Mechanism of Changes in Goaf Water Hydrogeochemistry: A Case Study of the Menkeqing Coal Mine
Abstract
1. Introduction
2. The Geological Setting
3. Materials and Methods
Subsec
4. Results and Discussion
4.1. Changes in Mineral Content of Rock Layers in Goaf
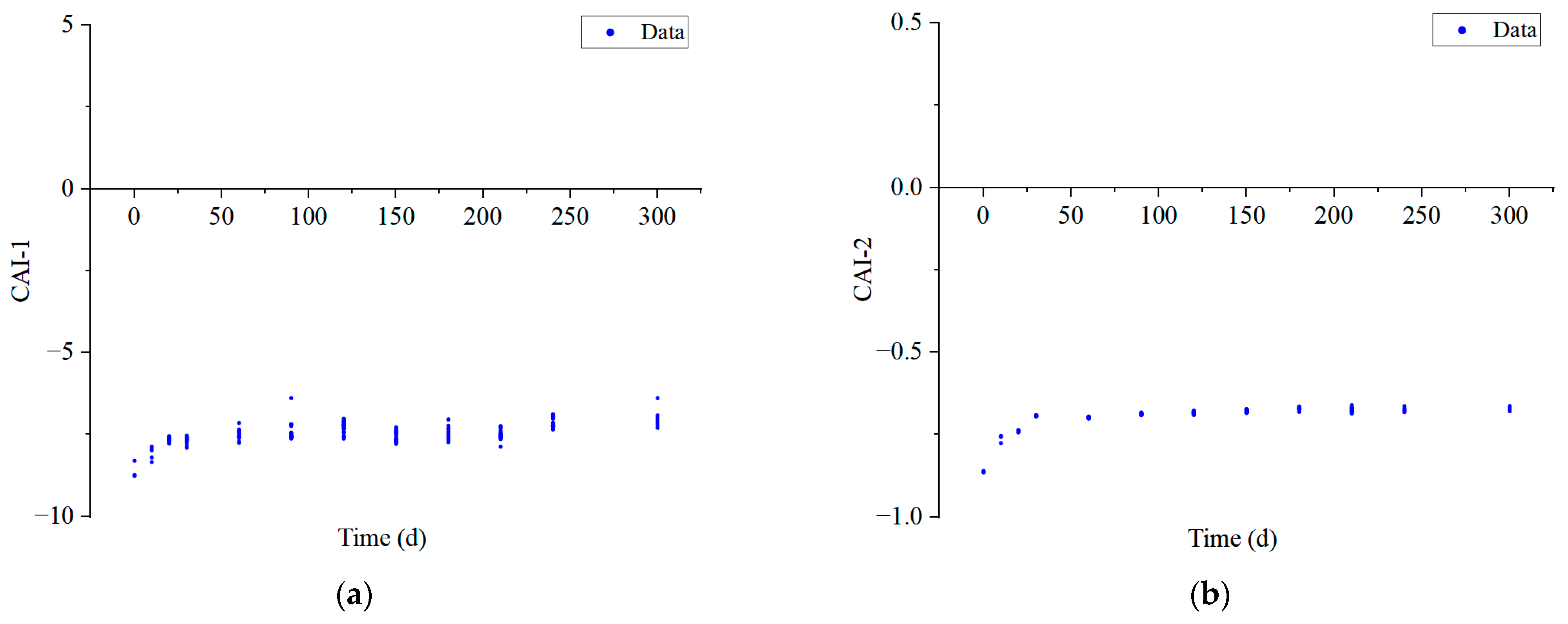
4.2. Changes in Hydrochemical Characteristics of Goaf Water
4.3. Mechanisms Controlling Goaf Water Composition
4.3.1. The Chemical Weathering Processes of Minerals
4.3.2. Action of Biochemistry
4.3.3. Ion Exchange Process
4.3.4. Inverse Geochemical Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Nie, R. An evaluating system for scientific mining of China’s coal resources. Res. Policy 2017, 53, 317–327. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Wu, Q. Innovation and future of mining rock mechanics. J. Rock Mech. Geotech. 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Greassidis, S.; Trinh Quoc, V.; Bromme, K.; Stolpe, H. Waterminer—A regional spatio-temporal approach to water reuse management in mining areas in Vietnam. J. Water Reuse. Desal. 2020, 10, 527–534. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, R.; Qin, Y. Scenario analysis of mine water inrush hazard using Bayesian networks. Safety Sci. 2016, 89, 231–239. [Google Scholar] [CrossRef]
- Chang, Q.; Sun, X.; Zhou, H.; Shi, X. A multivariate matrix model of analysing mine water bursting and its application. Desalin. Water Treat. 2018, 123, 20–26. [Google Scholar] [CrossRef]
- Tammetta, P. Estimation of the Change in Storage Capacity above Mined Longwall Panels. Groundwater 2016, 54, 646–655. [Google Scholar] [CrossRef]
- Younger, P.L. Mine water pollution in Scotland: Nature, extent and preventative strategies. Sci. Total Environ. 2001, 265, 309–326. [Google Scholar] [CrossRef]
- Luo, B.; Sun, Y.; Xu, Z.; Chen, G.; Zhang, L.; Lu, W.; Zhao, X.; Yuan, H. Damage Characteristics and Mechanism of the 2017 Groundwater Inrush Accident That Occurred at Dongyu Coalmine in Taiyuan, Shanxi, China. Water 2021, 13, 368. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Long, J.; Fan, J.; Wu, P.; Chen, M.; Liu, P.; Li, T. Migration mechanism of pollutants in karst groundwater system of tailings impoundment and management control effect analysis: Gold mine tailing impoundment case. J. Clean Prod. 2022, 350, 131434. [Google Scholar] [CrossRef]
- Belmer, N.; Wright, I.A. The regulation and impact of eight Australian coal mine waste water discharges on downstream river water quality: A regional comparison of active versus closed mines. Water Environ. J. 2020, 34, 350–363. [Google Scholar] [CrossRef]
- Bomberg, M.; Arnold, M.; Kinnunen, P. Characterization of the Bacterial and Sulphate Reducing Community in the Alkaline and Constantly Cold Water of the Closed Kotalahti Mine. Minerals 2015, 5, 452–472. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Gao, Y.; Zhu, L. Coal Mining Activities Driving the Changes in Microbial Community and Hydrochemical Characteristics of Underground Mine Water. Int. J. Environ. Res. Public Health 2022, 19, 13359. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.A.; Paciuszkiewicz, K.; Belmer, N. Increased Water Pollution After Closure of Australia’s Longest Operating Underground Coal Mine: A 13-Month Study of Mine Drainage, Water Chemistry and River Ecology. Water Air Soil. Poll. 2018, 229, 55. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States—An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Jiang, B.; Ji, K.; Xu, D.; Cao, Z.; Wen, S.; Song, K.; Ma, L. Effects of Coal Gangue on the Hydrochemical Components under Different Types of Site Karst Water in Closed Mines. Water 2022, 14, 3110. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, X.; Gao, J.; Liu, S.; Wang, F.; Han, J. Insight into the Process and Mechanism of Water-Rock Interaction in Underground Coal Mine Reservoirs Based on Indoor Static Simulation Experiments. ACS Omega 2022, 7, 36387–36402. [Google Scholar] [CrossRef] [PubMed]
- Liwei, Z.; Dong, S.; Tang, S.; Ji, Y.; Luo, J.; Li, H.; Li, X.; Liu, C.; Zeng, M. Molecular structure characterization of coal under the water-rock interaction in acid mine drainage (AMD). J. Mol. Struct. 2022, 1251, 132043. [Google Scholar] [CrossRef]
- Fan, R.; Qian, G.; Li, Y.; Short, M.D.; Schumann, R.C.; Chen, M.; Smart, R.S.C.; Gerson, A.R. Evolution of pyrite oxidation from a 10-year kinetic leach study: Implications for secondary mineralisation in acid mine drainage control. Chem. Geol. 2022, 588, 120653. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, X.; Hou, B.; Zhang, S.; Zhang, J.; Li, C.; Wang, W. Occurrence and environmental impact of coal mine goaf water in karst areas in China. J. Clean Prod. 2020, 275, 123813. [Google Scholar] [CrossRef]
- Wanda, E.M.M.; Chavula, G.; Tembo, F.M. Hydrogeochemical characterization of water quality evolution within Livingstonia coalfield mining areas in Rumphi district, northern Malawi. Phys. Chem. Earth 2021, 123, 103045. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Chen, Y.; Huang, L.; Hua, Z.; Hu, M.; Shu, W. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ. Microbiol. 2013, 15, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.M.; Gong, W.Q.; Yuan, C.X. Application of microbial oxidation of pyrite. J. Wuhan Univ. Technol. 2000, 15, 49–53. [Google Scholar]
- Qiao, W.; Li, W.; Li, T.; Chang, J.; Wang, Q. Effects of Coal Mining on Shallow Water Resources in Semiarid Regions: A Case Study in the Shennan Mining Area, Shaanxi, China. Mine Water Environ. 2017, 36, 104–113. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Li, T.; Li, X.; Liu, S. Goaf water storage and utilization in arid regions of northwest China: A case study of Shennan coal mine district. J. Clean. Prod. 2018, 202, 33–44. [Google Scholar] [CrossRef]
- Bai, E.; Guo, W.; Zhang, H.; Tan, Y.; Ma, Z.; Wu, D.; Guo, M.; Wen, P. Coal mining method with near-zero impact on the ecological environment in a high-intensity mining area of Northwest China. B Eng. Geol. Environ. 2022, 81, 80. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Bai, Q. Underground space utilization of coalmines in China: A review of underground water reservoir construction. Tunn. Undergr. Sp. Tech. 2021, 107, 103657. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.; Sun, Y.; Sui, W.; Li, X.; Zhao, X.; Liu, Q. Minewater deep transfer and storage. J. Clean Prod. 2022, 332, 129848. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Long, J.; Fan, J.; Chen, M.; Li, T.; Liu, P. Multi-source information fusion technology for risk assessment of water inrush from coal floor karst aquifer. Geomat. Nat. Haz. Risk 2022, 13, 2086–2106. [Google Scholar] [CrossRef]
- Grawunder, A.; Merten, D.; Buechel, G. Origin of middle rare earth element enrichment in acid mine drainage-impacted areas. Environ. Sci. Pollut. Res. 2014, 21, 6812–6823. [Google Scholar] [CrossRef]
- Zha, H.; Liu, W.; Liu, Q. Self-Designed Hydrophilicity-Related Geomaterials and Their Testing Utility in Simulation of Overlying Strata Instability. Adv. Mater. Sci. Eng. 2019, 2019, 5290524. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L. Experimental study on distribution law of residual fissure under multiple mining conditions. Rev. Constr. 2015, 14, 77–81. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, J.; Men, D.; Zhao, X.; Wu, S. Insight into the heavy metal binding properties of dissolved organic matter in mine water affected by water-rock interaction of coal seam goaf. Chemosphere 2021, 265, 129134. [Google Scholar] [CrossRef] [PubMed]
- Olds, W.E.; Weber, P.A.; Pizey, M.H.; Pope, J. Acid mine drainage analysis for the Reddale Coal Mine, Reefton, New Zealand. N. Z. J. Geol. Geop. 2016, 59, 341–351. [Google Scholar] [CrossRef][Green Version]
- Pope, J.; Weber, P.; Olds, W. Control of acid mine drainage by managing oxygen ingress into waste rock dumps at bituminous coal mines in New Zealand. In Proceedings of the Mining Meets Water—Conflicts and Solutions, Leipzig, Germany, 11–15 July 2016; pp. 368–376. [Google Scholar]
- Elena Garcia-Arreola, M.; Maria Flores-Velez, L.; Loredo-Tovias, M.; Aguillon-Robles, A.; Alfonso Lopez-Doncel, R.; Cano-Rodriguez, I.; Hortensia Soriano-Perez, S. Assessment of the acid drainage neutralization capacity and the toxic metals lixiviation of tailing from Guanajuato mining district, Mexico. Environ. Earth Sci. 2018, 77, 355. [Google Scholar] [CrossRef]
- Su, C.; Wang, Y.; Pan, Y. Hydrogeochemical and isotopic evidences of the groundwater regime in Datong Basin, Northern China. Environ. Earth Sci. 2013, 70, 877–885. [Google Scholar] [CrossRef]
- Omonona, O.V.; Amah, J.O.; Olorunju, S.B.; Waziri, S.H.; Ekwe, A.C.; Umar, D.N.; Olofinlade, S.W. Hydrochemical characteristics and quality assessment of groundwater from fractured Albian carbonaceous shale aquifers around Enyigba-Ameri, southeastern Nigeria. Environ. Monit. Assess 2019, 191, 125. [Google Scholar] [CrossRef]
- Bozau, E.; Licha, T.; Liessmann, W. Hydrogeochemical characteristics of mine water in the Harz Mountains, Germany. Chem. Erde-Geochem. 2017, 77, 614–624. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Liang, X.; Cui, L.; Ma, L.; Xu, Q. Hydrochemical and Stable Isotope (delta D and delta O-18) Characteristics of Groundwater and Hydrogeochemical Processes in the Ningtiaota Coalfield, Northwest China. Mine Water Environ. 2018, 37, 119–136. [Google Scholar] [CrossRef]
- Kumar, P.J.S.; James, E.J. Identification of hydrogeochemical processes in the Coimbatore district, Tamil Nadu, India. Hydrolog. Sci. J. 2016, 61, 719–731. [Google Scholar] [CrossRef]
- Nasher, G.; Al-Sayyaghi, A.; Al-Matary, A. Identification and evaluation of the hydrogeochemical processes of the lower part of Wadi Siham catchment area, Tihama plain, Yemen. Arab. J. Geosci. 2013, 6, 2131–2146. [Google Scholar] [CrossRef]
- Wang, M.; Gui, H.; Hu, R.; Zhao, H.; Li, J.; Yu, H.; Fang, H. Hydrogeochemical Characteristics and Water Quality Evaluation of Carboniferous Taiyuan Formation Limestone Water in Sulin Mining Area in Northern Anhui, China. Int. J. Environ. Res. Public Health 2019, 16, 2512. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tian, H.; Huang, Y.; Ding, Z.; Yin, Z. Pyrite oxidation mechanism in aqueous medium. J. Chin. Chem. Soc.-Taip. 2019, 66, 345–354. [Google Scholar] [CrossRef]
- Pachana, P.K.; Rattanasak, U.; Nuithitikul, K.; Jitsangiam, P.; Chindaprasirt, P. Sustainable utilization of water treatment residue as a porous geopolymer for iron and manganese removals from groundwater. J. Environ. Manag. 2022, 302, 114036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xian, H.; Lin, X.; Tang, H.; Du, R.; Yang, Y.; Zhu, R.; Liang, X.; Wei, J.; Teng, H.H.; et al. Surface structure-dependent pyrite oxidation in relatively dry and moist air: Implications for the reaction mechanism and sulfur evolution. Geochim. Cosmochim. Ac. 2018, 228, 259–274. [Google Scholar] [CrossRef]
- Bhandari, P.; Choudhary, S. Insights on the Role of Sulfur Oxidizing Bacteria in Acid Mine Drainage Biogeochemistry. Geomicrobiol. J. 2022, 39, 270–281. [Google Scholar] [CrossRef]
- Roesler, A.J.; Gammons, C.H.; Druschel, G.K.; Oduro, H.; Poulson, S.R. Geochemistry of flooded underground mine workings influenced by bacterial sulfate reduction. Aquat. Geochem. 2007, 13, 211–235. [Google Scholar] [CrossRef]
- Bilgin, A.A.; Harrington, J.M.; Silverstein, J. Enhancement of bacterial iron and sulfate respiration for in situ bioremediation of acid mine drainage sites: A case study. Miner. Metall. Process. 2007, 24, 139–144. [Google Scholar] [CrossRef]
- Pott, A.S.; Dahl, C. Sirohaem sulfite reductase and other proteins encoded by genes at the dsr locus of Chromatium vinosum are involved in the oxidation of intracellular sulfur. Microbiology 1998, 144 Pt. 7, 1881–1894. [Google Scholar] [CrossRef]
- Chiang, Y.; Hsieh, Y.; Fang, J.; Liu, E.; Huang, Y.; Chuankhayan, P.; Jeyakanthan, J.; Liu, M.; Chan, S.I.; Chen, C. Crystal Structure of Adenylylsulfate Reductase from Desulfovibrio gigas Suggests a Potential Self-Regulation Mechanism Involving the C Terminus of the beta-Subunit. J. Bacteriol. 2009, 191, 7597–7608. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major Ion Chemistry of Shallow Groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Liu, R. Solute Geochemistry and Multivariate Analysis of Water Quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expos. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Chung, S.Y.; Rajendran, R.; Senapathi, V.; Sekar, S.; Ranganathan, P.C.; Oh, Y.Y.; Elzain, H.E. Processes and characteristics of hydrogeochemical variations between unconfined and confined aquifer systems: A case study of the Nakdong River Basin in Busan City, Korea. Environ. Sci. Pollut. Res. 2020, 27, 10087–10102. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zang, H.; Hobbs, P.; Zheng, X.; Xu, Y.; Wang, K. Application of inverse modeling in a study of the hydrogeochemical evolution of karst groundwater in the Jinci Spring region, northern China. Environ. Earth Sci. 2017, 76, 312. [Google Scholar] [CrossRef]
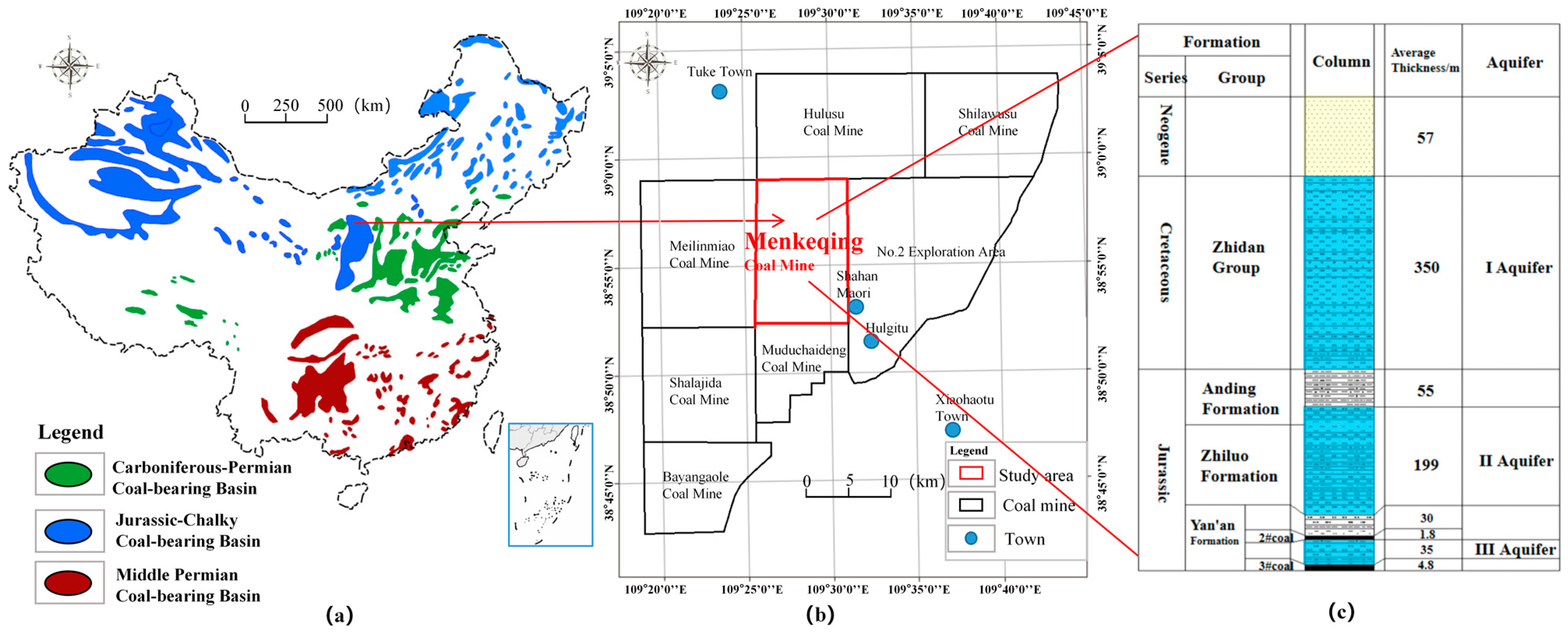
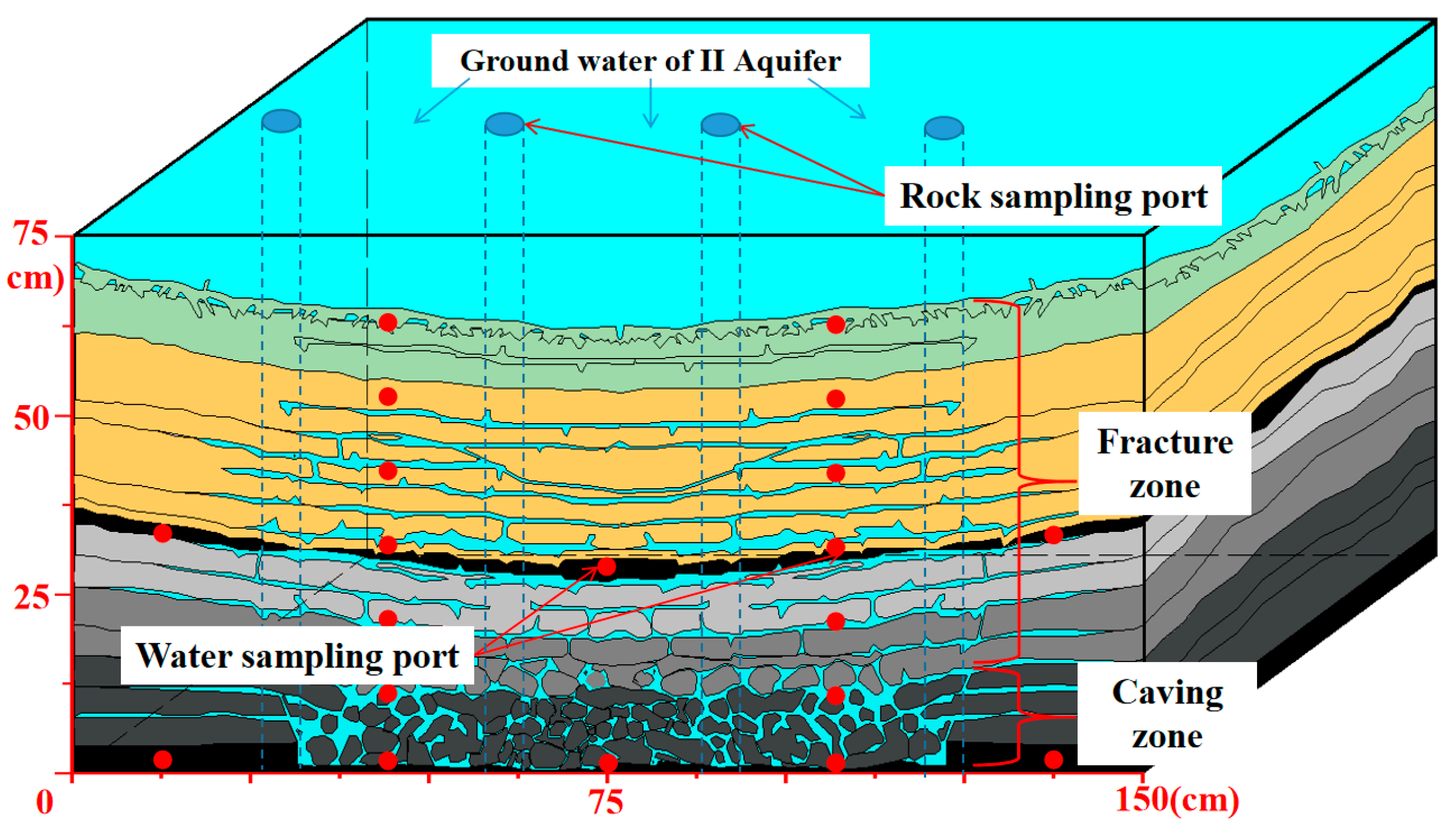
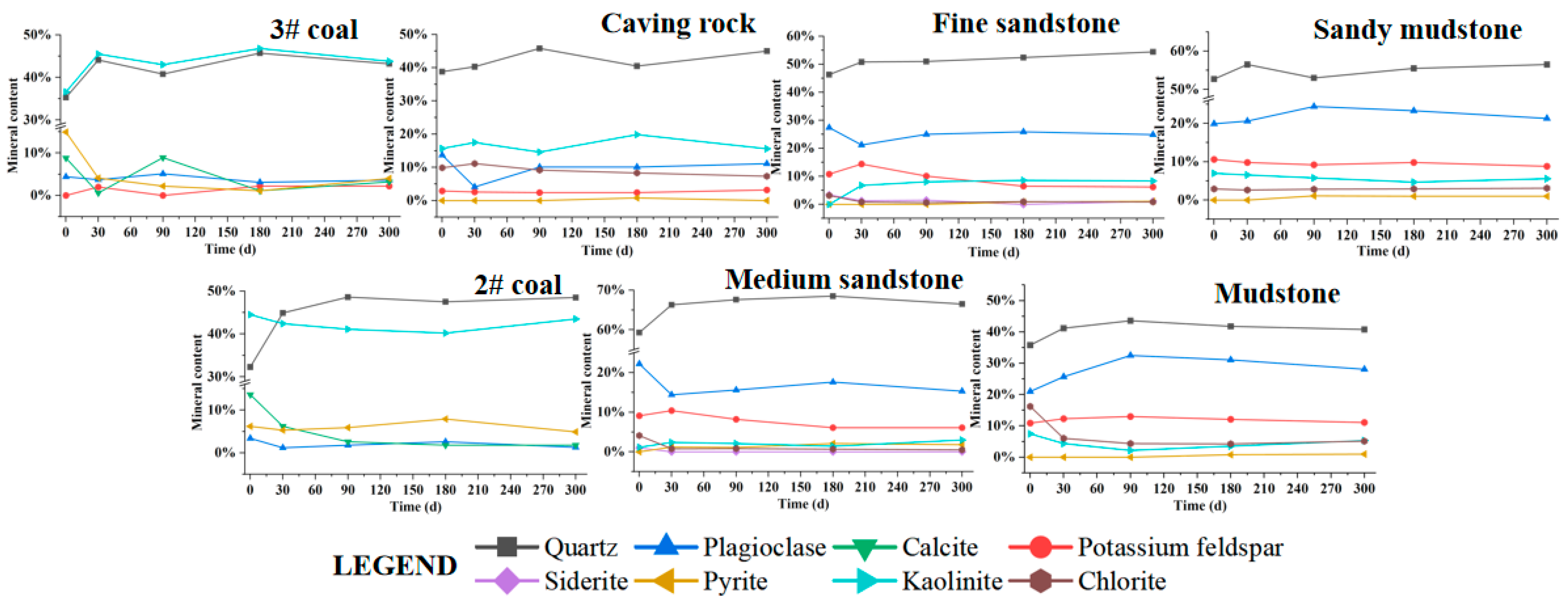

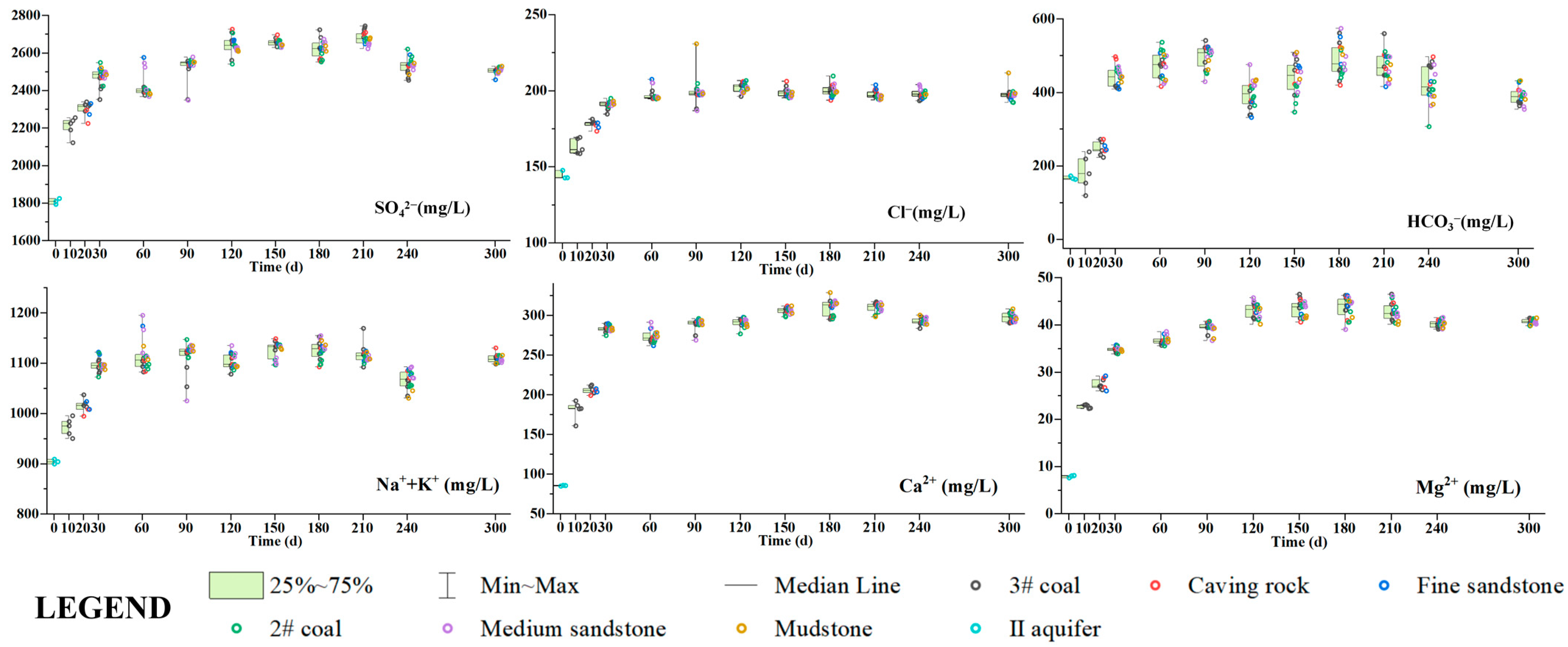
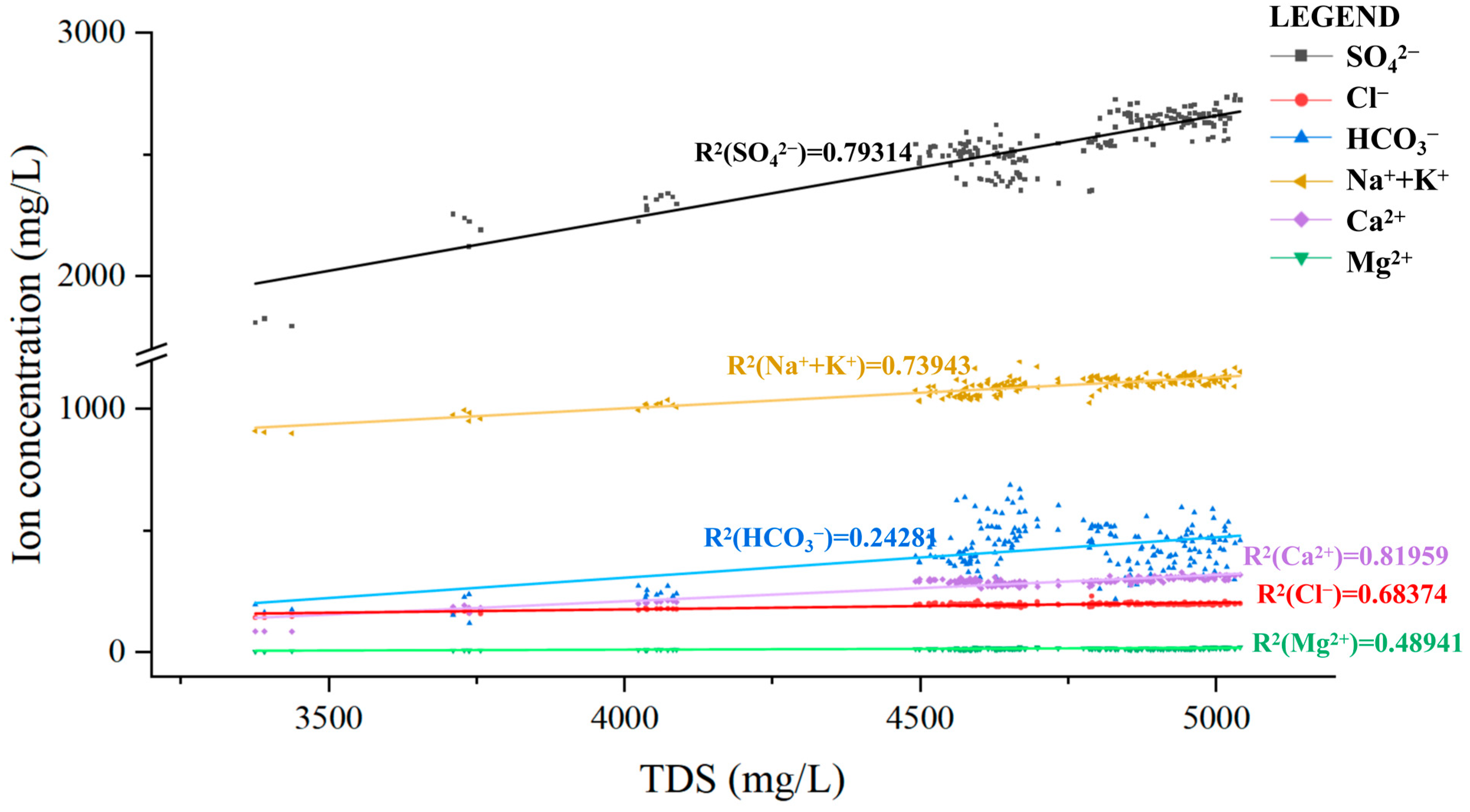
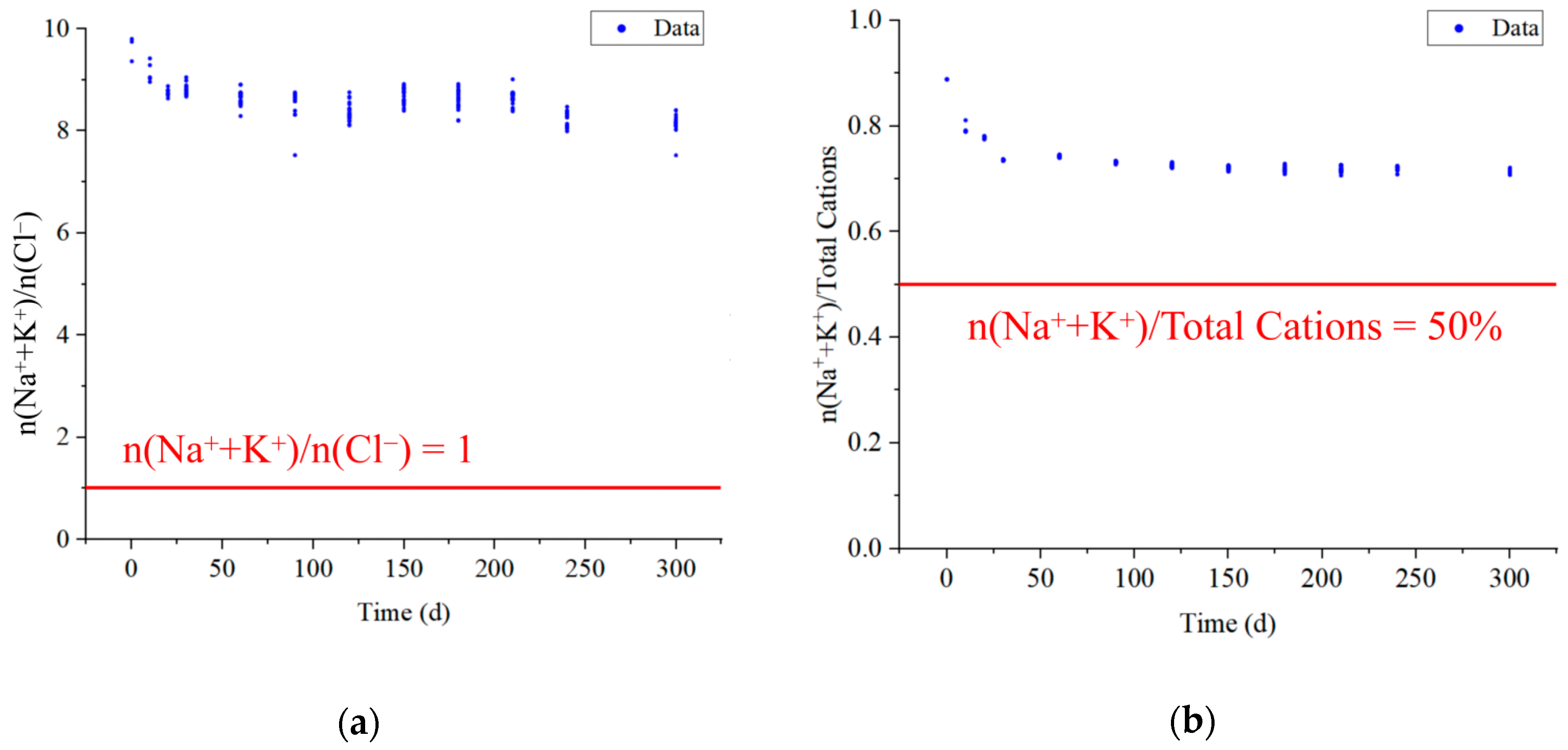
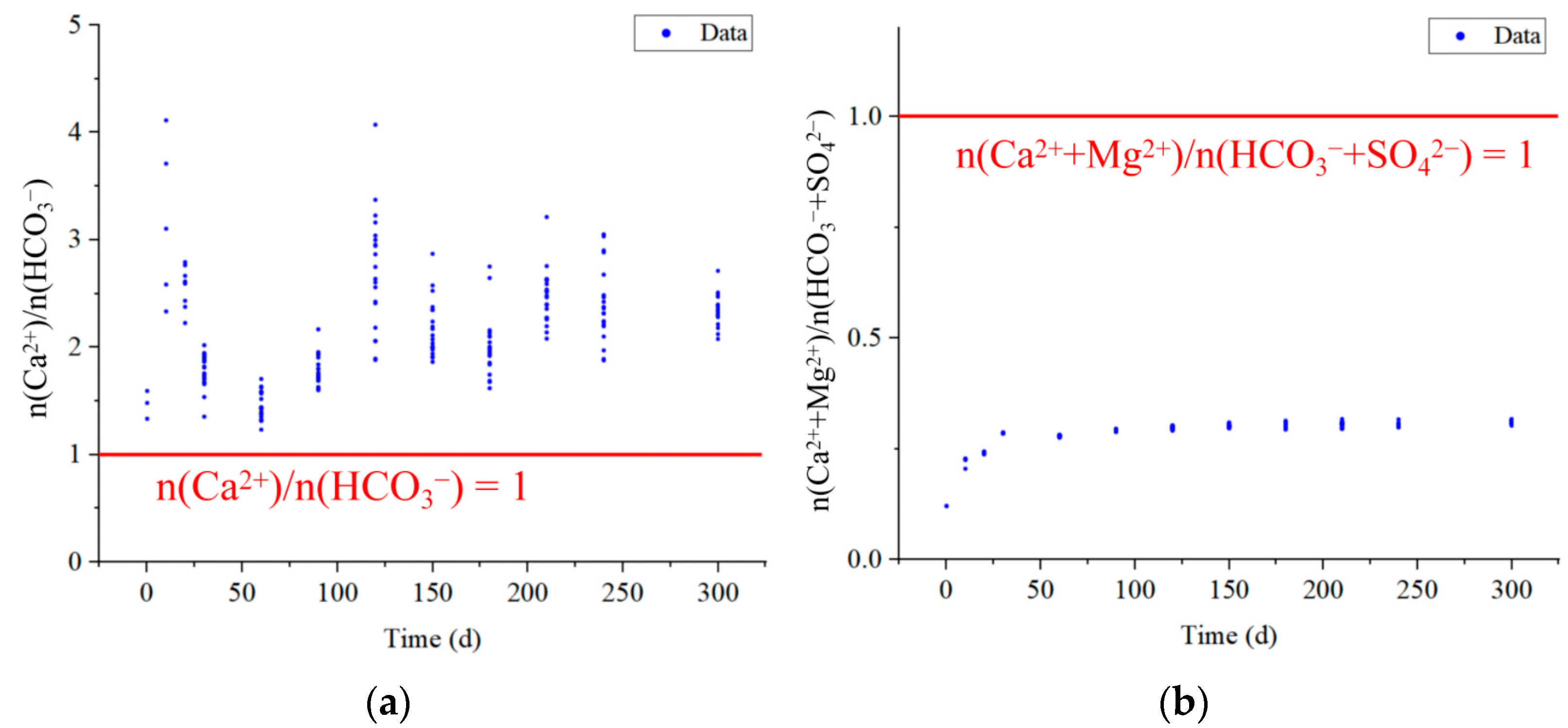


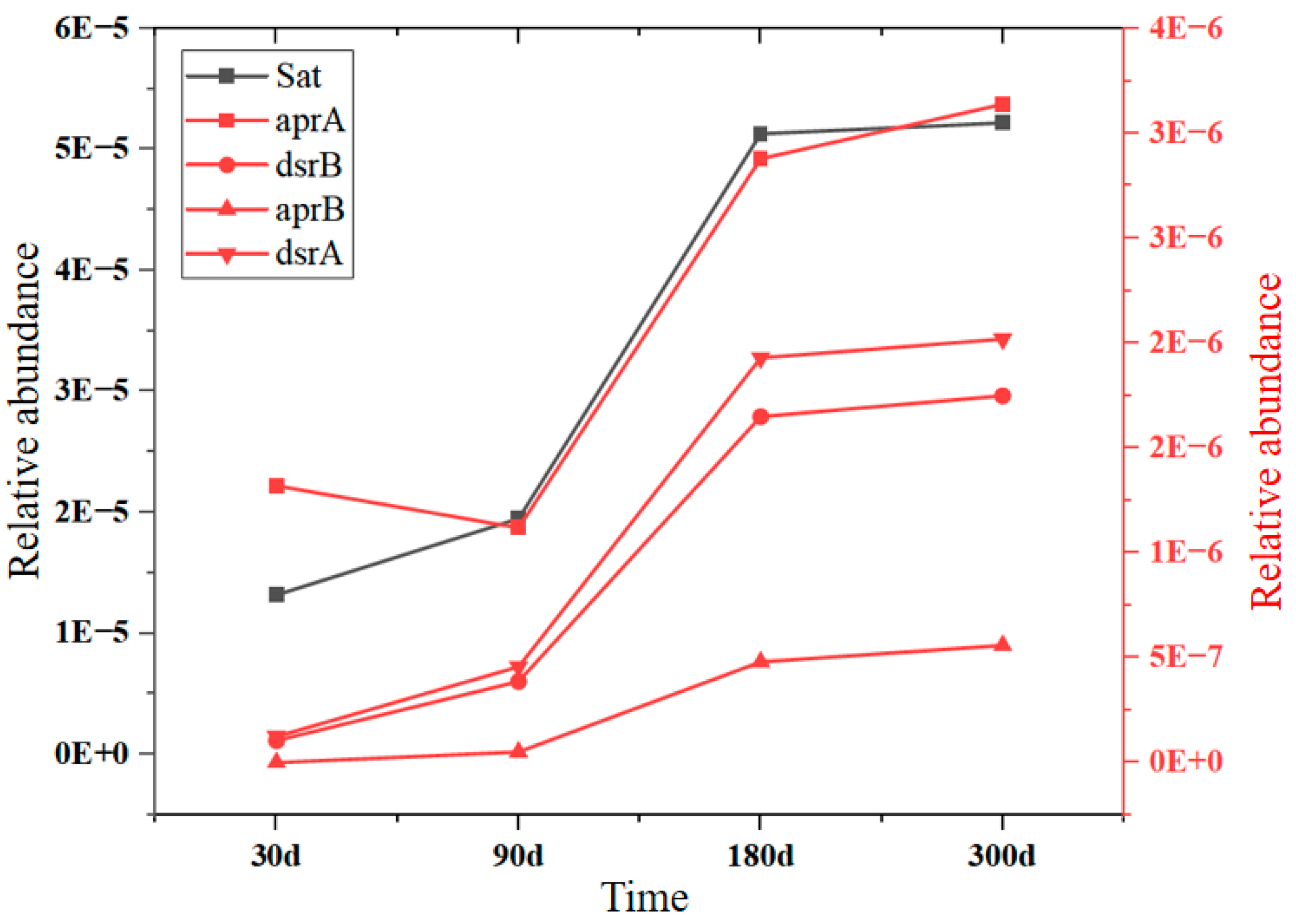
| Goaf | Layers | Fissure Rate (%) | Thickness (cm) | Relative Level (cm) | Number of Sampling Ports |
|---|---|---|---|---|---|
| Caving zone | 3# coal | 22.25 | 0.5 | 0–0.5 | 5 |
| Caving rock | 16 | 0.5–16.5 | |||
| Fracture zone | Fine sandstone | 11.89 | 13.5 | 16.5–30 | 2 |
| Sandy mudstone | 7.65 | 9 | 30–39 | 2 | |
| 2# coal | 14.79 | 2 | 39–41 | 5 | |
| Medium sandstone | 7.12 | 20 | 41–61 | 4 | |
| Mudstone | 5.82 | 9 | 61–70 | 2 |
| Layers | Mineral Content (%) | Total Content of Clay Mineral (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Potassium Feldspar | Plagioclase | Calcite | Siderite | Pyrite | Total Content of Clay Mineral | Illite/Smectite Mixed | Illite/Smectite Ratio | Illite | Kaolinite | Chlorite | |
| 3# coal | 35.3 | \ | 4.4 | 8.8 | \ | 14.9 | 36.6 | \ | \ | \ | 100 | \ |
| Caving rock | 38.8 | 2.9 | 13.7 | \ | \ | \ | 44.6 | 19 | 15 | 24 | 35 | 22 |
| Fine sandstone | 46.3 | 10.8 | 27.4 | \ | 3.4 | 12.1 | 27 | 45 | 26 | 20 | 26 | |
| Sandy mudstone | 52.7 | 10.6 | 19.9 | \ | \ | \ | 16.8 | 20 | 15 | 21 | 42 | 17 |
| 2# coal | 32.3 | \ | 3.4 | 13.6 | \ | 6.2 | 44.5 | \ | \ | \ | 100 | \ |
| Medium sandstone | 59.3 | 9.1 | 22.2 | \ | 1.0 | \ | 8.4 | 17 | 60 | 20 | 14 | 49 |
| Mudstone | 35.8 | 10.9 | 21.0 | \ | \ | \ | 32.3 | 17 | 15 | 10 | 23 | 50 |
| Simulation Path | Initial | Final | Phase Mole Transfers (mmol/L) | Redox Mole Transfers (mmol/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcite | Albite | Potassium Feldspar | Pyrite | Kaolinite | Chlorite | Quartz | Halite | O2(g) | CaX2 | NaX | O(0) | S(2) | |||
| 1 | 1 d | 30 d | 8.02 | 5.75 | 0.307 | 3.64 | −0.21 | 0.23 | −12.27 | 1.33 | 12.72 | −0.34 | 0.68 | 25.45 | 7.27 |
| 2 | 30 d | 300 d | 0.37 | 1.51 | 0.086 | 0.79 | −0.76 | 0.049 | −2.91 | 0.19 | 2.76 | −0.045 | 0.089 | 5.52 | 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Xu, Z.; Sun, Y. Mechanism of Changes in Goaf Water Hydrogeochemistry: A Case Study of the Menkeqing Coal Mine. Int. J. Environ. Res. Public Health 2023, 20, 536. https://doi.org/10.3390/ijerph20010536
Zhao X, Xu Z, Sun Y. Mechanism of Changes in Goaf Water Hydrogeochemistry: A Case Study of the Menkeqing Coal Mine. International Journal of Environmental Research and Public Health. 2023; 20(1):536. https://doi.org/10.3390/ijerph20010536
Chicago/Turabian StyleZhao, Xianming, Zhimin Xu, and Yajun Sun. 2023. "Mechanism of Changes in Goaf Water Hydrogeochemistry: A Case Study of the Menkeqing Coal Mine" International Journal of Environmental Research and Public Health 20, no. 1: 536. https://doi.org/10.3390/ijerph20010536
APA StyleZhao, X., Xu, Z., & Sun, Y. (2023). Mechanism of Changes in Goaf Water Hydrogeochemistry: A Case Study of the Menkeqing Coal Mine. International Journal of Environmental Research and Public Health, 20(1), 536. https://doi.org/10.3390/ijerph20010536








