Comparison between the American and the European Systems of Monitoring Adverse Effects of Dietary Supplements and Their Usefulness on the Polish Market
Abstract
:1. Introduction
- (1)
- to determine the needs for creating, in the future, a national register of adverse effects caused by dietary supplements by determining the degree of the presence of borderline-type supplements on the Polish market, including their potential for causing adverse effects;
- (2)
- to prepare a general concept of a register of adverse effects for Poland, considering the various conclusions drawn from European and American experiences in the area of the functioning of such registers.
2. Materials and Methods
2.1. The French Register
2.2. Recognising Selected Ingredients as Borderline
2.3. Borderline Products in the American Register
2.4. Assessment of Dietary Supplements Containing Borderline Ingredients
2.5. Data Comparison
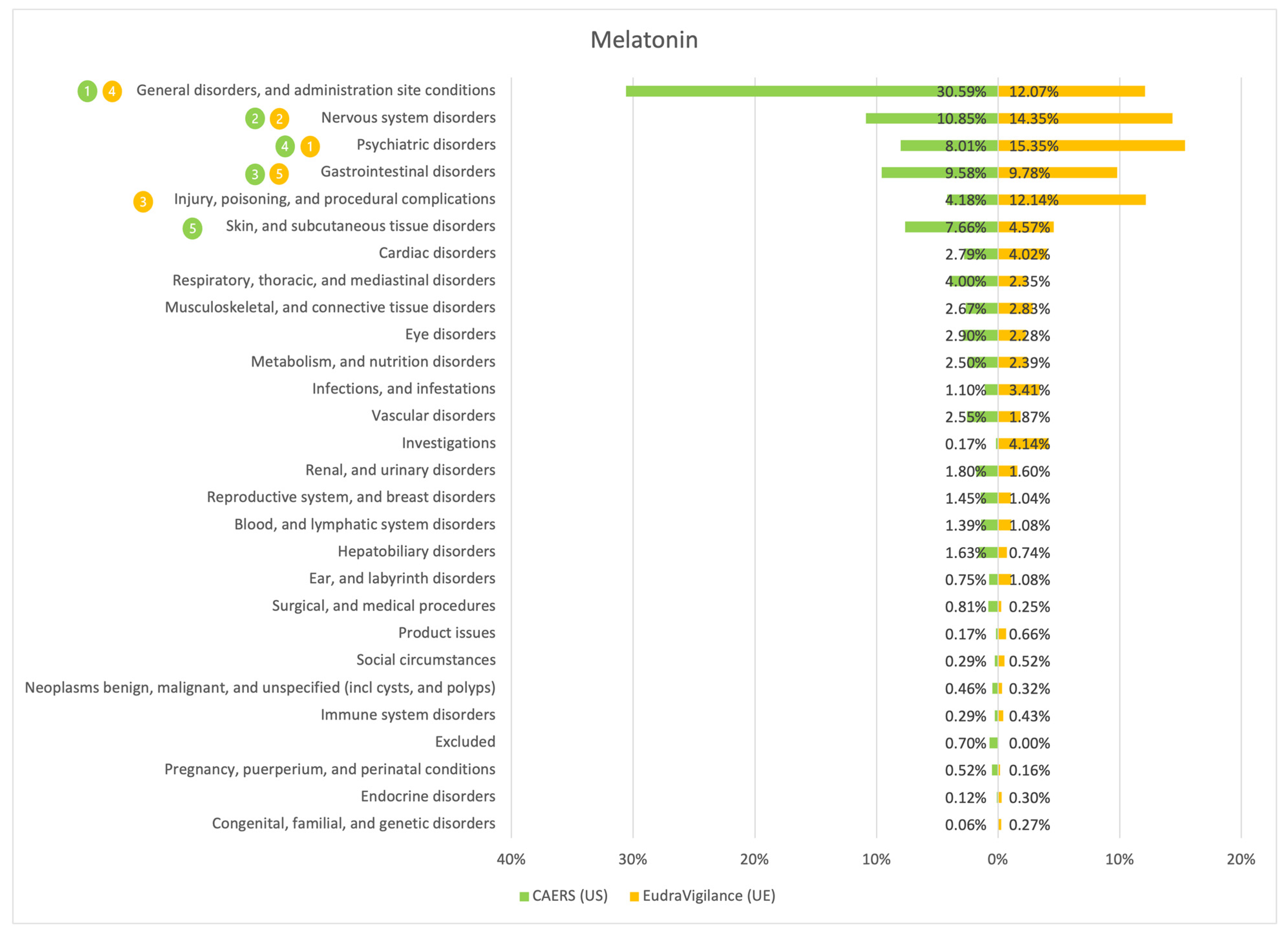
3. Results
4. Discussion
Limitations
5. Conclusions
- Considering the system of notification of dietary supplements newly placed on the market in force in Poland and its current flaws and imperfections, it is reasonable to undertake work on creating a register of adverse effects caused by dietary supplements.
- The studied ingredients are commonly present in dietary supplements placed on the Polish market and may be simultaneously available as OTC medicines or plant-based medicines. The number of notifications recorded in Poland’s national register indicates a real risk of producing the adverse effects identified in the CAERS register.
- Non-professional entities generate more general-type notifications, whose scientific and substantive value is lower, but which may nonetheless identify certain trends and needs for further, more in-depth studies.
- Owing to the multidrug approach of patients who also consume dietary supplements, as well as the role that dietary supplements play in causing adverse effects, the concept of a national register should include the possibility to report other concurrently used medicinal preparations and functional food products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dietz, P.; Ulrich, R.; Niess, A.; Best, R.; Simon, P.; Striegel, H. Prediction Profiles for Nutritional Supplement Use among Young German Elite Athletes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplements: A Framework for Evaluating Safety; National Academies Press: Washington, DC, USA, 2005; p. 10882. ISBN 978-0-309-09110-7.
- Palmer, M.E.; Haller, C.; McKinney, P.E.; Klein-Schwartz, W.; Tschirgi, A.; Smolinske, S.C.; Woolf, A.; Sprague, B.M.; Ko, R.; Everson, G.; et al. Adverse Events Associated with Dietary Supplements: An Observational Study. Lancet 2003, 361, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Sousa, C.; Pedro, M.; Fernandes, M. Dangerous Mistake: An Accidental Caffeine Overdose. BMJ Case Rep. 2018, 2018, bcr2018224185. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Lindsey, T. Fatal Caffeine Overdose: Two Case Reports. Forensic Sci. Int. 2005, 153, 67–69. [Google Scholar] [CrossRef]
- Mathews, N.M. Prohibited Contaminants in Dietary Supplements. Sport. Health Multidiscip. Approach 2018, 10, 19–30. [Google Scholar] [CrossRef]
- Tarn, D.M.; Paterniti, D.A.; Good, J.S.; Coulter, I.D.; Galliher, J.M.; Kravitz, R.L.; Karlamangla, A.S.; Wenger, N.S. Physician–Patient Communication about Dietary Supplements. Patient Educ. Couns. 2013, 91, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Chiba, T.; Sato, Y.; Suzuki, S.; Umegaki, K. Concomitant Use of Dietary Supplements and Medicines in Patients Due to Miscommunication with Physicians in Japan. Nutrients 2015, 7, 2947–2960. [Google Scholar] [CrossRef] [Green Version]
- Simundic, A.-M.; Filipi, P.; Vrtaric, A.; Miler, M.; Nikolac Gabaj, N.; Kocsis, A.; Avram, S.; Gligorovic Barhanovic, N.; Bulo, A.; Cadamuro, J.; et al. Patient’s Knowledge and Awareness about the Effect of the over-the-Counter (OTC) Drugs and Dietary Supplements on Laboratory Test Results: A Survey in 18 European Countries. Clin. Chem. Lab. Med. CCLM 2018, 57, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Adam, K.; Agnieszka, B.; Kamila, B.; Katarzyna, K.B. Use of the Herbal OTC Products and Dietary Supplements by Patients Receiving Chemotherapy: Survey-Based Study. Indian J. Pharm. Educ. Res. 2017, 51, s675–s678. [Google Scholar] [CrossRef] [Green Version]
- Zalewski, A. Pharmaceutical marketing in Poland—Research on advertising messages for medicines, dietary supplements and medical devices. Rozpr. Społeczne 2020, 14, 107–127. [Google Scholar] [CrossRef]
- Rzymski, P.; Jaśkiewicz, M. Microalgal Food Supplements from the Perspective of Polish Consumers: Patterns of Use, Adverse Events, and Beneficial Effects. J. Appl. Phycol. 2017, 29, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICD-10 Version. 2019. Available online: https://icd.who.int/browse10/2019/en#/ (accessed on 10 November 2022).
- Haller, C.A.; Kearney, T.; Bent, S.; Ko, R.; Benowitz, N.L.; Olson, K. Dietary Supplement Adverse Events: Report of a One-Year Poison Center Surveillance Project. J. Med. Toxicol. 2008, 4, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.C.; Giordani, F.; Guaraldo, L.; Tognoni, G.; Rozenfeld, S. Adverse Drug Events Identified in Hospitalized Patients in Brazil by International Classification of Diseases (ICD-10) Code Listings. Cad. Saúde Pública 2018, 34, e00222417. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Zhu, K.; Prince, R.L. Adverse Events from Calcium Supplementation: Relationship to Errors in Myocardial Infarction Self-Reporting in Randomized Controlled Trials of Calcium Supplementation. J. Bone Miner. Res. 2012, 27, 719–722. [Google Scholar] [CrossRef]
- Ashar, A.; Bimal, H. Rowland-Seymour Advising Patients Who Use Dietary Supplements. Am. J. Med. 2008, 121, 91–97. [Google Scholar] [CrossRef]
- Kardas, P.; Dorożyński, J.; Zdanowicz, P.; Tyrna, D.; Lis, P.; Mroczka, M.; Lewek, P. Are Dietary Supplements Dangerous? Assessing How Society Perceives the Usage of Dietary Supplements—Results of the Preliminary Analysis of Nationwide Survey Data. Med. Sci. Pulse 2018, 12, 13–17. [Google Scholar] [CrossRef]
- Raschi, E.; Girardi, A.; Poluzzi, E.; Forcesi, E.; Menniti-Ippolito, F.; Mazzanti, G.; De Ponti, F. Adverse Events to Food Supplements Containing Red Yeast Rice: Comparative Analysis of FAERS and CAERS Reporting Systems. Drug Saf. 2018, 41, 745–752. [Google Scholar] [CrossRef]
- Morgovan, C. Nutrivigilance: A new activity in the field of dietary supplements. Farmacia. 2019, 67, 537–544. [Google Scholar] [CrossRef]
- van Grootheest, K.; de Graaf, L.; de Jong-van den Berg, L.T.W. Consumer Adverse Drug Reaction Reporting: A New Step in Pharmacovigilance? Drug Saf. 2003, 26, 211–217. [Google Scholar] [CrossRef]
- Xu, Y.; Patel, D.N.; Ng, S.-L.P.; Tan, S.-H.; Toh, D.; Poh, J.; Lim, A.T.; Chan, C.-L.; Low, M.-Y.; Koh, H.-L. Retrospective Study of Reported Adverse Events Due to Complementary Health Products in Singapore from 2010 to 2016. Front. Med. 2018, 5, 167. [Google Scholar] [CrossRef]
- Bilia, A.R. Herbal Medicinal Products versus Botanical-Food Supplements in the European Market: State of Art and Perspectives. Nat. Prod. Commun. 2015, 10, 1934578X1501000. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory Landscape of Dietary Supplements and Herbal Medicines from a Global Perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Coutrelis, N.; Corre, P.Y. Nutrivigilance in France. Eur. Food Feed Law Rev. EFFL 2011, 6, 57–58. [Google Scholar]
- ANSES Recommends That Certain Populations Avoid the Consumption of Food Supplements Containing Melatonin. Available online: https://www.anses.fr/en/content/anses-recommends-certain-populations-avoid-consumption-food-supplements-containing-melatonin (accessed on 12 October 2022).
- Food Supplements Containing Spirulina: The Importance of Choosing Trustworthy Supply Channels. Available online: https://www.anses.fr/en/content/food-supplements-containing-spirulina-importance-choosing-trustworthy-supply-channels (accessed on 12 October 2022).
- Red Yeast Rice-Based Food Supplements. Available online: https://www.anses.fr/en/content/red-yeast-rice-based-food-supplements (accessed on 12 October 2022).
- Dietary Supplements for Weight Loss Containing P-Synephrine. Available online: https://www.anses.fr/en/content/dietary-supplements-weight-loss-containing-p-synephrine (accessed on 12 October 2022).
- Food Supplements for Pregnant Women. Available online: https://www.anses.fr/en/content/food-supplements-pregnant-women (accessed on 12 October 2022).
- Food Supplements for Developing Muscle or Reducing Body Fat. Available online: https://www.anses.fr/en/content/food-supplements-developing-muscle-or-reducing-body-fat (accessed on 12 October 2022).
- What Are the Health Effects of Energy Drinks? Available online: https://www.anses.fr/en/content/what-are-health-effects-energy-drinks (accessed on 12 October 2022).
- Vasilakes, J.A.; Rizvi, R.F.; Zhang, J.; Zhang, R. Detecting Signals of Dietary Supplement Adverse Events from the CFSAN Adverse Event Reporting System (CAERS). AMIA Summits Transl. Sci. Proc. 2019, 2019, 258–266. [Google Scholar]
- EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance (accessed on 22 September 2022).
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Assessment of the Impact of Scientific Reports Published by EFSA and GIS on Functional Foods Newly Placed on the Market in Poland. Int. J. Environ. Res. Public. Health 2022, 19, 4057. [Google Scholar] [CrossRef]
- Dennehy, C.E.; Tsourounis, C.; Miller, A.E. Evaluation of Herbal Dietary Supplements Marketed on the Internet for Recreational Use. Ann. Pharmacother. 2005, 39, 1634–1639. [Google Scholar] [CrossRef]
- Benatrehina, P.A.; Pan, L.; Naman, C.B.; Li, J.; Kinghorn, A.D. Usage, Biological Activity, and Safety of Selected Botanical Dietary Supplements Consumed in the United States. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar] [CrossRef]
- Lipert, A.; Szadkowska, I.; Matusiak-Wieczorek, E.; Kochan, E. The Effect of Herbal Supplements on Blood Pressure: Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1419. [Google Scholar] [CrossRef]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.-M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Timbo, B.B.; Ross, M.P.; McCarthy, P.V.; Lin, C.-T.J. Dietary Supplements in a National Survey: Prevalence of Use and Reports of Adverse Events. J. Am. Diet. Assoc. 2006, 106, 1966–1974. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. The Impact of the COVID-19 Pandemic on the Composition of Dietary Supplements and Functional Foods Notified in Poland. Int. J. Environ. Res. Public. Health 2021, 18, 11751. [Google Scholar] [CrossRef]
- Wróbel, K.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Dietary Supplements Questioned in the Polish Notification Procedure upon the Basis of Data from the National Register of Functional Foods and the European System of the RASFF. Int. J. Environ. Res. Public. Health 2022, 19, 8161. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Coates, P.; Smith, M. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vujić, M.; Lušić, D.; Bošnir, J.; Pezo, L.L.; Kuharić, Ž.; Lasić, D.; Šabarić, J.; Barušić, L.; Lušić, D.V. Assessment of Metal Intake by Selected Food Supplements Based on Beehive Products. Foods 2022, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Vujić, M.; Pollak, L. Composition, Labelling, and Safety of Food Supplements Based on Bee Products in the Legislative Framework of the European Union–Croatian Experiences/Sastav, Označavanje i Zdravstvena Ispravnost Dodataka Prehrani Koji Se Temelje Na Pčelinjim Proizvodima u Pravnom Okviru Europske Unije–Hrvatska Iskustva. Arch. Ind. Hyg. Toxicol. 2015, 66, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Menniti-Ippolito, F.; Mazzanti, G.; Santuccio, C.; Angela Moro, P.; Calapai, G.; Firenzuoli, F.; Valeri, A.; Raschetti, R. Surveillance of Suspected Adverse Reactions to Natural Health Products in Italy. Pharmacoepidemiol. Drug Saf. 2008, 17, 626–635. [Google Scholar] [CrossRef]
- Jiang, T. Re-Thinking the Dietary Supplement Laws and Regulations 14 Years after the Dietary Supplement Health and Education Act Implementation. Int. J. Food Sci. Nutr. 2009, 60, 293–301. [Google Scholar] [CrossRef]
- Schmitz, S.M.; Lopez, H.L.; Mackay, D.; Nguyen, H.; Miller, P.E. Serious Adverse Events Reported with Dietary Supplement Use in the United States: A 2.5 Year Experience. J. Diet. Suppl. 2020, 17, 227–248. [Google Scholar] [CrossRef]
- Nishijima, C.; Sato, Y.; Chiba, T.; Umegaki, K. Nationwide Online Survey to Complement the Current Voluntary Reporting System for Adverse Events Associated with Dietary Supplements: Application to the Case of Skin Manifestations. J. Nutr. Sci. Vitaminol. 2018, 64, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Timbo, B.B.; Chirtel, S.J.; Ihrie, J.; Oladipo, T.; Velez-Suarez, L.; Brewer, V.; Mozersky, R. Dietary Supplement Adverse Event Report Data from the FDA Center for Food Safety and Applied Nutrition Adverse Event Reporting System (CAERS), 2004–2013. Ann. Pharmacother. 2018, 52, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.; Sarma, D.N.; Dog, T.L.; Barrett, M.L.; Chavez, M.L.; Ko, R.; Mahady, G.B.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I. The State of Dietary Supplement Adverse Event Reporting in the United States. Pharmacoepidemiol. Drug Saf. 2008, 17, 962–970. [Google Scholar] [CrossRef] [PubMed]
- European Database of Suspected Adverse Drug Reaction Reports—Understanding a Web Report. Available online: https://www.adrreports.eu/en/understanding_reports.html (accessed on 13 December 2022).
- Monnot, A.D.; Fung, E.S.; Compoginis, G.S.; Towle, K.M. An Evaluation of the FDA Adverse Event Reporting System and the Potential for Reporting Bias. J. Cosmet. Dermatol. 2021, 20, 1849–1854. [Google Scholar] [CrossRef] [PubMed]






| Date of Issue | Title of Document | Ingredients or Other Factors That Constitute a Probable Cause of the Adverse Effect |
|---|---|---|
| 14 December 2007 | Opinion of the French Food Safety Agency on the use of botanical food supplements which have been the subject of drug safety monitoring reports | Hoodia Gordoni Cimcifuga racemosa + Desmodium Viola tricolor Viola arvensis Echinacea angustifolia + Echincea pallida + Echinacea purpurea + Polygonum multiflorum |
| 7 June 2010 | Opinion of the French Food Safety Agency on the safety of use of a plant (Galega officinalis) in food supplements | Galega officinalis |
| 22 November 2010 | Opinion of the French Agency for Food, Environmental and Occupational Health and Safety regarding the safety of yam (Dioscorea) alcohol extracts in food supplements | Dioscorea |
| 25 February 2011 | Opinion of the French Agency for Food, Environmental and Occupational Health and Safety regarding the risk of drug eruption following the consumption of lutein and zeaxanthin in food supplements | Lutein Zeaxanthin |
| 7 August 2012 | ANSES OPINION regarding the safety of consumption of powdered green tea in food supplements | Green tea + |
| 14 February 2014 | OPINION of the French Agency for Food, Environmental and Occupational Health and Safety on the risks associated with the presence of “red yeast rice” in food supplements | Red yeast rice |
| 14 March 2014 | OPINION of the French Agency for Food, Environmental and Occupational Health and Safety on the risks associated with the presence in food supplements of p-synephrine or ingredients obtained from Citrus spp. fruits containing this substance | p-synephrine |
| 31 October 2014 | ANSES OPINION on allergy to a food supplement containing spirulina | Spirulina |
| 27 March 2015 | ANSES OPINION on a case of hepatitis following the consumption of a food supplement for joint conditions | Glucosamine + Chondroitin + |
| n/a | ANSES OPINION AND REPORT on the risks associated with the consumption of food supplements for sportsmen whose purpose is muscle building or adipose tissue reduction | n/a |
| 19 April 2017 | ANSES OPINION regarding photo-induced drug eruption following the consumption of a Bio-butter food supplement | Butyric acid |
| 4 August 2017 | ANSES OPINION on the risks associated with the consumption of food supplements containing spirulina | Spirulina |
| 23 February 2018 | ANSES OPINION on the risks associated with the consumption of food supplements containing melatonin | Melatonin + |
| 23 May 2018 | ANSES OPINION on allergy to the food supplement Nutrilin® | Linseed extract |
| 23 May 2018 | ANSES OPINION on three cases of allergy to food supplements containing pollen or hive products | Hive |
| 16 January 2019 | ANSES OPINION on a case of severe hypokalaemia following misuse of the food supplement Rhubarbe® containing liquorice | Liquorice + Rhubarb |
| 1 August 2019 | ANSES OPINION on the “safety of use of berberine-containing plants in the composition of food supplements” | Berberine |
| 16 October 2019 | ANSES OPINION on a case of severe hypokalaemia following misuse of an oral food supplement | Consumer’s error |
| 24 December 2019 | ANSES OPINION regarding anaphylaxis associated with consumption of a food supplement containing passiflora | Passiflora |
| 1 April 2020 | ANSES OPINION on a case of fatal fulminant hepatitis associated with consumption of the food supplement Slim Metabol® | Garcinia cambogia |
| 19 June 2020 | ANSES OPINION on two cases of severe acute life-threatening hepatitis associated with consumption of the food supplement Chewable Hair Vitamins® | Ingredient not determined |
| 26 January 2021 | ANSES OPINION regarding oesophageal perforation associated with the consumption of the food supplement Préservion 3 capsules | Large capsule |
| 28 July 2021 | ANSES OPINION regarding vitamin D poisoning in infants following misuse of food supplements | Vitamin D |
| 4 October 2021 | ANSES OPINION regarding epileptic seizures associated with consumption of the food supplement Novanuit® Triple Action | Melatonin + |
| US | UE | PL | |
|---|---|---|---|
| KEY WORD | Melatonin | Melatonin | Melatonin * |
| NUMBER OF REGISTERED DS | 1696 | 786 | |
| NUMBER OF CASES | 463 | 2658 | |
| AGE GROUP | |||
| Not Specified | 13.6% | 22.2% | |
| 0–1 Month | 0.3% | ||
| 2 Months–2 Years | 0.4% | 0.6% | |
| 3–11 Years | 4.1% | 10.5% | |
| 12–17 Years | 2.4% | 9.2% | |
| 18–64 Years | 51.2% | 37.7% | |
| 65–85 Years | 25.9% | 16.3% | |
| More than 85 Years | 2.4% | 3.2% | |
| SEX | |||
| Female | 67.6% | 57.8% | |
| Male | 28.5% | 38.3% | |
| Not Specified | 3.9% | 3.8% | |
| REPORTER GROUP | |||
| Healthcare Professional | 65.2% | ||
| Non-Healthcare Professional | 34.7% | ||
| Not Specified | 100% | 0.1% | |
Key Word | US Liquorice | UE Glycyrrhiza Glabra L. Radix | PL Lukrecj * |
|---|---|---|---|
| NUMBER OF REGISTERED DS | 3091 | 1191 | |
| NUMBER OF CASES | 27 | 234 | |
| AGE GROUP | |||
| Not Specified | 22.2% | 2.1% | |
| 0–1 Month | |||
| 2 Months–2 Years | 0.9% | ||
| 3–11 Years | 3.7% | 1.7% | |
| 12–17 Years | 1.3% | ||
| 18–64 Years | 44.4% | 55.1% | |
| 65–85 Years | 29.6% | 32.9% | |
| More than 85 Years | 6.0% | ||
| SEX | |||
| Female | 70.4% | 56.4% | |
| Male | 25.9% | 42.7% | |
| Not Specified | 3.7% | 0.9% | |
| REPORTER GROUP | |||
| Healthcare Professional | 93.6% | ||
| Non-Healthcare Professional | 5.6% | ||
| Not Specified | 100% | 0.9% | |
| US | UE | PL | |
|---|---|---|---|
| Key Word | Green Tea | Camellia Sinensis * | Zielon * + Herbat * |
| NUMBER OF REGISTERED DS | 6259 | 3595 | |
| NUMBER OF CASES | 223 | 171 | |
| AGE GROUP | |||
| Not Specified | 24.7% | 21.6% | |
| 0–1 Month | |||
| 2 Months–2 Years | 1.9% | ||
| 3–11 Years | |||
| 12–17 Years | 0.9% | 0.6% | |
| 18–64 Years | 55.6% | 70.2% | |
| 65–85 Years | 18.4% | 7.6% | |
| More than 85 Years | 0.4% | ||
| SEX | |||
| Female | 65.9% | 60.8% | |
| Male | 31.8% | 35.7% | |
| Not Specified | 2.2% | 3.5% | |
| REPORTER GROUP | |||
| Healthcare Professional | 69.6% | ||
| Non-Healthcare Professional | 29.8% | ||
| Not Specified | 100% | 0.6% | |
| US | UE | PL | |
|---|---|---|---|
| Key Word | Glucosamine * | Glucosamine * | Glukozamin * |
| NUMBER OF REGISTERED DS | 3221 | 1408 | |
| NUMBER OF CASES | 673 | 1543 | |
| AGE GROUP | |||
| Not Specified | 14.7% | 16.3% | |
| 0–1 Month | 0.1% | ||
| 2 Months–2 Years | 0.4% | ||
| 3–11 Years | 0.6% | ||
| 12–17 Years | 0.6% | ||
| 18–64 Years | 33.9% | 33.1% | |
| 65–85 Years | 44.9% | 45.4% | |
| More than 85 Years | 6.5% | 3.5% | |
| SEX | |||
| Female | 64.2% | 70.8% | |
| Male | 34.2% | 26.9% | |
| Not Specified | 1.6% | 2.3% | |
| REPORTER GROUP | |||
| Healthcare Professional | 72.10% | ||
| Non-Healthcare Professional | 27.50% | ||
| Not Specified | 100% | 0.40% | |
| US | UE | PL | |
|---|---|---|---|
| Key Word | Echinacea * | Echinacea * | Jeżówk * |
| NUMBER OF REGISTERED DS | 2901 | 498 | |
| NUMBER OF CASES | 82 | 347 | |
| AGE GROUP | |||
| Not Specified | 15.9% | 15.9% | |
| 0–1 Month | 0.3% | ||
| 2 Months–2 Years | 1.2% | 3.7% | |
| 3–11 Years | 3.7% | 4.9% | |
| 12–17 Years | 2.0% | ||
| 18–64 Years | 43.9% | 57.6% | |
| 65–85 Years | 32.9% | 14.7% | |
| More than 85 Years | 2.4% | 0.9% | |
| SEX | |||
| Female | 65.9% | 70.0% | |
| Male | 34.1% | 27.4% | |
| Not Specified | 2.6% | ||
| REPORTER GROUP | |||
| Healthcare Professional | 68.6% | ||
| Non-Healthcare Professional | 29.1% | ||
| Not Specified | 100% | 2.3% | |
| US | UE | PL | |
|---|---|---|---|
| Key Word | Chondroitin | Chondroitin * | Chondroityn * |
| NUMBER OF REGISTERED DS | 1713 | 1120 | |
| NUMBER OF CASES | 362 | 758 | |
| AGE GROUP | |||
| Not Specified | 14.4% | 20.1% | |
| 0–1 Month | |||
| 2 Months–2 Years | 0.4% | ||
| 3–11 Years | 0.4% | ||
| 12–17 Years | 0.7% | ||
| 18–64 Years | 32.0% | 35.4% | |
| 65–85 Years | 46.1% | 39.1% | |
| More than 85 Years | 7.5% | 4.1% | |
| SEX | |||
| Female | 68.8% | 66.6% | |
| Male | 37.6% | 27.6% | |
| Not Specified | 1.7% | 5.8% | |
| REPORTER GROUP | |||
| Healthcare Professional | 62.3% | ||
| Non-Healthcare Professional | 36.8% | ||
| Not Specified | 100% | 0.9% | |
| US | UE | PL | |
|---|---|---|---|
| Key Word | Black Cohosh | Cimicifuga * | Pluskwic * |
| NUMBER OF REGISTERED DS | 947 | 96 | |
| NUMBER OF CASES | 85 | 558 | |
| AGE GROUP | |||
| Not Specified | 20.0% | 13.6% | |
| 0–1 Month | 0.2% | ||
| 2 Months–2 Years | |||
| 3–11 Years | |||
| 12–17 Years | 0.4% | ||
| 18–64 Years | 67.1% | 79.4% | |
| 65–85 Years | 12.9% | 6.3% | |
| More than 85 Years | 0.2% | ||
| SEX | |||
| Female | 95.3% | 96.8% | |
| Male | 1.8% | ||
| Not Specified | 4.7% | 1.4% | |
| REPORTER GROUP | |||
| Healthcare Professional | 66.7% | ||
| Non-Healthcare Professional | 32.4% | ||
| Not Specified | 100% | 0.9% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, K.; Zastawna, B.; Milewska, A.J.; Marczak, M.; Kozłowski, R. Comparison between the American and the European Systems of Monitoring Adverse Effects of Dietary Supplements and Their Usefulness on the Polish Market. Int. J. Environ. Res. Public Health 2023, 20, 902. https://doi.org/10.3390/ijerph20020902
Wróbel K, Zastawna B, Milewska AJ, Marczak M, Kozłowski R. Comparison between the American and the European Systems of Monitoring Adverse Effects of Dietary Supplements and Their Usefulness on the Polish Market. International Journal of Environmental Research and Public Health. 2023; 20(2):902. https://doi.org/10.3390/ijerph20020902
Chicago/Turabian StyleWróbel, Kacper, Beata Zastawna, Anna Justyna Milewska, Michał Marczak, and Remigiusz Kozłowski. 2023. "Comparison between the American and the European Systems of Monitoring Adverse Effects of Dietary Supplements and Their Usefulness on the Polish Market" International Journal of Environmental Research and Public Health 20, no. 2: 902. https://doi.org/10.3390/ijerph20020902






