older adults Age: 50 years old and older | Blood samples were collected by venipuncture after overnight fasting, and then the serum was separated and stored at −80 °C until analysis. Serum levels of 25(OH)D were measured using kits from Immunodiagnostic Systems. | Serum 25(OH)D levels showed a substantial positive correlation with cognitive domains, including attention, language, registration, and naming (p < 0.001), whereas, there was a moderately positive correlation with domains such as orientation, recall, remote memory, visuospatial, and verbal fluency (p < 0.001). |
| Chang et al., 2010 [42] Taiwan | Cross-sectional study | Subjects: 215 community-dwelling older adults (128 females, 87 males) Age: 65–79 years old | Fasting bloods were collected and the serum specimens were stored at −80 °C until analysis. Serum 25(OH)D was measured by DiaSorin 25-Hydroxyvitamin D 125I RIA. | Insufficient 25(OH)D status was strongly linked to frailty syndrome using the Fried Frailty Index (FFI) (OR = 10.74, 95% CI: 2.60–44.31). |
| Dokuzlar et al., 2017 [43] Turkey | Cross-sectional study | Subjects: 335 patients who attended geriatric polyclinics (88 frail, 156 prefrail, and controls) Age: 60 years old and older | Serum 25-hydroxy-vitamin D [25(OH)D] level was measured using the radioimmunoassay technique. | Level of 25(OH)D decreased as severity of frailty increased (p < 0.05). |
| Gutiérrez-Robledo et al., 2016 [21] Mexico | Cross-sectional study | Subjects: 331 community-dwelling older adults Age: 70 years old and older | Peripheral blood samples were drawn, processed, and stored at −70 °C until analyses. 25(OH)-vitamin D serum levels were measured by a commercially available enzyme-linked immunosorbent assay (ELISA). | Low 25(OH)-vitamin D levels were significantly associated with the probability of being frail as compared with those with sufficient vitamin D levels (OR = 8.95, 95% CI: 2.41–33.30). |
| Hirani et al., 2013 [44] Australia | Cross-sectional study | Subjects: 1659 community-dwelling older adults (males) Age: 70 years old and older | Fasting blood samples were collected from participants in the morning of their clinic visit. Serum 25D and 1,25D levels were measured by manual radioimmunoassay using single batch reagents. | Low serum 25-hydroxyvitamin D (OR = 2.66, 95% CI: 1.32–5.36, p = 0.006) and 1,25-dihydroxyvitamin D (OR = 1.86, 95% CI: 1.04–3.59, p = 0.04) levels were independently linked to frailty in older adults. |
| Pabst et al., 2015 [45] Germany | Cross-sectional study | Subjects: 940 older adults (478 males and 462 females) from KORA (Cooperative health research in the Region of Augsburg) Age study Age: 65–90 years old | Serum total 25(OH)D was measured using enhanced chemiluminescence immunoassay. | High levels of 25(OH)D were inversely associated with being prefrail or frail. The odds ratios (OR) with 95% confidence intervals (CI) were 0.52 (0.34–0.78) for levels of 15 to <20 ng/mL, 0.55 (0.37 to 0.81) for normal 25(OH)D levels of 20 to <30 ng/mL, and 0.32 (0.21 to 0.51) for serum levels in the highest range ≥30 ng/mL. |
| Alvarez-Ríos et al., 2015 [46] Spain | Cross-sectional study | Subjects: 592 participants Median age: 74 years old | Serum concentrations of 25(OH)D from fasting blood were determined by a fully automated immunoassay 149 electrochemiluminescence system. | Low levels of 25(OH)D were significantly associated with frailty (OR = 1.65, 95% CI: 1.02–2.67, p = 0.042). |
| O’Halloran et al., 2020 [47] Ireland | Cross-sectional study | Subjects: 4068 participants Age: 50 years old and older | Non-fasting whole blood samples were collected between 09:30 AM and 16:30 PM by venipuncture and the plasma was then separated from the blood samples within 48 h of collection and archived at −80 °C until assayed. Plasma 25-hydroxyvitamin D (25(OH)D) concentrations were quantified using liquid chromatography tandem mass spectrometry. | All 3 measures of frailty were associated with lower levels of vitamin D (relative risk ratios (RRRs) = 0.51–0.75). |
| Wilhelm-Leen et al., 2010 [48] United States | Cross-sectional study | Subjects: 5048 participants from the Third National Health and Nutrition Survey (NHANES III) Age: 60 years old and older | Blood samples were collected, processed, and stored at −70 °C until analysis. Serum 25-hydroxyvitamin D was measured using the DiaSorin radioimmune assay kit. | Low serum 25-hydroxyvitamin D concentrations were associated with frailty amongst older adults (OR = 3.7, 95% CI: 2.1–6.8 amongst white older adults and OR = 4.0, 95% CI: 1.7–9.2 amongst non-white older adults). |
| Graf et al., 2014 [53] Switzerland | Prospective study | Subjects: 428 inpatients from the Geneva geriatric hospital Age: 75 years old and older | The 25(OH)D level was performed from frozen plasma obtained at the day of inclusion in the study and stored at −80 °C. Participants were followed-up for two years. Plasma 25(OH)D levels were measured by electrochemiluminesence immunoassay using Cobas E601. | Vitamin D was not associated with cognitive status (RRRs = 0.96, 95% CI: 0.25–3.61, p = 0.948). |
| Granic et al., 2015 [49] United Kingdom | Prospective study | Subjects: 775 participants in the Newcastle 85 + Study Age: 85 years old and older | Serum 25(OH)D was obtained from fasting morning blood samples. Participants were followed-up at 1.5 and 3 years. Serum 25(OH)D was measured using the DiaSorin radioimmune assay kit. | Both low and high season-specific concentrations of 25(OH)D were linked with the increased risk of prevalent cognitive impairment (OR = 1.66, 95% CI: 1.06–2.60, p = 0.03; and OR = 1.62, 95% CI: 1.02 to 2.59, p = 0.04, respectively). |
| Matchar et al., 2016 [50] China | Prospective study | Subjects: 1202 cognitively intact older adults Age: 60 years old and older | Fasting venous blood was collected, processed, and stored at −80 °C until analysis. The mean follow-up duration was 2.0 ± 0.2 years. Plasma 25(OH)D3 was measured using an enzyme-linked immunoassay by Immunodiagnostic Systems Limited. | Low vitamin D levels were significantly associated with an increased risk of subsequent cognitive decline (OR = 2.0, 95% CI: 1.2–3.3) and impairment (OR = 3.2, 95% CI: 1.5–6.6). |
| Moon et al., 2015 [51] Korea | Prospective study | Subjects: 405 older adults from the Korean Longitudinal Study on Health and Aging (KLoSHA) Age: 65 years old and older | Serum 25(OH)D concentrations were measured with ultra HPLC–tandem mass spectrometry. Participants were followed-up for 5 years. | Severe vitamin D deficiency was independently associated with the future risk of MCI (hazard ratio (HR) = 7.13, 95% CI: 1.54–32.9, p = 0.012). |
| Slinin et al., 2012 [52] United States | Prospective study | Subjects: 6257 older adults (females) Age: 65 years old and older | Fasting morning blood was collected, processed, and stored at −70 °C until analysis. Participants were followed-up for 4 years, and 25(OH)D 2 (ergocalciferol) and 25(OH)D 3 (cholecalciferol) were measured using mass spectrometry. | Low 25(OH)D levels among older women were associated with a higher odd of global cognitive impairment (OR = 1.60, 95% CI: 1.05–2.42) and a higher risk of global cognitive decline (OR = 1.58, 95% CI: 1.12–2.22). |
| Buchebner et al., 2019 [54] Sweden | Prospective study | Subjects: 1044 community-dwelling women, aged 75 years with reassessments at ages 80 (n = 715) and 85 (n = 382) years | Non-fasting serum samples were collected and stored at −80 °C. Participants were followed for 10 years. Serum 25(OH)D was assayed using liquid chromatography-mass spectrophotometry linked to an HPLC system. | The 25(OH)D insufficiency was associated with increased frailty in age 75 and 80 years (0.23 vs. 0.18, p < 0.001; and 0.32 vs. 0.25, p = 0.001, respectively). No association between 25(OH)D and frailty was observed at age 85 years (0.38 vs. 0.34, p = 0.187). |
| Buta et al., 2016 [56] United States | Prospective study | Subjects: 369 women from the Women’s Health and Aging Study II Age: 70–79 years old at baseline | Serum 25-hydroxyvitamin D (25[OH]D) was measured using a radioreceptor assay. The mean duration of follow-up was 8.5 ± 3.7 years. | Low serum vitamin D concentration was associated with incident frailty in older women (HR = 2.77, 95% CI: 1.14–6.71, p = 0.02), but the relationship was not evident after accounting for the presence of cardiometabolic diseases (HR = 2.29, 95% CI: 0.92–5.69, p = 0.07). |
| Vogt et al., 2015 [55] Germany | Prospective study | Subjects: 727 older adults from KORA-Age study Age: 65 years old and older | Non-fasting blood samples were collected at baseline and stored at −80 °C until analysis. Participants were followed for 2.9 ± 0.1 years. Serum total 25(OH)D were measured using enhanced chemiluminescence immunoassay. | Participants with very low 25(OH)D levels (<15 ng/mL vs. ≥30 ng/mL) had significantly higher odds of prefrailty (OR = 2.43, 95% CI: 1.17–5.03) and prefrailty/frailty combined (OR = 2.53, 95% CI: 1.23–5.22), but not exclusively for frailty (OR = 2.63, 95% CI: 0.39–17.67). |
3.3. Association of B Vitamins with Mild Cognitive Impairment, Frailty, and Cognitive Frailty
3.4. Association of Antioxidants with Mild Cognitive Impairment, Frailty, and Cognitive Frailty
3.5. Association of Protein with Mild Cognitive Impairment, Frailty, and Cognitive Frailty
3.6. Association of Lipids with Mild Cognitive Impairment, Frailty, and Cognitive Frailty
4. Discussion
4.1. Research Gaps
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; Abellan van Kan, G.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive Frailty: Rational and Definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Feng, L.; Shwe, M.; Nyunt, Z.; Gao, Q.; Feng, L. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies ( SLAS ). J. Am. Med. Dir. Assoc. 2017, 18, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild Cognitive Impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Nyunt, M.S.Z.; Gao, Q.; Feng, L.; Lee, T.S.; Tsoi, T.; Chong, M.S.; Lim, W.S.; Collinson, S.; Yap, P.; et al. Physical Frailty, Cognitive Impairment, and the Risk of Neurocognitive Disorder in the Singapore Longitudinal Ageing Studies. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2017, 72, 369–375. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; William Molloy, D.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of Frailty in 62 Countries across the World: A Systematic Review and Meta-Analysis of Population-Level Studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global Cognitive Impairment Prevalence and Incidence in Community Dwelling Older Adults—A Systematic Review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Vanoh, D.; Shahar, S.; Din, N.C.; Omar, A.; Vyrn, C.A.; Razali, R.; Ibrahim, R.; Hamid, T.A. Predictors of Poor Cognitive Status among Older Malaysian Adults: Baseline Findings from the LRGS TUA Cohort Study. Aging Clin. Exp. Res. 2017, 29, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kiiti Borges, M.; Oiring de Castro Cezar, N.; Silva Santos Siqueira, A.; Yassuda, M.; Cesari, M.; Aprahamian, I. The Relationship between Physical Frailty and Mild Cognitive Impairment in the Elderly: A Systematic Review. J. Frailty Aging 2019, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Daumas, M.; Lilamand, M.; Sourdet, S.; Delrieu, J.; Vellas, B.; Abellan van Kan, G. Association Between Frailty and Cognitive Impairment: Cross-Sectional Data from Toulouse Frailty Day Hospital. J. Am. Med. Dir. Assoc. 2017, 18, 990.e1–990.e5. [Google Scholar] [CrossRef] [PubMed]
- Rivan, N.F.M.; Shahar, S.; Rajab, N.F.; Singh, D.K.A.; Din, N.C.; Mahadzir, H.; Sakian, N.I.M.; Ishak, W.S.; Rahman, M.H.A.; Mohammed, Z.; et al. Incidence and Predictors of Cognitive Frailty among Older Adults: A Community-Based Longitudinal Study. Int. J. Environ. Res. Public Health 2020, 17, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.S.; Husin, M.H.; Ahmad, N.H.; Yun Hua, W.; Wan Chien, H.; Shahar, S.; Ismail, M.; Ajit Singh, D.K. Association between Nutritional Status, Food Insecurity and Frailty among Elderly with Low Income. J. Sains Kesihat. Malaysia 2017, 15, 51–60. [Google Scholar] [CrossRef]
- Badrasawi, M.; Shahar, S.; Kaur Ajit Singh, D. Risk Factors of Frailty Among Multi-Ethnic Malaysian Older Adults. Int. J. Gerontol. 2017, 11, 154–160. [Google Scholar] [CrossRef]
- Khairiah, K.; Mooi, C.S.; Hamid, T.A. Prevalence and Factors Associated with Mild Cognitive Impairment on Screening in Older Malaysians. Dusunen Adam 2016, 29, 298–306. [Google Scholar] [CrossRef]
- Hussin, N.M.; Shahar, S.; Yahya, H.M.; Din, N.C.; Singh, D.K.A.; Omar, M.A. Incidence and Predictors of Mild Cognitive Impairment (MCI) within a Multi-Ethnic Asian Populace: A Community-Based Longitudinal Study. BMC Public Health 2019, 19, 1159. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the Prot-Age Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, R.; Bianco, M.; De Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are Dietary Supplements and Nutraceuticals Effective for Musculoskeletal Health and Cognitive Function? A Scoping Review. J. Nutr. Health Aging 2016, 21, 527–538. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; De Nucci, S.; Sila, A.; Aresta, S.; Buscemi, C.; Randazzo, C.; Buscemi, S.; Triggiani, V.; De Pergola, G.; et al. Role of Dietary Carotenoids in Frailty Syndrome: A Systematic Review. Biomedicines 2022, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, Frailty, and Sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Lee, S.L.; Thomas, P.; Fenech, M. Genome Instability Biomarkers and Blood Micronutrient Risk Profiles Associated with Mild Cognitive Impairment and Alzheimer’s Disease. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2015, 776, 54–83. [Google Scholar] [CrossRef]
- Gutiérrez-Robledo, L.M.; Ávila-Funes, J.A.; Amieva, H.; Meillon, C.; Acosta, J.L.; Navarrete-Reyes, A.P.; Torres-Carrillo, N.; Muñoz-Valle, J.F.; Torres-Carrillo, N.M. Association of Low Serum 25-Hydroxyvitamin D Levels with the Frailty Syndrome in Mexican Community-Dwelling Elderly. Aging Male 2016, 19, 58–63. [Google Scholar] [CrossRef]
- Adachi, Y.; Ono, N.; Imaizumi, A.; Muramatsu, T.; Andou, T.; Shimodaira, Y.; Nagao, K.; Kageyama, Y.; Mori, M.; Noguchi, Y.; et al. Plasma Amino Acid Profile in Severely Frail Elderly Patients in Japan. Int. J. Gerontol. 2018, 12, 290–293. [Google Scholar] [CrossRef]
- Darwish, H.; Zeinoun, P.; Ghusn, H.; Khoury, B.; Tamim, H.; Khoury, S.J. Serum 25-Hydroxyvitamin D Predicts Cognitive Performance in Adults. Neuropsychiatr. Dis. Treat. 2015, 11, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Feng, L.; Nyunt, M.S.Z.; Feng, L.; Niti, M.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; Yap, P.; et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal among Older Adults: A Randomized Controlled Trial. Am. J. Med. 2015, 128, 1225–1236.e1. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Vitamins Associated with Brain Aging, Mild Cognitive Impairment, and Alzheimer Disease: Biomarkers, Epidemiological and Experimental Evidence, Plausible Mechanisms, and Knowledge Gaps. Adv. Nutr. 2017, 8, 958–970. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. Theory Pract. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- van Niekerk, L.; Manderson, L.; Balabanova, D. The Application of Social Innovation in Healthcare: A Scoping Review. Infect. Dis. Poverty 2021, 10, 26. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Nihms-237355. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Chei, C.L.; Raman, P.; Yin, Z.X.; Shi, X.; Zeng, Y.; Matchar, D.B. Vitamin D Levels and Cognition in the Elderly Population in China. J. Am. Geriatr. Soc. 2014, 141, 520–529. [Google Scholar] [CrossRef]
- Chhetri, J.K.; de Souto Barreto, P.; Soriano, G.; Gennero, I.; Cantet, C.; Vellas, B. Vitamin D, Homocysteine and N−3PUFA Status According to Physical and Cognitive Functions in Older Adults with Subjective Memory Complaint: Results from Cross-Sectional Study of the MAPT Trial. Exp. Gerontol. 2018, 111, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Lökk, J.; Solomon, A.; Mangialasche, F.; Miralbell, J.; Spulber, G.; Annerbo, S.; Andreasen, N.; Winblad, B.; Cedazo-Minguez, A.; et al. Vitamin D in Relation to Cognitive Impairment, Cerebrospinal Fluid Biomarkers, and Brain Volumes. Journals Gerontol.-Ser. A Biol. Sci. Med. Sci. 2014, 69, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, S.J.; Kim, K.M.; Yun, Y.M.; Song, B.M.; Kim, J.E.; Kim, H.C.; Rhee, Y.; Youm, Y.; Kim, C.O. Association of Metabolic Syndrome and 25-Hydroxyvitamin D with Cognitive Impairment among Elderly Koreans. Geriatr. Gerontol. Int. 2017, 17, 1069–1075. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Melzer, D. Vitamin D and Cognitive Impairment in the Elderly U.S. Population. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2011, 66 A, 59–65. [Google Scholar] [CrossRef]
- Pavlovic, A.; Abel, K.; Barlow, C.E.; Farrell, S.W.; Weiner, M.; DeFina, L.F. The Association between Serum Vitamin d Level and Cognitive Function in Older Adults: Cooper Center Longitudinal Study. Prev. Med. 2018, 113, 57–61. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, M.I.; Beck, W.O.; Colonetti, T.; Budni, J.; Falchetti, A.C.B.; Colonetti, L.; Coral, A.S.; Meller, F.O. Association of Vitamin D and Vitamin B12 with Cognitive Impairment in Elderly Aged 80 Years or Older: A Cross-Sectional Study. J. Hum. Nutr. Diet. 2019, 32, 518–524. [Google Scholar] [CrossRef]
- Sakuma, M.; Kitamura, K.; Endo, N.; Ikeuchi, T.; Yokoseki, A.; Onodera, O.; Oinuma, T.; Momotsu, T.; Sato, K.; Nakamura, K.; et al. Low Serum 25-Hydroxyvitamin D Increases Cognitive Impairment in Elderly People. J. Bone Miner. Metab. 2019, 37, 368–375. [Google Scholar] [CrossRef]
- Vedak, T.K.; Ganwir, V.; Shah, A.B.; Pinto, C.; Lele, V.R.; Subramanyam, A.; Shah, H.; Deo, S.S. Vitamin D as a Marker of Cognitive Decline in Elderly Indian Population. Ann. Indian Acad. Neurol. 2015, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.I.; Chan, D.C.; Kuo, K.N.; Hsiung, C.A.; Chen, C.Y. Vitamin D Insufficiency and Frailty Syndrome in Older Adults Living in a Northern Taiwan Community. Arch. Gerontol. Geriatr. 2010, 50, S17–S21. [Google Scholar] [CrossRef]
- Dokuzlar, Ö. Association between Serum Vitamin B12 Levels and Frailty in Older Adults. North. Clin. Istanb. 2017, 4, 22–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirani, V.; Naganathan, V.; Cumming, R.G.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Associations between Frailty and Serum 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Concentrations in Older Australian Men: The Concord Health and Ageing in Men Project. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Zimmermann, A.K.; Huth, C.; Koenig, W.; Ludwig, T.; Zierer, A.; Peters, A.; Thorand, B. Association of Low 25-Hydroxyvitamin D Levels with the Frailty Syndrome in an Aged Population: Results from the KORA-Age Augsburg Study. J. Nutr. Health Aging 2015, 19, 258–264. [Google Scholar] [CrossRef]
- Alvarez-Ríos, A.I.; Guerrero, J.M.; García-García, F.J.; Rodríguez-Mañas, L.; Medrano-Campillo, P.; de la Torre Lanza, M.A.; Alvarez-Sánchez, N.; Carrillo-Vico, A. Associations between Frailty and Serum N-Terminal Propeptide of Type I Procollagen and 25-Hydroxyvitamin D in Older Spanish Women: The Toledo Study for Healthy Aging. Exp. Gerontol. 2015, 69, 79–84. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, A.M.; Laird, E.J.; Feeney, J.; Healy, M.; Moran, R.; Beatty, S.; Nolan, J.M.; Molloy, A.M.; Kenny, R.A. Circulating Micronutrient Biomarkers Are Associated With 3 Measures of Frailty: Evidence from the Irish Longitudinal Study on Ageing. J. Am. Med. Dir. Assoc. 2020, 21, 240–247.e5. [Google Scholar] [CrossRef]
- Wilhelm-Leen, E.R.; Hall, Y.N.; Deboer, I.H.; Chertow, G.M. Vitamin D Deficiency and Frailty in Older Americans. J. Intern. Med. 2010, 268, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Kirkwood, T.B.L.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; von Zglinicki, T.; Saxby, B.K.; Wesnes, K.A.; Collerton, D.; et al. Serum 25-Hydroxyvitamin D and Cognitive Decline in the Very Old: The Newcastle 85+ Study. Eur. J. Neurol. 2015, 22, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; Chei, C.L.; Yin, Z.X.; Koh, V.; Chakraborty, B.; Shi, X.M.; Zeng, Y. Vitamin D Levels and the Risk of Cognitive Decline in Chinese Elderly People: The Chinese Longitudinal Healthy Longevity Survey. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2016, 71, 1363–1368. [Google Scholar] [CrossRef]
- Moon, J.H.; Lim, S.; Han, J.W.; Kim, K.M.; Choi, S.H.; Kim, K.W.; Jang, H.C. Serum 25-Hydroxyvitamin D Level and the Risk of Mild Cognitive Impairment and Dementia: The Korean Longitudinal Study on Health and Aging (KLoSHA). Clin. Endocrinol. 2015, 83, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Slinin, Y.; Paudel, M.; Taylor, B.C.; Ishani, A.; Rossom, R.; Yaffe, K.; Blackwell, T.; Lui, L.Y.; Hochberg, M.; Ensrud, K.E. Association between Serum 25(OH) Vitamin D and the Risk of Cognitive Decline in Older Women. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2012, 67 A, 1092–1098. [Google Scholar] [CrossRef]
- Graf, C.E.; Rossi, C.; Giannelli, S.V.; Nobari, B.H.; Gold, G.; Herrmann, F.R.; Zekry, D. Vitamin D Is Not Associated with Cognitive Status in a Cohort of Very Old Hospitalized Patients. J. Alzheimer’s Dis. 2014, 42, S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Buchebner, D.; Bartosch, P.; Malmgren, L.; McGuigan, F.E.; Gerdhem, P.; Akesson, K.E. Association between Vitamin D, Frailty, and Progression of Frailty in Community-Dwelling Older Women. J. Clin. Endocrinol. Metab. 2019, 104, 6139–6147. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Decke, S.; de Las Heras Gala, T.; Linkohr, B.; Koenig, W.; Ladwig, K.H.; Peters, A.; Thorand, B. Prospective Association of Vitamin D with Frailty Status and All-Cause Mortality in Older Adults: Results from the KORA-Age Study. Prev. Med. 2015, 73, 40–46. [Google Scholar] [CrossRef]
- Buta, B.; Choudhury, P.P.; Xue, Q.L.; Chaves, P.; Bandeen-Roche, K.; Shardell, M.; Semba, R.D.; Walston, J.; Michos, E.D.; Appel, L.J.; et al. The Association of Vitamin D Deficiency and Incident Frailty in Older Women: The Role of Cardiometabolic Diseases. J. Am. Geriatr. Soc. 2017, 65, 619–624. [Google Scholar] [CrossRef]
- Castillo-Lancellotti, C.; Margozzini, P.; Valdivia, G.; Padilla, O.; Uauy, R.; Rozowski, J.; Tur, J.A. Serum Folate, Vitamin B12 and Cognitive Impairment in Chilean Older Adults. Public Health Nutr. 2015, 18, 2600–2608. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Kim, K.N.; Son, J.I.; Kim, S.Y.; Tamura, T.; Chang, N. Relationship of Cognitive Function with B Vitamin Status, Homocysteine, and Tissue Factor Pathway Inhibitor in Cognitively Impaired Elderly: A Cross-Sectional Survey. J. Alzheimer’s Dis. 2013, 33, 853–862. [Google Scholar] [CrossRef]
- Baroni, L.; Bonetto, C.; Rizzo, G.; Bertola, C.; Caberlotto, L.; Bazzerla, G. Association between Cognitive Impairment and Vitamin B12, Folate, and Homocysteine Status in Elderly Adults: A Retrospective Study. J. Alzheimer’s Dis. 2019, 70, 441–451. [Google Scholar] [CrossRef]
- Mendonça, N.; Granic, A.; Mathers, J.C.; Martin-Ruiz, C.; Wesnes, K.A.; Seal, C.J.; Jagger, C.; Hill, T.R. One-Carbon Metabolism Biomarkers and Cognitive Decline in the Very Old: The Newcastle 85+ Study. J. Am. Med. Dir. Assoc. 2017, 18, 806.e19–806.e27. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.; An, P.; Du, Y.; Zhao, J.; Song, A.; Huang, G. Relationship between Folate, Vitamin B12, Homocysteine, Transaminase and Mild Cognitive Impairment in China: A Case-Control Study. Int. J. Food Sci. Nutr. 2020, 71, 315–324. [Google Scholar] [CrossRef]
- Moore, E.M.; Ames, D.; Mander, A.G.; Carne, R.P.; Brodaty, H.; Woodward, M.C.; Boundy, K.; Ellis, K.A.; Bush, A.I.; Faux, N.G.; et al. Among Vitamin B12 Deficient Older People, High Folate Levels Are Associated with Worse Cognitive Function: Combined Data from Three Cohorts. J. Alzheimer’s Dis. 2014, 39, 661–668. [Google Scholar] [CrossRef]
- Senger, J.; Bruscato, N.M.; Werle, B.; Moriguchi, E.H.; Pattussi, M.P. Nutritional Status and Cognitive Impairment among the Very Old in a Community Sample from Southern Brazil. J. Nutr. Health Aging 2019, 23, 923–929. [Google Scholar] [CrossRef]
- Soh, Y.; Lee, D.H.; Won, C.W. Association between Vitamin B12 Levels and Cognitive Function in the Elderly Korean Population. Medicine 2020, 99, e21371. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, Y.M.; Choi, B.Y.; Kim, M.K.; Roh, S.; Kim, K.; Yang, Y.J. Associations of Serum Levels of Vitamins A, C, and E with the Risk of Cognitive Impairment among Elderly Koreans. Nutr. Res. Pract. 2018, 12, 160–165. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Kåreholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum Levels of Vitamin E Forms and Risk of Cognitive Impairment in a Finnish Cohort of Older Adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, J.; Ma, W.; Dong, L.; Wang, W.; Che, R.; Xiao, R. Dietary Pattern and Antioxidants in Plasma and Erythrocyte in Patients with Mild Cognitive Impairment from China. Nutrition 2016, 32, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Lee, L.K.; Rajab, N.; Lim, C.L.; Harun, N.A.; Noh, M.F.N.M.; Mian-Then, S.; Jamal, R. Association between Vitamin A, Vitamin E and Apolipoprotein E Status with Mild Cognitive Impairment among Elderly People in Low-Cost Residential Areas. Nutr. Neurosci. 2013, 16, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rietman, M.L.; Spijkerman, A.M.W.; Wong, A.; van Steeg, H.; Bürkle, A.; Moreno-Villanueva, M.; Sindlinger, T.; Franceschi, C.; Grubeck-Loebenstein, B.; Bernhardt, J.; et al. Antioxidants Linked with Physical, Cognitive and Psychological Frailty: Analysis of Candidate Biomarkers and Markers Derived from the MARK-AGE Study. Mech. Ageing Dev. 2019, 177, 135–143. [Google Scholar] [CrossRef]
- Rivan, N.F.M.; Shahar, S.; Rajab, N.F.; Singh, D.K.A.; Din, N.C.; Hazlina, M.; Hamid, T.A.T.A. Cognitive Frailty among Malaysian Older Adults: Baseline Findings from the LRGS TUA Cohort Study. Clin. Interv. Aging 2019, 14, 1343–1352. [Google Scholar] [CrossRef]
- Llewellyn, D.J. Serum Albumin Concentration and Cognitive Impairment. Curr. Alzheimer Res. 2010, 7, 91–96. [Google Scholar] [CrossRef]
- Supasitthumrong, T.; Tunvirachaisakul, C.; Aniwattanapong, D.; Tangwongchai, S.; Chuchuen, P.; Tawankanjanachot, I.; Snabboon, T.; Hemrungrojn, S.; Carvalho, A.F.; Maes, M. Peripheral Blood Biomarkers Coupled with the Apolipoprotein E4 Genotype Are Strongly Associated with Semantic and Episodic Memory Impairments in Elderly Subjects with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 797–811. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Liu, J.; Zhang, Q.; Lei, P. Inverse Relationship between Baseline Serum Albumin Levels and Risk of Mild Cognitive Impairment in Elderly: A Seven-Year Retrospective Cohort Study. Tohoku J. Exp. Med. 2018, 246, 51–57. [Google Scholar] [CrossRef]
- Lukaschek, K.; Von Schacky, C.; Kruse, J.; Ladwig, K.H. Cognitive Impairment Is Associated with a Low Omega-3 Index in the Elderly: Results from the KORA-Age Study. Dement. Geriatr. Cogn. Disord. 2016, 42, 236–245. [Google Scholar] [CrossRef]
- Yuan, L.; Zhen, J.; Ma, W.; Cai, C.; Huang, X.; Xiao, R. The Erythrocyte Fatty Acid Profile and Cognitive Function in Old Chinese Adults. Nutrients 2016, 8, 385. [Google Scholar] [CrossRef]
- Orsitto, G.; Fulvio, F.; Tria, D.; Turi, V.; Venezia, A.; Manca, C. Nutritional Status in Hospitalized Elderly Patients with Mild Cognitive Impairment. Clin. Nutr. 2009, 28, 100–102. [Google Scholar] [CrossRef]
- Shawky Khater, M.; Fawzy Abouelezz, N. Nutritional Status in Older Adults with Mild Cognitive Impairment Living in Elderly Homes in Cairo, Egypt. J. Nutr. Health Aging 2011, 15, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Etgen, T.; Sander, D.; Bickel, H.; Sander, K.; Förstl, H. Vitamin D Deficiency, Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2012, 33, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimer’s Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Sorice, G.P.; Ajjan, R.; Mezza, T.; Pilz, S.; Prioletta, A.; Scragg, R.; Volpe, S.L.; Witham, M.D.; Giaccari, A. Can Vitamin D Deficiency Cause Diabetes and Cardiovascular Diseases? Present Evidence and Future Perspectives. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 81–87. [Google Scholar] [CrossRef]
- Leritz, E.C.; McGlinchey, R.E.; Kellison, I.; Rudolph, J.L.; Milberg, W.P. Cardiovascular Disease Risk Factors and Cognition in the Elderly. Curr. Cardiovasc. Risk Rep. 2011, 5, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New Clues about Vitamin D Functions in the Nervous System. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1,25-Dihydroxyvitamin D3 Induces Nerve Growth Factor, Promotes Neurite Outgrowth and Inhibits Mitosis in Embryonic Rat Hippocampal Neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Masoumi, A.; Goldenson, B.; Ghirmai, S.; Avagyan, H.; Zaghi, J.; Abel, K.; Zheng, X.; Espinosa-Jeffrey, A.; Mahanian, M.; Liu, P.T.; et al. 1α, 25-Dihydroxyvitamin D 3 interacts with curcuminoids to stimulate amyloid-β clearance by macrophages of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2009, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, J.; Martel, F.; Borges, N.; Manuel, J.; Keating, E. Folates and Aging: Role in Mild Cognitive Impairment, Dementia and Depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, G.; Liu, D.; Yang, Y.; Li, X. The Association Between Folate and Alzheimer’ s Disease: A Systematic Review and Meta-Analysis. Front. Neurosci. 2021, 15, 661198. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Vitamin B-12 and Cognition in the Elderly 1–4. Am. J. Clin. Nutr. 2009, 89, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.; Yuan, C.; Ding, D. Vitamin B12, B6, or Folate and Cognitive Function in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 77, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, Folate, and Vitamin B-12 in Mild Cognitive Impairment, Alzheimer Disease, and Vascular Dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic Studies of Modifiable Factors Associated with Cognition and Dementia: Systematic Review and Meta-Analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S. The Role of B Vitamins in Preventing and Treating Cognitive Impairment and Decline. Adv. Nutr. 2012, 3, 801–812. [Google Scholar] [CrossRef]
- Mischoulon, D.; Raab, M.F. The Role of Folate in Depression and Dementia. J. Clin. Psychiatry 2007, 68, 28–33. [Google Scholar]
- Ho, P.I.; Collins, S.C.; Dhitavat, S.; Ortiz, D.; Ashline, D.; Rogers, E.; Shea, T.B. Homocysteine Potentiates β-Amyloid Neurotoxicity: Role of Oxidative Stress. J. Neurochem. 2001, 78, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of Homocysteine Neurotoxicity in Neurodegenerative Diseases with Special Reference to Dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, M.; Lucassen, P.; Perry, G.; Smith, M.; Zhu, X. Alzheimer’s Disease: Cerebrovascular Dysfunction, Oxidative Stress, and Advanced Clinical Therapies. J. Alzheimers. Dis. 2008, 15, 199–210. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary Patterns and Risk of Dementia: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Argellati, F.; Pronzato, M.A.; Domenicotti, C. Vitamin E and Neurodegenerative Diseases. Mol. Aspects Med. 2007, 28, 591–606. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-Inflammatory Properties of α- and γ-Tocopherol. Mol. Aspects Med. 2007, 28, 668–691. [Google Scholar] [CrossRef]
- Wołoszynowska-Fraser, M.U.; Kouchmeshky, A.; McCaffery, P. Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 2020, 40, 247–272. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Singh, N. All-Trans Retinoic Acid Rescues Memory Deficits and Neuropathological Changes in Mouse Model of Streptozotocin-Induced Dementia of Alzheimer’s Type. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Stoney, P.N.; McCaffery, P.J. The Evidence for a Beneficial Role of Vitamin A in Multiple Sclerosis. CNS Drugs 2014, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yamada, M. Vitamin A and Alzheimer’s Disease. Geriatr. Gerontol. Int. 2012, 12, 180–188. [Google Scholar] [CrossRef]
- Brooke, J.; Ojo, O. Enteral Nutrition in Dementia: A Systematic Review. Nutrients 2015, 7, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Faxén-Irving, G.; Basun, H.; Cederholm, T. Nutritional and Cognitive Relationships and Long-Term Mortality in Patients with Various Dementia Disorders. Age Ageing 2005, 34, 136–141. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Covinsky, M.H.; Palmer, R.M.; Sehgal, A.R. Serum Albumin Concentration and Clinical Assessments of Nutritional Status in Hospitalized Older People: Different Sides of Different Coins? J. Am. Geriatr. Soc. 2002, 50, 631–637. [Google Scholar] [CrossRef]
- Karakis, I.; Pase, M.; Beiser, A.; Booth, S.; Jacques, P.; Rogers, G.; DeCarli, C.; Vasan, R.; Wang, T.; Himali, J.; et al. Association of Serum Vitamin D with the Risk of Incident Dementia and Subclinical Indices of Brain Aging: The Framingham Heart Study. J. Alzheimers Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef]
- Ohlsson, O.; Henningsen, N.C.; Malmquist, I. Blood Pressure, Heart Rate and Plasma Albumin in Relatives of Hypertensive Patients. Acta Med. Scand. 1981, 209, 445–450. [Google Scholar] [CrossRef]
- Mao, P. Oxidative Stress and Its Clinical Applications in Dementia. J. Neurodegener. Dis. 2013, 2013, 319898. [Google Scholar] [CrossRef]
- Guo, J.; Jono, H.; Kugimiya, T.; Saito, S.; Maruyama, T.; Misumi, Y.; Hoshii, Y.; Su, Y.; Shono, M.; Ueda, M.; et al. Antioxidative Effect of Albumin on Amyloid Fibril Formation in Transthyretin-Related Amyloidosis. Amyloid 2011, 18 (Suppl. 1), 12–13. [Google Scholar] [CrossRef]
- Cole, G.M.; Ma, Q.L.; Frautschy, S.A. Omega-3 Fatty Acids and Dementia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 213–221. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish Consumption, Long-Chain Omega-3 Fatty Acids and Risk of Cognitive Decline or Alzheimer Disease: A Complex Association. Nat. Clin. Pract. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Kiliaan, A.J. Long-Chain Polyunsaturated Fatty Acids (LCPUFA) from Genesis to Senescence: The Influence of LCPUFA on Neural Development, Aging, and Neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Bazan, N.G. Docosahexaenoic Acid and the Aging Brain. J. Nutr. 2008, 138, 2510–2514. [Google Scholar] [CrossRef]
- Huang, T.L. Omega-3 Fatty Acids, Cognitive Decline, and Alzheimer’s Disease: A Critical Review and Evaluation of the Literature. J. Alzheimer’s Dis. 2010, 21, 673–690. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Maseda, A.; De Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional Determinants of Frailty in Older Adults: A Systematic Review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Maturitas Association of Vitamin D Deficiency and Frailty: A Systematic Review and Meta-Analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef]
- Guilbaud, A.; Puisieux, F.; Dauchet, L. Circulating Biomarkers Characterizing Physical Frailty: CRP, Hemoglobin, Albumin, 25OHD and Free Testosterone as Best Biomarkers. Results of a meta-analysis. Exp. Gerontol. 2021, 139, 111014. [Google Scholar] [CrossRef]
- Houston, D.K.; Neiberg, R.H.; Tooze, J.A.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Shea, M.K.; Schwartz, G.G.; Williamson, J.D.; et al. Low 25-Hydroxyvitamin D Predicts the Onset of Mobility Limitation and Disability in Community-Dwelling Older Adults: The Health ABC Study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 181–187. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and Skeletal Muscle Tissue and Function. Mol. Aspects Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Shardell, M.; Hicks, G.E.; Miller, R.R.; Kritchevsky, S.; Andersen, D.; Bandinelli, S.; Cherubini, A.; Ferrucci, L. Association of Low Vitamin D Levels with the Frailty Syndrome in Men and Women. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2009, 64, 69–75. [Google Scholar] [CrossRef]
- Hubbard, R.E.; O’Mahony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and Frailty Measures in Older People. J. Cell. Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids Activate the Antioxidant Response Element Transcription System. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High Serum Carotenoids Associated with Lower Risk for Bone Loss and Osteoporosis in Post-Menopausal Japanese Female Subjects: Prospective Cohort Study. PLoS ONE 2012, 7, e52643. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, D. Toward an Understanding of Frailty. Ann. Intern. Med. 1999, 130, 945–950. [Google Scholar] [CrossRef]
- Chojkier, M. Inhibition of Albumin Synthesis in Chronic Diseases: Molecular Mechanisms. J. Clin. Gastroenterol. 2005, 39, 143–146. [Google Scholar] [CrossRef]
- Wu, I.C.; Shiesh, S.C.; Kuo, P.H.; Lin, X.Z. High Oxidative Stress Is Correlated with Frailty in Elderly Chinese. J. Am. Geriatr. Soc. 2009, 57, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Ponvel, P.; Shahar, S.; Kaur, D.; Singh, A.; Fitri, A.; Ludin, M. Multidomain Intervention for Reversal of Cognitive Frailty, Towards a Personalized Approach (AGELESS Trial): Study Design. J. Alzheimer’s Dis. 2021, 82, 673–687. [Google Scholar] [CrossRef]

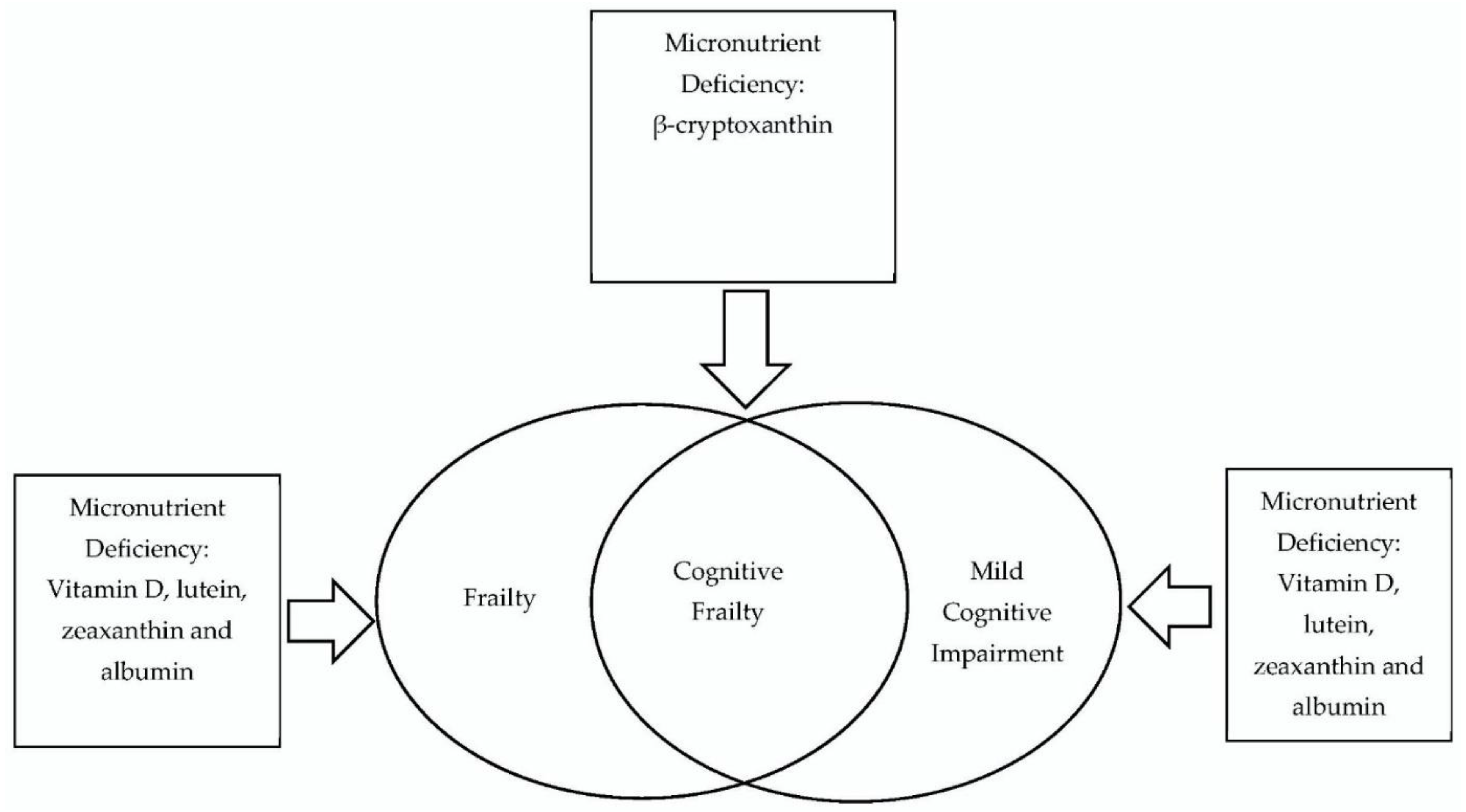
| Blood Micronutrient Profiles | Cognitive Frailty | Older Adult |
|---|---|---|
| Albumin | Cognitive impairments | Aging population |
| Homocysteine | Cognitive dysfunction | Older population |
| Amino acids | Mild cognitive impairments | Aging |
| Fatty acids | Physical frailty | Elderly |
| Minerals | Frailty syndrome | |
| Vitamins | Debility | |
| Antioxidants |
| Search strings | “Blood micronutrient profiles” OR “Albumin” OR “Homocysteine” OR “Amino Acids” OR “Fatty Acids” OR “Minerals” OR “Vitamins” OR “Antioxidants” AND “Cognitive frailty” OR “Cognitive impairment” OR “Cognitive dysfunction” OR “Mild cognitive impairments” OR “Physical frailty” OR “Frailty syndrome” OR “Debility” AND “Older adults” OR “Aging population” OR “Older population” OR “Ageing” OR “Elderly” |
| Author, Year, Country | Study Design | Participant Characteristics | Micronutrient in Blood Profiles’ Outcomes and Methods of Measurement | Findings |
|---|---|---|---|---|
| Castillo-Lancellotti et al., 2014 [57] Chile | Cross-sectional study | Subjects: 1051 older adults who participated in the National Health Survey 2009–2010 Age: 65 years old and older | The blood samples were obtained from fasting individuals and were processed within 4 h of venepuncture. Serum folate and vitamin B12 levels were determined by competitive immunoassay using direct chemiluminescence. | Low serum folate levels significantly increased the risk of cognitive impairment (p = 0.026). |
| Kim et al., 2013 [58] South Korea | Cross-sectional study | Subjects: 321 older adults in the Songpa district Age: 60 years old and older | A fasting blood sample was drawn from each subject and the plasma samples were stored at −80 °C until analysis. Plasma folate and vitamin B12 were analyzed using a radioimmunoassay kit and a gamma counter. Plasma homocysteine was measured using HPLC with a fluorescence detector. | Plasma folate level was positively associated with the cognitive function test scores in the mild cognitive impairment (MCI) group (β = 0.840, p = 0.040). |
| Moore et al., 2014 [62] Australia | Cross-sectional study | Subjects: 1354 older adults from the Prospective Research in Memory (PRIME) clinics study and the Australian Imaging, Biomarkers and Lifestyle (AIBL) study and from patients attending for assessment or management of memory problems Age: 60 years old and older | Blood samples were taken within six months of cognitive testing. Serum vitamin B12 and red cell folate (RCF) levels were measured using the ADVIA Centaur chemiluminescent microparticle immunoassay, Tosoh immunoassay analyzer AIA600, the Roche Cobas 8000 electrochemiluminescence immunoassay, and Siemens Healthcare Diagnostic Immulite 2000 immunoassay. | Participants with low serum vitamin B12 (<250 pmol/L) and high red cell folate (>1594 nmol/L) levels were more likely to have impaired cognitive performance (adjusted odds ratio (AOR) = 3.45, 95% CI: 1.60–7.43, p = 0.002; and AOR = 1.74, 95% CI: 1.03–2.95, p = 0.04, respectively) as compared to participants with biochemical measurements that were within the normal range. |
| Rosa et al., 2019 [39] Brazil | Cross-sectional study | Subjects: 165 older adults Age: 80 years old and older | All blood samples were collected after a 12 h fast, and the serum was stored at −20 °C until analysis. Serum vitamin B12 was measured using the chemiluminescent microparticle immunoassay on the BitLab system. | A high concentration of B12 levels (≥496 pg mL−1) indicated a risk factor for cognitive decline (prevalence ratio = 1.90, 95% CI: 1.08–3.36). |
| Senger et al., 2019 [63] Brazil | Cross-sectional study | Subjects: 153 older adults Age: 80 years and older | Serum albumin levels were determined with the calorimetric method. Serum vitamin B12 was measured with the immunoassay technique. | Low vitamin B12 concentration positively associated with cognitive impairment (AOR = 5.37, 95% CI: 1.44–19.97, p = 0.012). |
| Soh et al., 2020 [64] Korea | Cross-sectional study | Subjects: 2991 older adults (1416 males and 1575 females) Age: 70–84 years old | Serum samples were collected, and vitamin B12 was measured with an Architect vitamin kit. | The association between the B12 group and cognitive function was not statistically significant (p > 0.05). |
| Dokuzlar et al., 2017 [43] Turkey | Cross-sectional study | Subjects: 335 patients who attended geriatric polyclinics (88 frail, 156 prefrail, and controls) Age: 60 years old and older | Serum vitamin B12 was measured using a diagnostic modular system autoanalyzer. | No association between vitamin B12 level and frailty (p > 0.05). |
| Mendonça et al., 2017 [60] United Kingdom | Prospective study | Subjects: 765 community-dwelling and institutionalized older adults Age: 85 years old and older | Blood was drawn between 7:00 and 10:30 AM after an overnight fast, and red blood cells (RBC) folate and plasma vitamin B12 were quantified by chemiluminescence. Total homocysteine (tHcy) was measured with an Abbot Imx immunoassay. Data were collected at baseline; 18 months, 36 months, and 60 months. | RBC folate and plasma tHcy were significantly associated with better global cognition at baseline (β = +1.02, SE = 0.43, p = 0.02; and β = −1.05, SE = 0.46, p = 0.02, respectively). |
| Zhou et al., 2020 [61] China | Case-control study | Subjects: 118 subjects with MCI and 118 subjects without MCI Age: 60 years old and older | Blood samples were collected, processed, and stored at −80 °C until analysis. The concentrations of serum folate and vitamin B12 were determined using the Abbott Architect-i2000SR automated chemiluminescence immunoassay system. The concentrations of plasma Hcy were determined by HPLC. | Increased Hcy levels and lower folate levels were independently associated with the risk of MCI (OR = 3.93, 95% CI: 1.54–10.07, p = 0.004; and OR = 0.24, 95% CI: 0.11–0.52, p = 0.000, respectively). |
| Baroni et al., 2019 [59] Italy | Retrospective | Subjects: 569 older adults attended the Centre for Diagnosis and Treatment of Cognitive Disorders (226 males and 343 females) Age: 60–96 years old | A 6-year observational, retrospective study was conducted by collecting routine blood analyses. Serum vitamin B12 and folate were measured by Immulite 2000 immunoassay system and plasma homocysteine by Cobas c702 chemistry analyzer. | Higher folate concentrations were significantly correlated with better cognitive performances (beta = 0.144, p = 0.001). |
| Author, Year, Country | Study Design | Participant Characteristics | Micronutrient in Blood Profiles’ Outcomes and Methods of Measurement | Findings |
|---|---|---|---|---|
| Kim et al., 2018 [65] Korea | Cross-sectional study | Subjects: 230 older adults from Yangpyeong cohort Age: 60–79 years old | Participants provided blood specimens after overnight fasting. Serum levels of vitamins A, C, and E (alpha tocopherol, beta tocopherol, and gamma tocopherol) were measured by high performance liquid chromatography (HPLC). | There was no significant association between the risk of cognitive impairment and serum levels of vitamin A and vitamin C (p > 0.05). β-gamma tocopherol levels were inversely associated with cognitive impairment (OR = 0.37, 95% CI: 0.14–0.98, p for trend = 0.051). |
| Shahar et al., 2013 [70] Malaysia | Cross-sectional study | Subjects: 333 older adults Age: 60 years old and older | Fasting venous blood was obtained, and the serum was stored at −40 °C until analysis. Vitamin A (serum retinol) and vitamin E (alpha tocopherol) statuses were determined in subsamples using HPLC. | Vitamin A deficiency was associated with an increased risk of MCI (AOR = 3.253, 95% CI: 0.972–10.886, p < 0.05). |
| O’Halloran et al., 2020 [47] Ireland | Cross-sectional study | Subjects: 4068 participants Age: 50 years old and older | Non-fasting whole blood samples were collected between 09:30 AM and 16:30 PM by venipuncture, and the plasma was separated from the blood samples within 48 h of collection and archived at −80 °C until assayed. Lutein and zeaxanthin were measured by the reverse-phase high performance liquid chromatography method. | All 3 measures of frailty were associated with lower levels of lutein (RRR = 0.43–0.63) and zeaxanthin (RRR = 0.49–0.63). |
| Rietman et al., 2019 [69] Europe | Cross-sectional study | Subjects: 2220 participants from Randomly Recruited Age-Stratified Individuals from the General Population (RASIG) study population Age: 35–74 years old | Analysis by the Universitaet Hohenheim. | Levels of β-cryptoxanthin and zeaxanthin were inversely associated with the risk of being cognitively frail (OR = 0.742, 95% CI: 0.604–0.911, p = 0.0043; and OR = 0.752, 95% CI: 0.588–0.960, p = 0.0225, respectively). |
| Mangialasche et al., 2013 [66] Finland | Prospective study | Subjects: 140 non-cognitively impaired older adult subjects derived from the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study Age: 65–79 years old | Blood samples were taken after a minimum of 2 h fasting, and all serum samples were stored at −70 °C until analysis. The mean duration of follow-up was 8.2 years. Serum tocopherols, tocotrienols, αTQ, and 5-NO2-γ-tocopherol were measured with reverse-phase HPLC. | Elevated levels of tocopherol and tocotrienol forms were significantly associated with reduced risk of cognitive impairment in older adults (OR = 0.33, 95% CI: 0.11–0.97 for γ-tocopherol; OR = 0.21, 95% CI: 0.06–0.71 for β-tocotrienol; and OR = 0.33, 95% CI: 0.10–1.06 for γ-tocotrienol). |
| Yuan et al., 2016 [67] China | Case-control study | Subjects: 138 MCI patients and 138 age- and sex-matched healthy controls Age: 55–75 years old | Fasting venous blood samples were collected, processed, and stored at −80 °C until analysis. Plasma retinol and α-tocopherol were determined by using HPLC with UV detection. | Lower α-tocopherol was detected in the MCI patients compared to the control group (p < 0.05). |
| Author, Year, Country | Study Design | Participant Characteristics | Micronutrient in Blood Profiles’ Outcomes and Methods of Measurement | Findings |
|---|---|---|---|---|
| Llewellyn et al., 2009 [71] United Kingdom | Cross-sectional study | Subjects: 1752 older adults (699 males and 1053 females) from the Health Survey for England 2000 Age: 65 years old and older | Non-fasting blood samples were collected, and serum albumin was measured using the DAX system. | Low serum albumin was independently associated with increased odds of cognitive impairment in the elderly population (OR = 2.5, 95% CI: 1.3–5.1, p for linear trend = 0.002). |
| Supasitthumrong et al., 2019 [72] Thailand | Cross-sectional study | Subjects: 182 older adults (60 with amnestic mild cognitive impairment (aMCI), 60 with Alzheimer’s disease (AD), and 62 normal controls (NC). Age: 55–90 years old | Fasting blood was collected between 8.00 and 8.30 AM, and serum albumin was measured using Architect C8000. | Serum albumin significantly affected cognitive functions (B = 0.148, SE = 0.061, p = 0.017), including episodic and semantic memory among elderly with MCI. |
| Dokuzlar et al., 2017 [43] Turkey | Cross-sectional study | Subjects: 335 patients who attended geriatric polyclinics (88 frail, 156 prefrail, and controls) Age: 60 years old and older | Serum albumin was measured using a diagnostic modular system autoanalyzer. | Level of albumin decreased as severity of frailty increased (p < 0.05). |
| Wang et al., 2018 [73] China | Retrospective study | Subjects: 1800 older adults Age: 60 years old and older | A 7-year retrospective cohort study was conducted by collecting data from medical records including serum levels of albumin. The method for measurement of serum albumin was not stated. | Low serum albumin levels at baseline (<40.5 g/L) were associated with the increased risk of MCI (HR = 2.18, 95% CI: 1.67–2.82). |
| Author, Year, Country | Study Design | Participant Characteristics | Micronutrient in Blood Profiles’ Outcomes and Methods of Measurement | Findings |
|---|---|---|---|---|
| Lukaschek et al., 2016 [74] Germany | Cross-sectional study | Subjects: 720 older adults from (Cooperative Health Research in the Region of Augsburg) KORA-Age study Age: 68–92 years old | Erythrocyte fatty acid composition was measured using gas chromatography. | Low omega-3 index levels were significantly associated with cognitive impairment (OR = 1.77, 95% CI: 1.14–2.76, p = 0.01). |
| Chhetri et al., 2018 [34] France | Cross-sectional study | Subjects: 1680 older adults from the Multi-domain Alzheimer Disease Preventive Trial (MAPT) Age: 70 years old and older | Blood samples were taken during enrollment. Erythrocyte membrane fatty acids were measured using gas chromatography. | Low n−3PUFA showed higher likelihood of physical limitation (OR = 1.55, 95% CI: 1.12 to 2.15, p = 0.009). |
| Yuan et al., 2016 [75] China | Case-control study | Subjects: 60 MCI subjects and 60 age- and gender-matched control adults Age: 55 years old and older | Fasting venous blood samples were collected between 8:00 and 9:00 AM from each subject. A fatty acid analysis was performed using gas chromatography (GC). | Lower erythrocyte unsaturated fatty acid and higher saturated fatty acid proportions might predict cognitive function decline in elderly Chinese adults. The percentage of erythrocyte DHA was positively correlated with the total MoCA score (r = 0.356, p < 0.05), while 12:0 fatty acid was inversely associated with the total MoCA score (r = 0.450, p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa Khalid, N.; Haron, H.; Shahar, S.; Fenech, M. Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15722. https://doi.org/10.3390/ijerph192315722
Mustafa Khalid N, Haron H, Shahar S, Fenech M. Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(23):15722. https://doi.org/10.3390/ijerph192315722
Chicago/Turabian StyleMustafa Khalid, Norhayati, Hasnah Haron, Suzana Shahar, and Michael Fenech. 2022. "Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 23: 15722. https://doi.org/10.3390/ijerph192315722
APA StyleMustafa Khalid, N., Haron, H., Shahar, S., & Fenech, M. (2022). Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review. International Journal of Environmental Research and Public Health, 19(23), 15722. https://doi.org/10.3390/ijerph192315722







