Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection and Measurements
2.3. DNA Extraction from Fecal Samples and 16S rRNA Gene Sequencing
2.4. Measurement of SCFA in Fecal Samples
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Participants
3.2. Gut Microbial Diversity within and between FMS and the Control Groups
3.3. Abundance of Microbial Composition and FMS
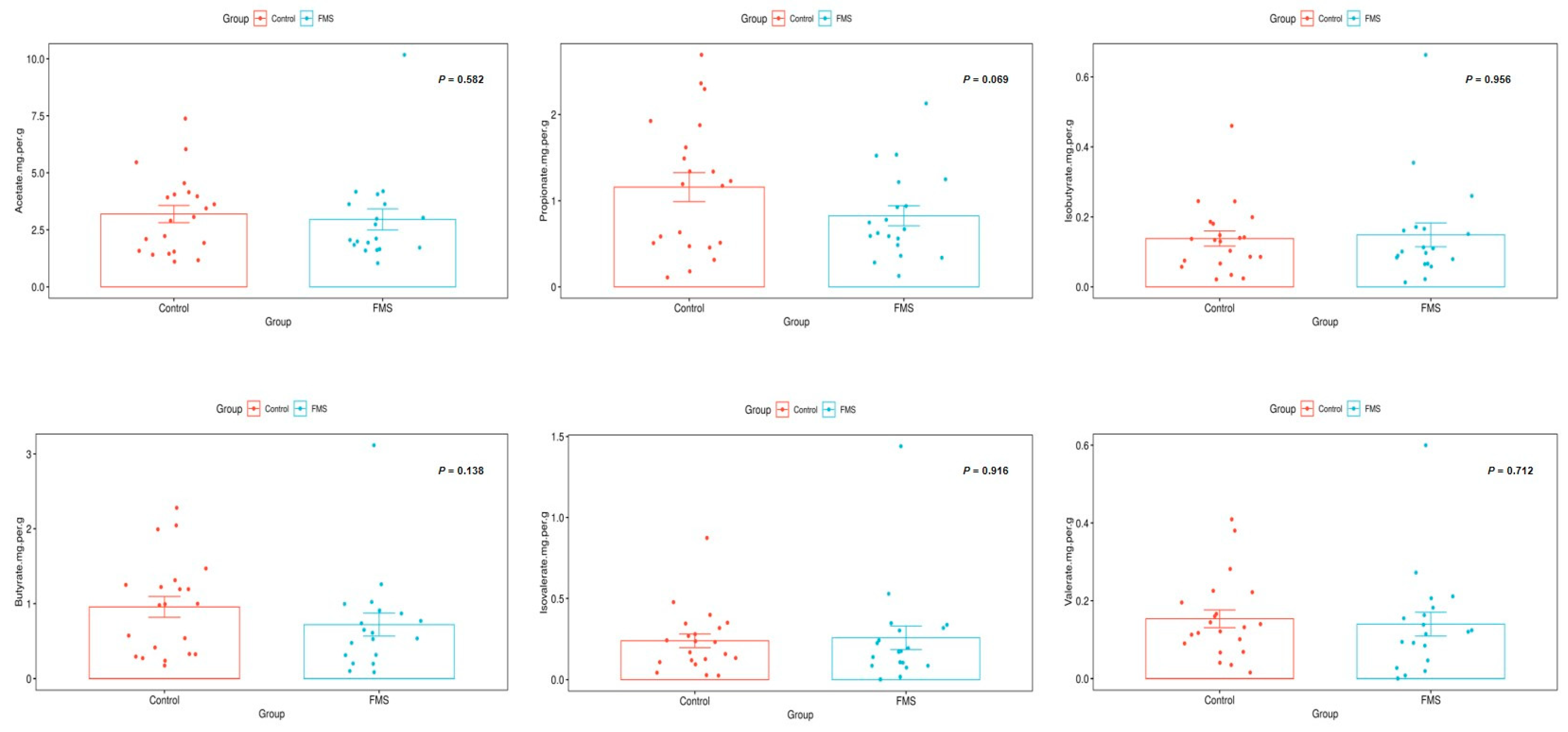
3.4. Association of Stool SCFA and FMS
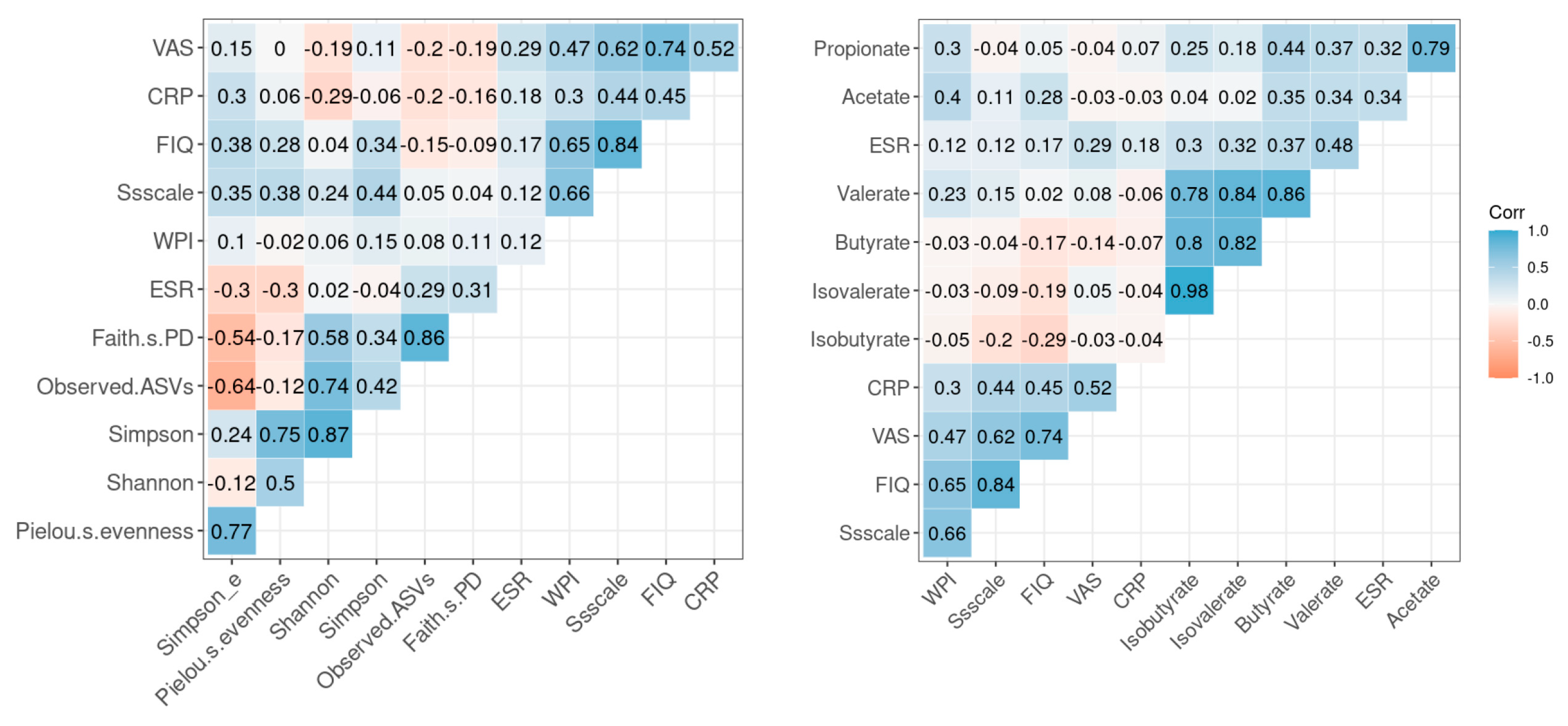
3.5. Correlation between Inflammatory Markers, Symptomatic Scales of FMS and Microbial Diversity and SCFA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, F. New American College of Rheumatology criteria for fibromyalgia: A twenty-year journey. Arthritis Care Res. 2010, 62, 583–584. [Google Scholar] [CrossRef]
- Queiroz, L.P. Worldwide Epidemiology of Fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.-A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.-H.; Zhao, J.; Lai, W.-T.; Wang, M.-B.; Xu, D.; Liu, Y.-H.; Guo, Y.-Y.; Xu, S.-X.; Deng, W.-F.; et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep Med. 2021, 87, 1–7. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to brain dysbiosis: Mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andres-Marin, N.; Fernandez-Eulate, G.; Abecia, L.; Lavin, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.; Tatzl, E.; Kashofer, K.; Holter, M.; Trajanoski, S.; Berghold, A.; Heinemann, A.; Holzer, P.; Herbert, M.K. Fibromyalgia-associated hyperalgesia is related to psychopathological alterations but not to gut microbiome changes. PLoS ONE 2022, 17, e0274026. [Google Scholar] [CrossRef] [PubMed]
- Conteh, A.R.; Huang, R. Targeting the gut microbiota by Asian and Western dietary constituents: A new avenue for diabetes. Toxicol. Res. 2020, 9, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sarmer, S.; Ergin, S.; Yavuzer, G. The validity and reliability of the Turkish version of the Fibromyalgia Impact Questionnaire. Rheumatol. Int. 2000, 20, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yun, K.E.; Chu, J.M.; Lee, J.Y.; Hong, C.P.; Nam, Y.D.; Jeong, J.; Han, K.; Ahn, Y.J. Performance comparison of fecal preservative and stock solutions for gut microbiome storage at room temperature. J. Microbiol. 2020, 58, 703–710. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). Wiley Statsref Stat. Ref. Online 2014, 1–15. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466.e454. [Google Scholar] [CrossRef]
- Michielan, A.; D’Incà, R. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Bengelsdorf, F.R.; Poehlein, A.; Daniel, R.; Dürre, P. Genome sequence of the caproic acid-producing bacterium Caproiciproducens galactitolivorans BS-1T (JCM 30532). Microbiol. Resour. Announc. 2019, 8, e00346-19. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Shorten, G.D.; O’Mahony, S.M. Postoperative pain and the gut microbiome. Neurobiol. Pain 2021, 10, 100070. [Google Scholar] [CrossRef] [PubMed]
- Studer, N.; Desharnais, L.; Beutler, M.; Brugiroux, S.; Terrazos, M.A.; Menin, L.; Schürch, C.M.; McCoy, K.D.; Kuehne, S.A.; Minton, N.P. Functional intestinal bile acid 7α-dehydroxylation by Clostridium scindens associated with protection from Clostridium difficile infection in a gnotobiotic mouse model. Front. Cell. Infect. Microbiol. 2016, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef]
- Rodríguez, D.F.G.; Mendoza, C.A. Fisiopatología de la fibromialgia. Reumatol. Clin. 2020, 16, 191–194. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park. Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [Green Version]
- Karen, C.; Shyu, D.J.; Rajan, K.E. Lactobacillus paracasei supplementation prevents early life stress-induced anxiety and depressive-like behavior in maternal separation model-possible involvement of microbiota-gut-brain axis in differential regulation of microrna124a/132 and glutamate receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [PubMed]
- Chen, J.; Wang, Q.; Wang, A.; Lin, Z. Structural and functional characterization of the gut microbiota in elderly women with migraine. Front. Cell. Infect. Microbiol. 2020, 9, 470. [Google Scholar] [CrossRef]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Ivashkin, V.; Zolnikova, O.; Potskherashvili, N.; Trukhmanov, A.; Kokina, N.; Dzhakhaya, N.; Sedova, A.; Bueverova, E. Metabolic activity of intestinal microflora in patients with bronchial asthma. Clin. Pract. 2019, 9, 1126. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; He, F.; Zuo, D.; Li, F.; Fan, H.; Yin, Z.; Liang, H.; Li, Q. Sodium propionate improves rheumatoid arthritis by inhibiting survivin mediated proliferation of fibroblast like synoviocytes by promoting miR-140-5p. Autoimmunity 2022, 55, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s disease. Front. Aging Neurosci. 2021, 12, 580001. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Shi, H.; Xu, Y.; Ji, L. The gut microbiota metabolite propionate ameliorates intestinal epithelial barrier dysfunction-mediated Parkinson’s disease via the AKT signaling pathway. Neuroreport 2021, 32, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wang, T.-Y.; Zhao, C.; Wang, H.-J. Oxidative stress bridges the gut microbiota and the occurrence of frailty syndrome. World J. Gastroenterol. 2022, 28, 5547. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Bonfiglio, F.; Belheouane, M.; Vallier, M.; Sauer, S.; Bang, C.; Bujanda, L.; Andreasson, A.; Agreus, L.; Engstrand, L.; et al. Faecal microbiota composition associates with abdominal pain in the general population. Gut 2018, 67, 778–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Control (n = 21) | FMS (n = 19) | p-Value | |
|---|---|---|---|
| Age | 46.6 (8.7) | 51.4 (7.4) | 0.070 |
| BMI | 22.1 (2.7) | 27.8 (4.8) | <0.001 |
| Smoking status | 0.043 | ||
| Non-smokers | 21 (100.0) | 14 (3.7) | |
| Former smokers | 0 (0) | 1 (5.3) | |
| Current smokers | 0 (0) | 4 (21.1) | |
| Hypertension | 0.085 | ||
| Yes | 20 (95.1) | 14 (73.7) | |
| No | 1 (4.8) | 5 (26.3) | |
| Diabetes | 1.000 | ||
| Yes | 19 (90.5) | 17 (89.5) | |
| No | 2 (9.5) | 2 (10.5) | |
| Dyslipidemia | 0.003 | ||
| Yes | 0 (0) | 7 (36.8) | |
| No | 21 (100) | 12 (63.2) | |
| Total cholesterol | 187.2 (39.1) | 187.1 (34.1) | 0.991 |
| HDL-cholesterol | 62.9 (15.4) | 52.9 (12.8) | 0.031 |
| LDL-cholesterol | 118.2 (35.9) | 114.7 (17.3) | 0.698 |
| Triglyceride | 75.5 (32.9) | 182.2 (67.6) | <0.001 |
| ESR | 16.5 (13.9) | 19.9 (15.2) | 0.467 |
| CRP | 0.1 (0.2) | 0.2 (0.3) | 0.106 |
| WPI | 11.2 (5.2) | ||
| Symptom severity scale | 8.0 (2.6) | ||
| VAS | 5.9 (1.9) | ||
| FIQ | 61.5 (24.9) |
| Coefficient | SE | W-Value | p-Value | |
|---|---|---|---|---|
| Eubacterium eligens group | −3.67 | 0.84 | −4.36 | <0.001 |
| Eubacterium ruminantium group | −1.41 | 0.74 | −1.91 | <0.001 |
| Alloprevotella | −1.07 | 0.76 | −1.41 | <0.001 |
| Acinetobacter | −0.49 | 0.38 | −1.28 | <0.001 |
| Rikenellaceae RC9 gut group | −0.93 | 0.75 | −1.25 | <0.001 |
| Coprobacter | −0.81 | 0.66 | −1.23 | <0.001 |
| Adlercreutzia | −0.53 | 0.47 | −1.15 | <0.001 |
| Methanobrevibacter | −0.36 | 0.39 | −0.93 | <0.001 |
| Anaerococcus | −0.32 | 0.41 | −0.78 | <0.001 |
| Peptococcus | −0.26 | 0.46 | −0.56 | <0.001 |
| Butyricicoccaceae | −0.23 | 0.43 | −0.54 | <0.001 |
| Enterorhabdus | −0.14 | 0.32 | −0.44 | <0.001 |
| Allisonella | −0.19 | 0.45 | −0.43 | <0.001 |
| Gastranaerophilales | −0.33 | 0.84 | −0.39 | <0.001 |
| Methylobacterium-Methylorubrum | −0.13 | 0.33 | −0.38 | <0.001 |
| Granulicatella | −0.15 | 0.44 | −0.35 | <0.001 |
| Terrisporobacter | −0.18 | 0.54 | −0.33 | <0.001 |
| Gemella | −0.15 | 0.46 | −0.32 | <0.001 |
| Finegoldia | −0.11 | 0.43 | −0.27 | <0.001 |
| Campylobacter | −0.09 | 0.39 | −0.23 | <0.001 |
| Parvimonas | −0.07 | 0.38 | −0.19 | <0.001 |
| Prevotellaceae UCG-001 | −0.05 | 0.33 | −0.16 | <0.001 |
| Peptoniphilus | 0.01 | 0.40 | 0.03 | <0.001 |
| Porphyromonas | 0.03 | 0.28 | 0.10 | <0.001 |
| Ornithobacterium | 0.08 | 0.32 | 0.24 | <0.001 |
| Corynebacterium | 0.12 | 0.47 | 0.25 | <0.001 |
| Fenollaria | 0.10 | 0.32 | 0.31 | <0.001 |
| Staphylococcus | 0.15 | 0.44 | 0.35 | <0.001 |
| Actinomyces | 0.14 | 0.36 | 0.40 | <0.001 |
| Fournierella | 0.23 | 0.45 | 0.50 | <0.001 |
| Tuzzerella | 0.25 | 0.44 | 0.57 | <0.001 |
| Faecalitalea | 0.42 | 0.67 | 0.63 | <0.001 |
| Coprobacillus | 0.41 | 0.63 | 0.65 | <0.001 |
| Succinivibrio | 0.48 | 0.71 | 0.67 | <0.001 |
| Lachnospiraceae NC2004 group | 0.29 | 0.37 | 0.78 | <0.001 |
| Slackia | 0.39 | 0.47 | 0.84 | <0.001 |
| Victivallis | 0.43 | 0.48 | 0.89 | <0.001 |
| Clostridia | 0.35 | 0.33 | 1.06 | <0.001 |
| Eubacterium fissicatena group | 0.44 | 0.42 | 1.06 | <0.001 |
| Howardella | 0.64 | 0.60 | 1.08 | <0.001 |
| Megasphaera | 1.21 | 0.79 | 1.54 | <0.001 |
| Paludicola | 1.30 | 0.50 | 2.60 | <0.001 |
| Catenibacillus | 1.23 | 0.44 | 2.77 | <0.001 |
| Eisenbergiella | 2.00 | 0.71 | 2.81 | <0.001 |
| Caproiciproducens | 1.14 | 0.39 | 2.92 | <0.001 |
| Frisingicoccus | 1.89 | 0.63 | 2.98 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, G.-T.; Kang, J. Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia. Int. J. Environ. Res. Public Health 2023, 20, 3183. https://doi.org/10.3390/ijerph20043183
Kim Y, Kim G-T, Kang J. Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia. International Journal of Environmental Research and Public Health. 2023; 20(4):3183. https://doi.org/10.3390/ijerph20043183
Chicago/Turabian StyleKim, Yunkyung, Geun-Tae Kim, and Jihun Kang. 2023. "Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia" International Journal of Environmental Research and Public Health 20, no. 4: 3183. https://doi.org/10.3390/ijerph20043183
APA StyleKim, Y., Kim, G.-T., & Kang, J. (2023). Microbial Composition and Stool Short Chain Fatty Acid Levels in Fibromyalgia. International Journal of Environmental Research and Public Health, 20(4), 3183. https://doi.org/10.3390/ijerph20043183







