Obesity Status and Physical Fitness Levels in Male and Female Portuguese Adolescents: A Two-Way Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ethical Aspects
2.3. Data Collection
2.4. Measurements
2.5. Statistical Analysis
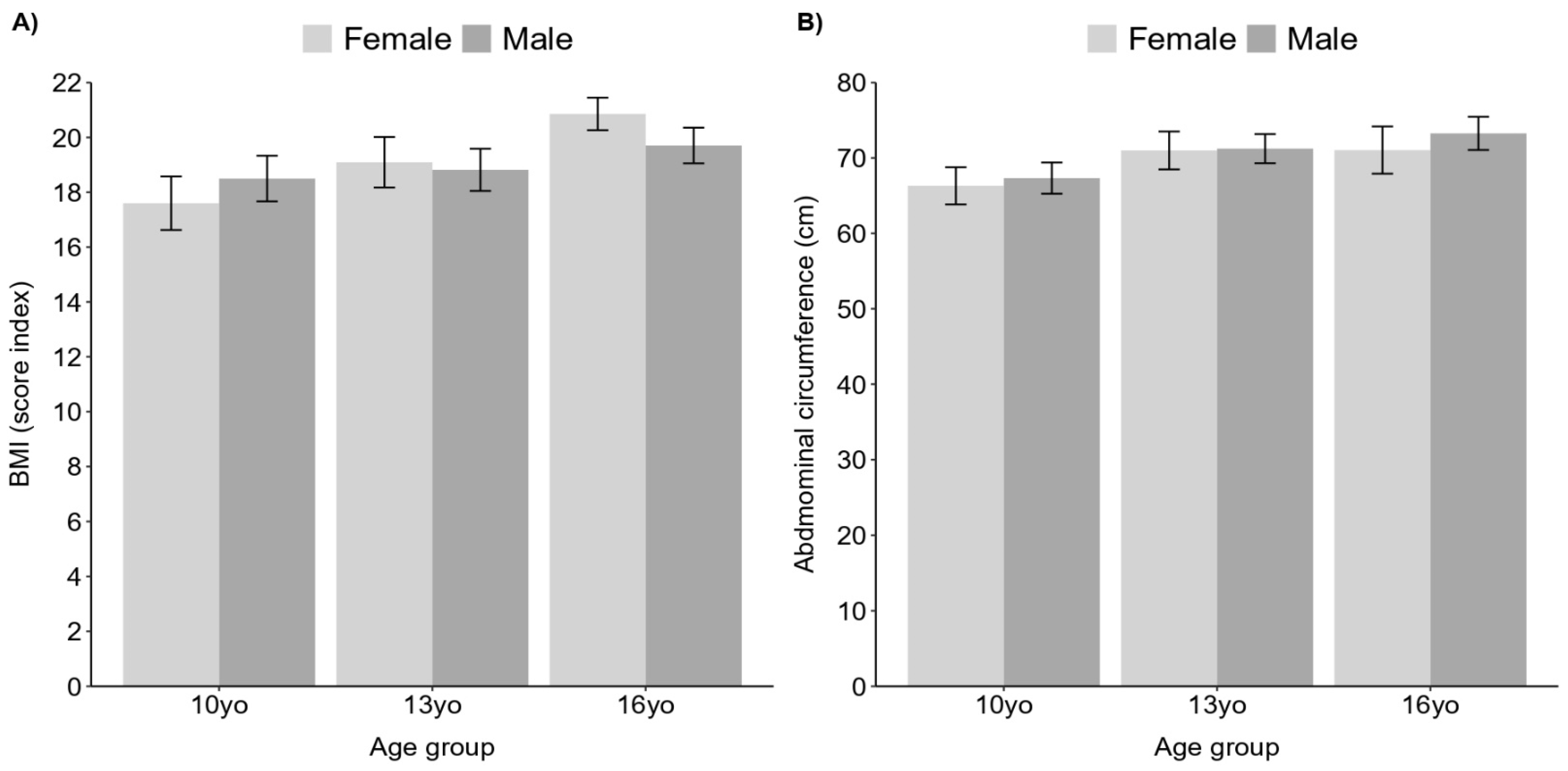
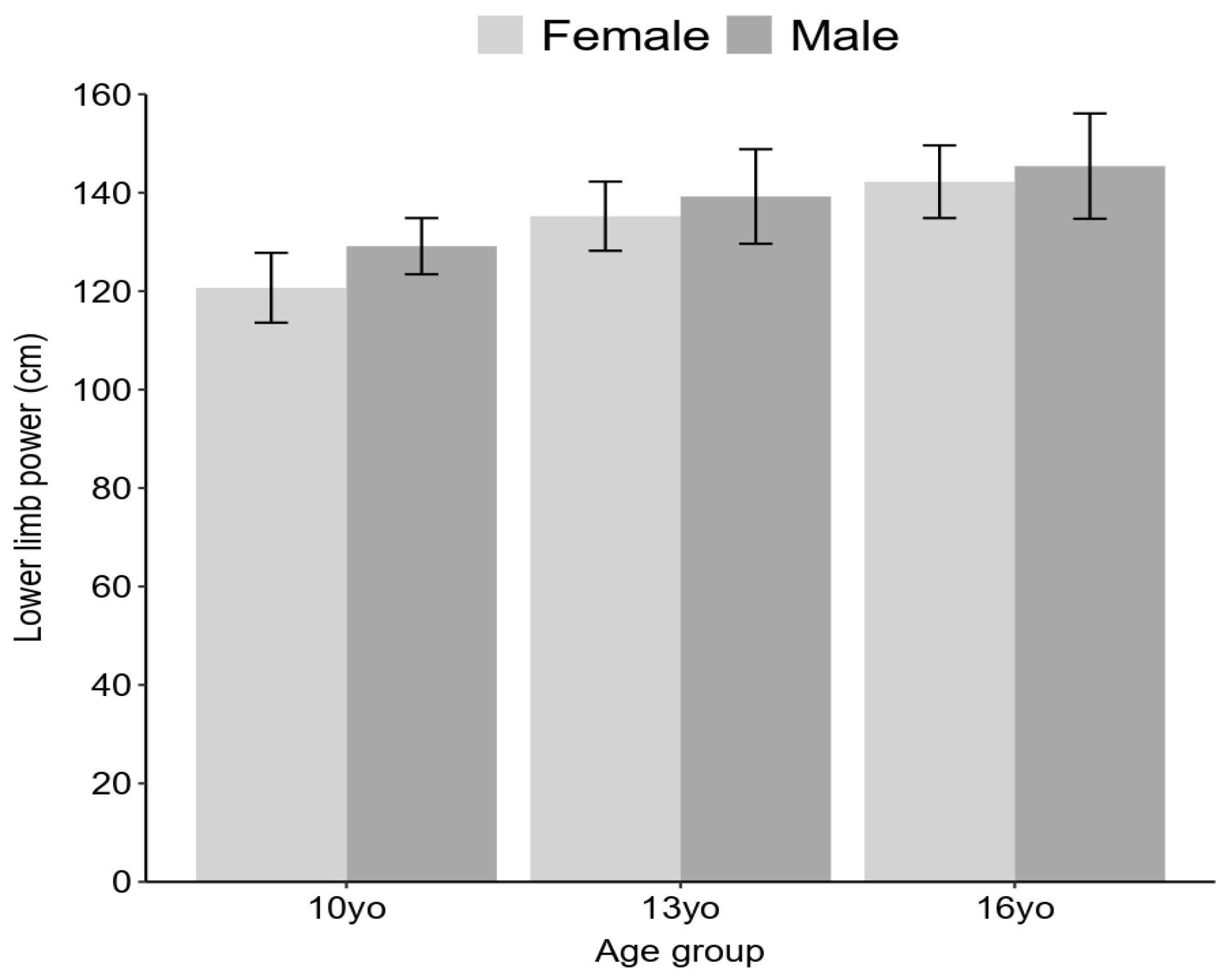
3. Results
4. Discussion
Limitations, Study Strengths, and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kansra, A.R.; Lakkunarajah, S.; Jay, M.S. Childhood and Adolescent Obesity: A Review. Front. Pediatr. 2021, 8, 581461. [Google Scholar] [CrossRef]
- Räisänen, L.; Lommi, S.; Engberg, E.; Kolho, K.; Viljakainen, H. Central Obesity in School-aged Children Increases the Likelihood of Developing Paediatric Autoimmune Diseases. Pediatr. Obes. 2022, 17, e12857. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.G.; Rodrigues, E.L.; da Silva Lopes, M.R.; do Nascimento, V.A.; Pott, A.; Guimarães, R.d.C.A.; Pegolo, G.E.; Freitas, K.d.C. Adiposity Metabolic Consequences for Adolescent Bone Health. Nutrients 2022, 14, 3260. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Furer, A.; Afek, A.; Sommer, A.; Keinan-Boker, L.; Derazne, E.; Levi, Z.; Tzur, D.; Tiosano, S.; Shina, A.; Glick, Y.; et al. Adolescent Obesity and Midlife Cancer Risk: A Population-Based Cohort Study of 2·3 Million Adolescents in Israel. Lancet Diabetes Endocrinol. 2020, 8, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Narciso, J.; Silva, A.J.; Rodrigues, V.; Monteiro, M.J.; Almeida, A.; Saavedra, R.; Costa, A.M. Behavioral, Contextual and Biological Factors Associated with Obesity during Adolescence: A Systematic Review. PLoS ONE 2019, 14, e0214941. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.M.; Moraes, Í.A.P.; Dantas, M.T.A.P.; Fernani, D.C.G.L.; Fontes, A.M.G.G.; Silveira, A.C.; Barnabé, V.; Fernandes, M.; Martinelli, P.M.; Monteiro, C.B.M.; et al. Influence of Chronic Exposure to Exercise on Heart Rate Variability in Children and Adolescents Affected by Obesity: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 11065. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Börnhorst, C.; Russo, P.; Veidebaum, T.; Tornaritis, M.; Molnár, D.; Lissner, L.; Mårild, S.; De Henauw, S.; Moreno, L.A.; Floegel, A.; et al. The Role of Lifestyle and Non-Modifiable Risk Factors in the Development of Metabolic Disturbances from Childhood to Adolescence. Int. J. Obes. 2020, 44, 2236–2245. [Google Scholar] [CrossRef]
- Marques, A.; Henriques-Neto, D.; Peralta, M.; Martins, J.; Demetriou, Y.; Schönbach, D.M.I.; Gaspar de Matos, M. Prevalence of Physical Activity among Adolescents from 105 Low, Middle, and High-Income Countries. Int. J. Environ. Res. Public Health 2020, 17, 3145. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Marphatia, A.A.; Cole, T.J.; McCoy, D. Associations of Economic and Gender Inequality with Global Obesity Prevalence: Understanding the Female Excess. Soc. Sci. Med. 2012, 75, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Padez, C.; Mourão, I.; Moreira, P.; Rosado, V. Prevalence and Risk Factors for Overweight and Obesity in Portuguese Children. Acta Paediatr. 2005, 94, 1550–1557. [Google Scholar] [CrossRef]
- da Encarnação, S.G.A.; Flores, P.; Magalhães, D.; Afonso, G.; Pereira, A.; Fonseca, R.B.; Ribeiro, J.; Silva-Santos, S.; Teixeira, J.E.; Monteiro, A.M.; et al. The Influence of Abdominal Adiposity and Physical Fitness on Obesity Status of Portuguese Adolescents. Int. J. Environ. Res. Public Health 2022, 19, 11213. [Google Scholar] [CrossRef] [PubMed]
- R project R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 January 2023).
- Zhong, P.-S.; Li, R.; Santo, S. Homogeneity Tests of Covariance Matrices with High-Dimensional Longitudinal Data. Biometrika 2019, 106, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Bathke, A.C.; Friedrich, S.; Pauly, M.; Konietschke, F.; Staffen, W.; Strobl, N.; Höller, Y. Testing Mean Differences among Groups: Multivariate and Repeated Measures Analysis with Minimal Assumptions. Multivar. Behav. Res. 2018, 53, 348–359. [Google Scholar] [CrossRef]
- FITescola FITescola: O Programa Dos Alunos Ativos. Available online: https://fitescola.dge.mec.pt/hometestes.aspx (accessed on 8 June 2023).
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, C.J.; Janssen, I. Development of Age-Specific Adolescent Metabolic Syndrome Criteria That Are Linked to the Adult Treatment Panel III and International Diabetes Federation Criteria. J. Am. Coll. Cardiol. 2007, 49, 891–898. [Google Scholar] [CrossRef]
- Huang, F.L. MANOVA: A Procedure Whose Time Has Passed? Gift. Child Q. 2020, 64, 56–60. [Google Scholar] [CrossRef]
- Muller, K.E. A New F Approximation for the Pillai–Bartlett Trace under H0. J. Comput. Graph. Stat. 1998, 7, 131–137. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Costa, T.; Murara, P.; Vancini, R.L.; de Lira, C.A.B.; Andrade, M.S. Influence of Biological Maturity on the Muscular Strength of Young Male and Female Swimmers. J. Hum. Kinet. 2021, 78, 67–77. [Google Scholar] [CrossRef]
- Santos, L.P.; Santos, I.S.; Matijasevich, A.; Barros, A.J.D. Changes in Overall and Regional Body Fatness from Childhood to Early Adolescence. Sci. Rep. 2019, 9, 1888. [Google Scholar] [CrossRef]
- Azzolino, D.; Spolidoro, G.C.I.; Saporiti, E.; Luchetti, C.; Agostoni, C.; Cesari, M. Musculoskeletal Changes Across the Lifespan: Nutrition and the Life-Course Approach to Prevention. Front. Med. 2021, 8, 697954. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity, and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S.; Baker, J.S. Relationship between Abdominal Adiposity, Cardiovascular Fitness, and Biomarkers of Cardiovascular Risk in British Adolescents. J. Sport Health Sci. 2020, 9, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Henao-Mejia, J.; Henrickson, S.E. Obesity and Immune Status in Children. Curr. Opin. Pediatr. 2020, 32, 805–815. [Google Scholar] [CrossRef]
- Duraccio, K.M.; Krietsch, K.N.; Chardon, M.L.; Van Dyk, T.R.; Beebe, D.W. Poor Sleep and Adolescent Obesity Risk: A Narrative Review of Potential Mechanisms. Adolesc. Health Med. Ther. 2019, 10, 117–130. [Google Scholar] [CrossRef]
- Ruiz, L.D.; Zuelch, M.L.; Dimitratos, S.M.; Scherr, R.E. Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors. Nutrients 2020, 12, 43. [Google Scholar] [CrossRef]
- Högström, G.; Ohlsson, H.; Crump, C.; Sundquist, J.; Sundquist, K. Aerobic Fitness in Late Adolescence and the Risk of Cancer and Cancer-Associated Mortality in Adulthood: A Prospective Nationwide Study of 1.2 Million Swedish Men. Cancer Epidemiol. 2019, 59, 58–63. [Google Scholar] [CrossRef]
- Belcher, B.R.; Zink, J.; Azad, A.; Campbell, C.E.; Chakravartti, S.P.; Herting, M.M. The Roles of Physical Activity, Exercise, and Fitness in Promoting Resilience During Adolescence: Effects on Mental Well-Being and Brain Development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 225–237. [Google Scholar] [CrossRef]
- Altaye, K.Z.; Mondal, S.; Legesse, K.; Abdulkedir, M. Effects of Aerobic Exercise on Thyroid Hormonal Change Responses among Adolescents with Intellectual Disabilities. BMJ Open Sport Exerc. Med. 2019, 5, e000524. [Google Scholar] [CrossRef]
- Minatto, G.; de Sousa, T.F.; de Carvalho, W.R.G.; Ribeiro, R.R.; Santos, K.D.; Petroski, E.L. Association between Cardiorespiratory Fitness and Body Fat in Girls. Rev. Paul. Pediatr. (Engl. Ed.) 2016, 34, 469–475. [Google Scholar] [CrossRef]
- Páez-Maldonado, J.A.; Reigal, R.E.; Morillo-Baro, J.P.; Carrasco-Beltrán, H.; Hernández-Mendo, A.; Morales-Sánchez, V. Physical Fitness, Selective Attention and Academic Performance in a Pre-Adolescent Sample. Int. J. Environ. Res. Public Health 2020, 17, 6216. [Google Scholar] [CrossRef] [PubMed]
- Al-Shenqiti, A.M.; Emara, H.A.; Algarni, F.S.; Khaled, O.A. Isokinetic Trunk Muscle Performance in Adolescents with Different Body Mass Indices. J. Taibah Univ. Med. Sci. 2021, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Szadvári, I.; Ostatníková, D.; Babková Durdiaková, J. Sex Differences Matter: Males and Females Are Equal but Not the Same. Physiol. Behav. 2023, 259, 114038. [Google Scholar] [CrossRef]
- Zemková, E.; Zapletalová, L. Back Problems: Pros and Cons of Core Strengthening Exercises as a Part of Athlete Training. Int. J. Environ. Res. Public Health 2021, 18, 5400. [Google Scholar] [CrossRef] [PubMed]
- Huxel Bliven, K.C.; Anderson, B.E. Core Stability Training for Injury Prevention. Sport Health 2013, 5, 514–522. [Google Scholar] [CrossRef]
- Fraser, B.J.; Blizzard, L.; Buscot, M.-J.; Schmidt, M.D.; Dwyer, T.; Venn, A.J.; Magnussen, C.G. Muscular Strength across the Life Course: The Tracking and Trajectory Patterns of Muscular Strength between Childhood and Mid-Adulthood in an Australian Cohort. J. Sci. Med. Sport 2021, 24, 696–701. [Google Scholar] [CrossRef]
- Evaristo, S.; Moreira, C.; Lopes, L.; Oliveira, A.; Abreu, S.; Agostinis-Sobrinho, C.; Oliveira-Santos, J.; Póvoas, S.; Santos, R.; Mota, J. Muscular Fitness and Cardiorespiratory Fitness Are Associated with Health-Related Quality of Life: Results from Labmed Physical Activity Study. J. Exerc. Sci. Fit. 2019, 17, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rauch, R.; Veilleux, L.-N.; Rauch, F.; Bock, D.; Welisch, E.; Filler, G.; Robinson, T.; Burrill, E.; Norozi, K. Muscle Force and Power in Obese and Overweight Children. J. Musculoskelet. Neuronal. Interact 2012, 12, 80–83. [Google Scholar] [PubMed]
- Heneweer, H.; Picavet, H.S.J.; Staes, F.; Kiers, H.; Vanhees, L. Physical Fitness, Rather than Self-Reported Physical Activities, Is More Strongly Associated with Low Back Pain: Evidence from a Working Population. Eur. Spine J. 2012, 21, 1265–1272. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- de Almeida-Neto, P.F.; Oliveira, V.M.M.; de Matos, D.G.; dos Santos, Í.K.; Baxter-Jones, A.; Pinto, V.C.M.; de Macêdo Cesário, T.; Aidar, F.J.; Silva Dantas, P.M.; Cabral, B.G.A.T. Factors Related to Lower Limb Performance in Children and Adolescents Aged 7 to 17 Years: A Systematic Review with Meta-Analysis. PLoS ONE 2021, 16, e0258144. [Google Scholar] [CrossRef]
- Vandoni, M.; Carnevale Pellino, V.; De Silvestri, A.; Lovecchio, N.; Rovida, A.; Gatti, A.; Biagioli, V.; Zuccotti, G.; Calcaterra, V. The Temporal Association between Body Characteristics and Speed Performance over Twenty-Five Years in Italian Adolescents. Children 2022, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Aerenhouts, D.; Clarys, P.; Taeymans, J.; Van Cauwenberg, J. Estimating Body Composition in Adolescent Sprint Athletes: Comparison of Different Methods in a 3 Years Longitudinal Design. PLoS ONE 2015, 10, e0136788. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.D.; Gómez-Arbeláez, D.; Camacho, P.A.; Pinzon, S.; Hormiga, C.; Trejos-Suarez, J.; Duperly, J.; Lopez-Jaramillo, P. Low Muscle Strength Is Associated with Metabolic Risk Factors in Colombian Children: The ACFIES Study. PLoS ONE 2014, 9, e93150. [Google Scholar] [CrossRef] [PubMed]
- Telama, R.; Yang, X.; Viikari, J.; Välimäki, I.; Wanne, O.; Raitakari, O. Physical Activity from Childhood to Adulthood: A 21-Year Tracking Study. Am. J. Prev. Med. 2005, 28, 267–273. [Google Scholar] [CrossRef]




| Variables | Normal Weight (n) | Obesity (n) | X2 | p-Value | ES |
|---|---|---|---|---|---|
| Sex | |||||
| Male (n = 85) | 48 (56%) | 37 (44%) | 20.20 | p < 0.001 | 0.15 |
| Girls (n = 85) | 50 (59%) | 35 (41%) | 31.40 | p < 0.001 | 0.19 |
| BMI by age | |||||
| 10 yo (n = 58) | 27 (46%) | 31 (58%) | 19.70 | p < 0.001 | 0.22 |
| 13 yo (n = 60) | 34 (57%) | 26 (43%) | 22.90 | p < 0.001 | 0.23 |
| 16 yo (n = 54) | 37 (71%) | 15 (29%) | 75.00 | p < 0.001 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnação, S.; Rodrigues, F.; Monteiro, A.M.; Gouili, H.; Hattabi, S.; Sortwell, A.; Branquinho, L.; Teixeira, J.E.; Ferraz, R.; Flores, P.; et al. Obesity Status and Physical Fitness Levels in Male and Female Portuguese Adolescents: A Two-Way Multivariate Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6115. https://doi.org/10.3390/ijerph20126115
Encarnação S, Rodrigues F, Monteiro AM, Gouili H, Hattabi S, Sortwell A, Branquinho L, Teixeira JE, Ferraz R, Flores P, et al. Obesity Status and Physical Fitness Levels in Male and Female Portuguese Adolescents: A Two-Way Multivariate Analysis. International Journal of Environmental Research and Public Health. 2023; 20(12):6115. https://doi.org/10.3390/ijerph20126115
Chicago/Turabian StyleEncarnação, Samuel, Filipe Rodrigues, António Miguel Monteiro, Hatem Gouili, Soukaina Hattabi, Andrew Sortwell, Luís Branquinho, José Eduardo Teixeira, Ricardo Ferraz, Pedro Flores, and et al. 2023. "Obesity Status and Physical Fitness Levels in Male and Female Portuguese Adolescents: A Two-Way Multivariate Analysis" International Journal of Environmental Research and Public Health 20, no. 12: 6115. https://doi.org/10.3390/ijerph20126115
APA StyleEncarnação, S., Rodrigues, F., Monteiro, A. M., Gouili, H., Hattabi, S., Sortwell, A., Branquinho, L., Teixeira, J. E., Ferraz, R., Flores, P., Silva-Santos, S., Ribeiro, J., Batista, A., & Forte, P. M. (2023). Obesity Status and Physical Fitness Levels in Male and Female Portuguese Adolescents: A Two-Way Multivariate Analysis. International Journal of Environmental Research and Public Health, 20(12), 6115. https://doi.org/10.3390/ijerph20126115












