An Assessment of the Ocular Toxicity of Two Major Sources of Environmental Exposure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, J.; Bessa, M.J.; Delerue-Matos, C.; Sarmento, B.; Santos-Silva, A.; Rodrigues, F.; Oliveira, M. Firefighters’ personal exposure to gaseous PAHs during controlled forest fires: A case study with estimation of respiratory health risks and in vitro toxicity. Sci. Total Environ. 2024, 908, 168364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Doherty, T.A.; James, C. Military burn pit exposure and airway disease: Implications for our Veteran population. Ann. Allergy Asthma Immunol. 2023, 131, 720–725. [Google Scholar] [CrossRef]
- Davis, C.W.; Rabin, A.S.; Jani, N.; Osterholzer, J.J.; Krefft, S.; Hines, S.E.; Arjomandi, M.; Robertson, M.W.; Sotolongo, A.M.; Falvo, M.J. Post-Deployment Cardiopulmonary Evaluation Network. Postdeployment Respiratory Health: The Roles of the Airborne Hazards and Open Burn Pit Registry and the Post-Deployment Cardiopulmonary Evaluation Network. Fed. Pract. 2022, 39, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Asbell, P.; Dogru, M.; Giannaccare, G.; Grau, A.; Gregory, D.; Kim, D.H.; Marini, M.C.; Ngo, W.; Nowinska, A.; et al. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul. Surf. 2023, 29, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, H.; Liu, G.; Tang, Y.; Li, W. In silico prediction of chemical aquatic toxicity by multiple machine learning and deep learning approaches. J. Appl. Toxicol. 2022, 42, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Manyepa, P.; Gani, K.M.; Seyam, M.; Banoo, I.; Genthe, B.; Kumari, S.; Bux, F. Removal and risk assessment of emerging contaminants and heavy metals in a wastewater reuse process producing drinkable water for human consumption. Chemosphere 2024, 361, 142396. [Google Scholar] [CrossRef]
- Biggi, G.; Giuliani, E.; Martinelli, A.; Benfenati, E. Patent Toxicity. Res. Policy 2022, 51, 104329. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Valadares, M.C.; de Oliveira, G.A.R.; de Ávila, R.I.; da Silva, A.C.G. Strategy Combining Nonanimal Methods for Ocular Toxicity Evaluation. Methods Mol. Biol. 2021, 2240, 175–195. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Klausner, M. In vitro reconstructed 3D corneal tissue models for ocular toxicology and ophthalmic drug development. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 207–237. [Google Scholar] [CrossRef]
- Jiménez Barbosa, I.A.; Rodríguez Alvarez, M.F.; Dussán Torres, G.A.; Khuu, S.K. Ocular surface and tear film changes in workers exposed to organic solvents used in the dry-cleaning industry. PLoS ONE 2019, 14, e0226042. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Vitoux, M.A.; Kessal, K.; Baudouin, C.; Laprévote, O.; Melik Parsadaniantz, S.; Achard, S.; Brignole-Baudouin, F. Formaldehyde Gas Exposure Increases Inflammation in an In Vitro Model of Dry Eye. Toxicol. Sci. 2018, 165, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Hidalgo, A.; Moore, A.L.; Ghafourian, T. Prediction of drug-induced mitochondrial dysfunction using succinate-cytochrome c reductase activity, QSAR and molecular docking. Toxicology 2023, 485, 153412. [Google Scholar] [CrossRef] [PubMed]
- Luechtefeld, T.; Maertens, A.; Russo, D.P.; Rovida, C.; Zhu, H.; Hartung, T. Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data. ALTEX 2016, 33, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Japertas, P.; Didziapetris, R.; Sazonovas, A. A rule based approach for prediction of the rabbit eye and skin irritation. Toxicol. Lett. 2007, 172, S80. [Google Scholar] [CrossRef]
- European Chemicals Inventory. Available online: https://echa.europa.eu/information-on-chemicals/ec-inventory (accessed on 1 June 2023).
- The Registry of Toxic Effects of Chemical Substances (RTECS). Available online: https://www.cdc.gov/niosh/docs/97-119/default.html (accessed on 1 June 2023).
- Szymański, P.; Skibiński, R.; Inglot, T.; Bajda, M.; Jończyk, J.; Malawska, B.; Mikiciuk-Olasik, E. New tacrine analogs as acetylcholinesterase inhibitors—Theoretical study with chemometric analysis. Molecules 2013, 8, 2878–2894. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.I.; Mansouri, K.; Phillips, K.A.; Grulke, C.M.; Richard, A.M.; Williams, A.J.; Rabinowitz, J.; Isaacs, K.K.; Yau, A.; Wambaugh, J.F. Rapid experimental measurements of physicochemical properties to inform models and testing. Sci. Total Environ. 2018, 636, 901–909. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6372, Chlorodifluoromethane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorodifluoromethane (accessed on 11 April 2024).
- Gupta, S.; Martin, L.M.; Zhang, E.; Sinha, P.R.; Landreneau, J.; Sinha, N.R.; Hesemann, N.P.; Mohan, R.R. Toxicological effects of ocular acrolein exposure to eyelids in rabbits in vivo. Exp. Eye Res. 2023, 234, 109575. [Google Scholar] [CrossRef]
- Ilhan, A.; Yolcu, U.; Uzun, S. Acute and long-term ocular effects of acrolein vapor on the eyes and potential therapies. Cutan. Ocul. Toxicol. 2016, 35, 87. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, B.; Gazzard, M.F.; Swanston, D.W. The ophthalmic toxicology of dichlorøomethane. Toxicology 1976, 6, 173–187. [Google Scholar] [CrossRef]
- Modi, Y.S.; Qurban, Q.; Zlotcavitch, L.; Echeverri, R.J.; Feuer, W.; Florez, H.; Galor, A. Ocular surface symptoms in veterans returning from operation Iraqi freedom and operation enduring freedom. Investig. Ophthalmol. Vis. Sci. 2014, 55, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Sun, W. The Devastating Health Consequences of the Ohio Derailment: A Closer Look at the Effects of Vinyl Chloride Spill. Int. J. Environ. Res. Public Health 2023, 20, 5032. [Google Scholar] [CrossRef] [PubMed]
- Vinyl Chloride: Toxicological Overview. Available online: https://assets.publishing.service.gov.uk/media/5a7dcf25ed915d2ac884daf8/hpa_vinyl_chloride_toxicological_overview_v1.pdf (accessed on 10 March 2024).
- Bashir, H.; Mahalwar, G.; Henry, T. The East Palestine Disaster: The Potential Toxic Effects of Vinyl Chloride Exposure on Cardiovascular Health. Cureus 2023, 15, e46835. [Google Scholar] [CrossRef]
- Bonneau, N.; Potey, A.; Vitoux, M.A.; Magny, R.; Guerin, C.; Baudouin, C.; Peyrin, J.M.; Brignole-Baudouin, F.; Réaux-Le Goazigo, A. Corneal neuroepithelial compartmentalized microfluidic chip model for evaluation of toxicity-induced dry eye. Ocul. Surf. 2023, 30, 307–319. [Google Scholar] [CrossRef]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy. Public Health Research and Surveillance Priorities from the East Palestine Train Derailment: Proceedings of a Workshop—In Brief; Wollek, S., Masiello, M., Snair, J., Eds.; National Academies Press: Washington, DC, USA, 2024. [Google Scholar]
- Sanchez, V.; Baksh, B.S.; Cabrera, K.; Choudhury, A.; Jensen, K.; Klimas, N.; Galor, A. Dry Eye Symptoms and Signs in US Veterans with Gulf War Illness. Am. J. Ophthalmol. 2022, 237, 32–40. [Google Scholar] [CrossRef]
- Berra, M.; Galperín, G.; Dawidowski, L.; Tau, J.; Márquez, I.; Berra, A. Impact of wildfire smoke in Buenos Aires, Argentina, on ocular surface. Arq. Bras. Oftalmol. 2015, 78, 110–114. [Google Scholar] [CrossRef]
- Patel, S.; Mittal, R.; Kumar, N.; Galor, A. The environment and dry eye-manifestations, mechanisms, and more. Front. Toxicol. 2023, 5, 1173683. [Google Scholar] [CrossRef]
- Latham, S.G.; Williams, R.L.; Grover, L.M.; Rauz, S. Achieving net-zero in the dry eye disease care pathway. Eye 2024, 38, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Heilenbach, N.; Hu, G.; Lamrani, R.; Prasad, J.; Ogunsola, T.; Iskander, M.; Elgin, C.Y.; McGowan, R.; Vieira, D.; Al-Aswad, L.A. Environmental influences on ophthalmic conditions: A scoping review. Clin. Exp. Ophthalmol. 2023, 51, 516–545. [Google Scholar] [CrossRef] [PubMed]
- McCann, P.; Kruoch, Z.; Lopez, S.; Malli, S.; Qureshi, R.; Li, T. Interventions for Dry Eye: An Overview of Systematic Reviews. JAMA Ophthalmol. 2024, 142, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ucakhan, O.O.; Celik-Buyuktepe, T.; Yang, L.; Wogu, B.; Asbell, P.A. Update on Dry Eye Disease Treatment: Evidence from Randomized Controlled Trials. Eye Contact Lens 2023, 49, 542–568. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, K.L.; Balmer, J. Airborne Hazards and Open Burn Pit Exposures. Workplace Health Saf. 2023, 71, 352. [Google Scholar] [CrossRef]
- Van De Graaff, J.; Poole, J.A. A Clinician’s Guide to Occupational Exposures in the Military. Curr. Allergy Asthma Rep. 2022, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bith-Melander, P.; Ratliff, J.; Poisson, C.; Jindal, C.; Ming Choi, Y.; Efird, J.T. Slow Burns: A Qualitative Study of Burn Pit and Toxic Exposures Among Military Veterans Serving in Afghanistan, Iraq and Throughout the Middle East. Ann. Psychiatry Clin. Neurosci. 2021, 4, 1042. [Google Scholar] [PubMed]
- Woskie, S.R.; Bello, A.; Rennix, C.; Jiang, L.; Trivedi, A.N.; Savitz, D.A. Burn Pit Exposure Assessment to Support a Cohort Study of US Veterans of the Wars in Iraq and Afghanistan. J. Occup. Environ. Med. 2023, 65, 449–457. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee to Reassess the Department of Veterans Affairs Airborne Hazards and Open Burn Pit Registry. Reassessment of the Department of Veterans Affairs Airborne Hazards and Open Burn Pit Registry; National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Toxic Exposure Table (In Reference to VA 10-03). Available online: https://cswab.org/wp-content/uploads/2021/02/Health-Effects-Service-Member-Environmental-Exposures10-03-Burn-Pits-360-April-2020.pdf (accessed on 17 March 2024).
- Woodall, B.D.; Yamamoto, D.P.; Gullett, B.K.; Touati, A. Emissions from small-scale burns of simulated deployed U.S. military waste. Environ. Sci. Technol. 2012, 46, 10997–11003. [Google Scholar] [CrossRef]
- Oladeji, O.; Saitas, M.; Mustapha, T.; Johnson, N.M.; Chiu, W.A.; Rusyn, I.; Robinson, A.L.; Presto, A.A. Air Pollutant Patterns and Human Health Risk following the East Palestine, Ohio, Train Derailment. Environ. Sci. Technol. Lett. 2023, 10, 680–685. [Google Scholar] [CrossRef]
- Nogueira, L.M.; Sherman, J.D.; Shultz, J.M. Derailing Carcinogens-Oncologists and the Ohio Train Derailment. JAMA Oncol. 2024, 10, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Motria, C. CDC/ATSDR Assessment of Chemical Exposures (ACE)/Epi Aid Investigation East Palestine Train Derailment. 6 November 2023. Available online: https://stacks.cdc.gov/view/cdc/147412 (accessed on 15 April 2024).
- Neimark, J. Experts weigh in on potential health hazards posed by chemicals in Ohio train derailment. STAT, 21 February 2023. Available online: https://www.statnews.com/2023/02/21/east-palestine-train-chemicals/(accessed on 17 March 2024).
- East Palestine, Ohio Train Derailment Air Monitoring and Sampling Data. Available online: https://www.epa.gov/east-palestine-oh-train-derailment/air-sampling-data (accessed on 17 March 2024).
- Dutescu, R.M.; Uthoff, D.; Panfil, C.; Schrage, N. High-frequency application of cationic agents containing lubricant eye drops causes cumulative corneal toxicity in an ex vivo eye irritation test model. J. Ocul. Pharmacol. Ther. 2020, 36, 725–731. [Google Scholar] [CrossRef]
- Wilson, S.L.; Ahearne, M.; Hopkinson, A. An overview of current techniques for ocular toxicity testing. Toxicology 2015, 327, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Rauchman, S.H.; Locke, B.; Albert, J.; De Leon, J.; Peltier, M.R.; Reiss, A.B. Toxic External Exposure Leading to Ocular Surface Injury. Vision 2023, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Lieto, K.; Skopek, R.; Lewicka, A.; Stelmasiak, M.; Klimaszewska, E.; Zelent, A.; Szymański, Ł.; Lewicki, S. Looking into the Eyes-In Vitro Models for Ocular Research. Int. J. Mol. Sci. 2022, 23, 9158. [Google Scholar] [CrossRef]
- Adriaens, E.; Barroso, J.; Eskes, C.; Hoffmann, S.; McNamee, P.; Alépée, N.; Bessou-Touya, S.; De Smedt, A.; De Wever, B.; Pfannenbecker, U.; et al. Retrospective analysis of the Draize test for serious eye damage/eye irritation: Importance of understanding the in vivo endpoints under UN GHS/EU CLP for the development and evaluation of in vitro test methods. Arch. Toxicol. 2014, 88, 701–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hwang, J.H.; Lim, K.M. Alternatives to In Vivo Draize Rabbit Eye and Skin Irritation Tests with a Focus on 3D Reconstructed Human Cornea—Like Epithelium and Epidermis Models. Toxicol. Res. 2017, 33, 191–203. [Google Scholar] [CrossRef]
- Sedykh, A.; Choksi, N.Y.; Allen, D.G.; Casey, W.M.; Shah, R.; Kleinstreuer, N.C. Mixtures-Inclusive In Silico Models of Ocular Toxicity Based on United States and International Hazard Categories. Chem. Res. Toxicol. 2022, 35, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, N.; Baudouin, C.; Réaux-Le Goazigo, A.; Brignole-Baudouin, F. An overview of current alternative models in the context of ocular surface toxicity. J. Appl. Toxicol. 2022, 42, 718–737. [Google Scholar] [CrossRef]
- Rim, K.T. In silico prediction of toxicity and its applications for chemicals at work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef]
- Dracheva, E.; Norinder, U.; Rydén, P.; Engelhardt, J.; Weiss, J.M.; Andersson, P.L. In Silico Identification of Potential Thyroid Hormone System Disruptors among Chemicals in Human Serum and Chemicals with a High Exposure Index. Environ. Sci. Technol. 2022, 56, 8363–8372. [Google Scholar] [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Khalil, A.A.; El Sheikh, R.H.; Bakr, M.A.; Eissa, M.G.; El Sayed, Y.M. New approaches for diagnosis of dry eye disease. Int. J. Ophthalmol. 2019, 12, 1618–1628. [Google Scholar] [CrossRef]
- Roy, N.S.; Wei, Y.; Ying, G.S.; Maguire, M.G.; Asbell, P.A.; Dry Eye Assessment and Management (DREAM) Study Group. Association of tear cytokine concentrations with symptoms and signs of dry eye disease: Baseline data from the Dry Eye Assessment and Management (DREAM) Study. Curr. Eye Res. 2023, 48, 339–347. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease, an immune mediated ocular surface disorder. Arch. Ophthalol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Araj, H.; Tumminia, S.J.; Yeung, D.T. Ocular Surface—Merging Challenges and Opportunities. Transl. Vis. Sci. Technol. 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, A.A.; Ting, D.S.J.; Figueiredo, F.C. Epidemiology, economic and humanistic burdens of Ocular Surface Chemical Injury: A narrative review. Ocul. Surf. 2021, 20, 199–211. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Snieder, H.; Hammond, C.J.; Vehof, J. The physical and mental burden of dry eye disease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul. Surf. 2021, 21, 107–117. [Google Scholar] [CrossRef]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- Chen, E.M.; Kombo, N.; Teng, C.C.; Mruthyunjaya, P.; Nwanyanwu, K.; Parikh, R. Ophthalmic Medication Expenditures and Out-of-Pocket Spending: An Analysis of United States Prescriptions from 2007 through 2016. Ophthalmology 2020, 127, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Luo, Y.; Wu, S.; Niu, X.; Yan, Y.; Qiao, C.; Ming, W.; Zhang, Y.; Wang, H.; Chen, D.; et al. Estimated Annual Economic Burden of Dry Eye Disease Based on a Multi-Center Analysis in China: A Retrospective Study. Front. Med. 2021, 8, 771352. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Hammond, C.J.; Vehof, J. The work-related burden of dry eye. Ocul. Surf. 2023, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Dana, R.; Meunier, J.; Markowitz, J.T.; Joseph, C.; Siffel, C. Patient-Reported Burden of Dry Eye Disease in the United States: Results of an Online Cross-Sectional Survey. Am. J. Ophthalmol. 2020, 216, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hoisington, A.J.; Stearns-Yoder, K.A.; Kovacs, E.J.; Postolache, T.T.; Brenner, L.A. Airborne Exposure to Pollutants and Mental Health: A Review with Implications for United States Veterans. Curr. Environ. Health Rep. 2024, 11, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.H.; Chen, L.J.; Young, A.L. Depression and anxiety in dry eye disease: A systematic review and meta-analysis. Eye. 2016, 30, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Ziai, S.; Myageri, V.; Burns, J.G.; Prokopich, C.L. Economic burden and loss of quality of life from dry eye disease in Canada. BMJ Open Ophthalmol. 2021, 6, e000709. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, W.; Qi, M.; Wang, Y.; Li, S.; Wang, M.; Zeng, Q. Annual direct economic burden and influencing factors of dry eye disease in Central China. Ophthalmic Epidemiol. 2021, 30, 121–128. [Google Scholar] [CrossRef]
- Conti, M.A.; Bardes, J.M.; Oury, J.; Goodboy, A.K.; Shin, M.; Wilson, A. Prevalence of Burn Pit Associated Symptoms Among US Veterans Who Utilize Non-VA Private Healthcare. J. Occup. Environ. Med. 2024, 66, 439–444. [Google Scholar] [CrossRef]
- Hua, R.; Yao, K.; Hu, Y.; Chen, L. Discrepancy between subjectively reported symptoms and objectively measured clinical findings in dry eye: A population based analysis. BMJ Open 2014, 28, e005296. [Google Scholar] [CrossRef]
- Inomata, T.; Iwagami, M.; Nakamura, M.; Shiang, T.; Yoshimura, Y.; Fujimoto, K.; Okumura, Y.; Eguchi, A.; Iwata, N.; Miura, M.; et al. Characteristics and Risk Factors Associated with Diagnosed and Undiagnosed Symptomatic Dry Eye Using a Smartphone Application. JAMA Ophthalmol. 2020, 138, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P.I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Rispens, J.R.; Jones, S.A.; Clemmons, N.S.; Ahmed, S.; Harduar-Morano, L.; Johnson, M.D.; Edge, C., 3rd; Vyas, A.; Bourgikos, E.; Orr, M.F. Anhydrous Ammonia Chemical Release—Lake County, Illinois, April 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gargano, L.M.; Mantilla, K.; Fairclough, M.; Yu, S.; Brackbill, R.M. Review of Non-Respiratory, Non-Cancer Physical Health Conditions from Exposure to the World Trade Center Disaster. Int. J. Environ. Res. Public Health 2018, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Moline, J.; Herbert, R.; Nguyen, N. Health consequences of the September 11 World Trade Center attacks: A review. Cancer Investig. 2006, 24, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.E.; Gauntlett, L.; Amlôt, R. Public Perceptions of the “Remove, Remove, Remove” Information Campaign Before and During a Hazardous Materials Incident: A Survey. Health Secur. 2021, 19, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Hashim, D.; Acquilla, S.; Basanets, A.; Bertazzi, P.A.; Bushmanov, A.; Crane, M.; Harrison, D.J.; Holden, W.; Landrigan, P.J.; et al. A comparative assessment of major international disasters: The need for exposure assessment, systematic emergency preparedness, and lifetime health care. BMC Public Health 2017, 17, 46. [Google Scholar] [CrossRef]
- Jaffe, S. Hazardous train spills prompt calls for tougher safety rules. Lancet 2023, 401, 1143–1144. [Google Scholar] [CrossRef]
- Bartels, M. Chemical Health Risks from the Ohio Train Accident—What We Know So Far. Scientific American, 16 August 2023. Available online: https://www.scientificamerican.com/article/chemical-health-risks-from-the-ohio-train-accident-what-we-know-so-far/(accessed on 17 March 2024).
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.W.; Ku, Y.A.; Shin, Y.; Rahman, M.M.; Chung, M.H.; Kim, Y.H.; Kim, D.H. Investigating the Anti-Inflammatory Effects of RCI001 for Treating Ocular Surface Diseases: Insight into the Mechanism of Action. Front. Immunol. 2022, 13, 850287. [Google Scholar] [CrossRef]
- Pathak, G.; Nichter, M.; Hardon, A.; Moyer, E. The Open Burning of Plastic Wastes is an Urgent Global Health Issue. Ann. Glob. Health 2024, 90, 3. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, R.K.A. A mini review of hazardous wastes generated by environmental analytical laboratories: A perspective from Sri Lanka as an economically developing country. Environ. Monit. Assess. 2023, 195, 1380. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M. Insights into the in-silico research: Current scenario, advantages, limits, and future perspectives. Life Silico 2023, 1, 13–25. [Google Scholar]
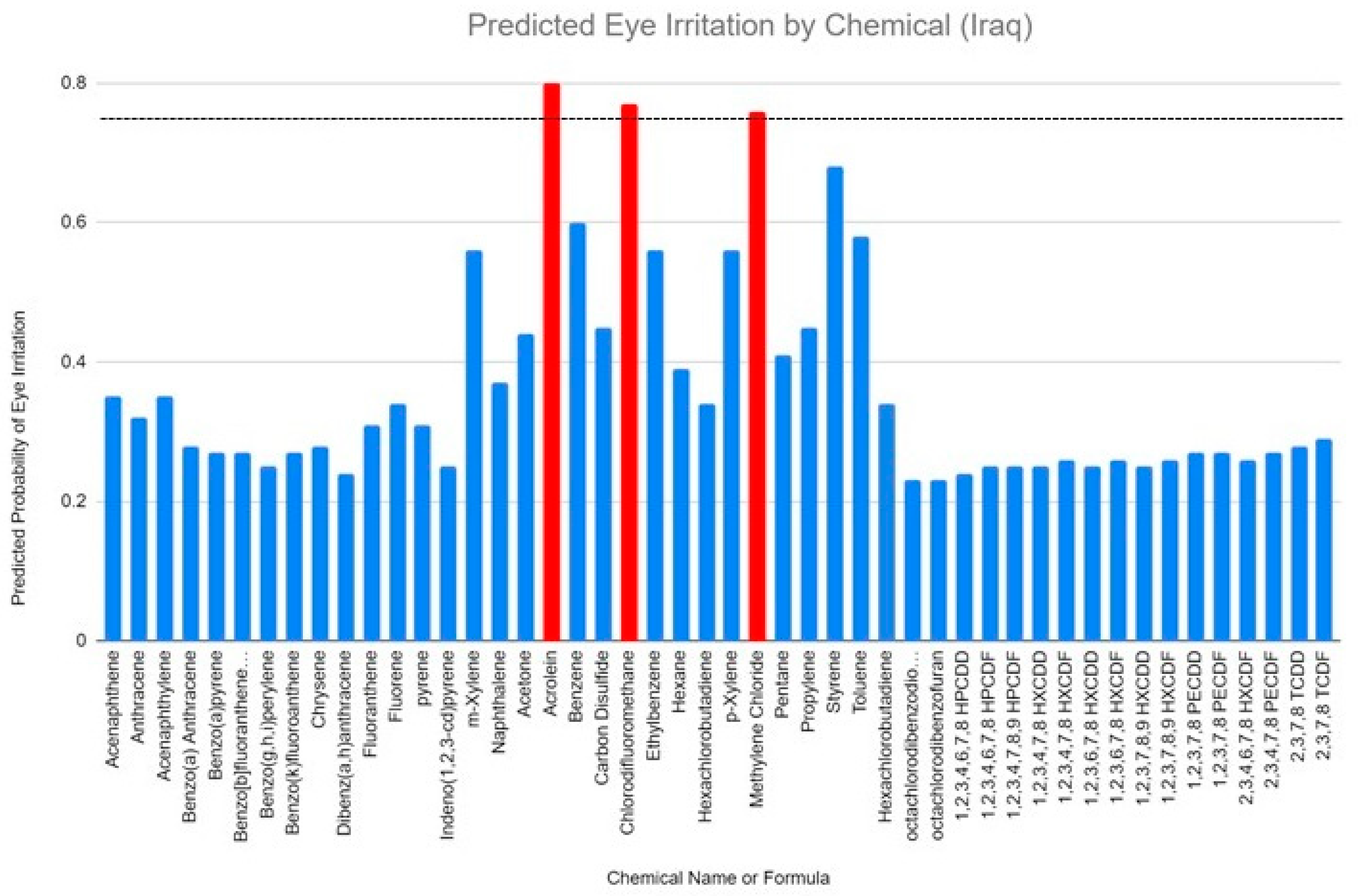
| ID | Name | SMILES | Probability of Eye Irritation | Chemical Formula |
|---|---|---|---|---|
| 1 | Acenaphthene | C1CC2=CC=CC3=C2C1=CC=C3 | 0.35 | C12H10 |
| 2 | Anthracene | C1=CC=C2C=C3C=CC=CC3=CC2=C1 | 0.32 | C14H10 |
| 3 | Acenaphthylene | C1=CC2=C3C(=C1)C=CC3=CC=C2 | 0.35 | C12H8 |
| 4 | Benzo(a) Anthracene | C1=CC=C2C(=C1)C=CC3=CC4=CC=CC=C4C=C32 | 0.28 | C18H12 |
| 5 | Benzo(a)pyrene | C1=CC=C2C3=C4C(=CC2=C1)C=CC5=C4C(=CC=C5)C=C3 | 0.27 | C20H12 |
| 6 | Benzo[b]fluoranthene-d12 | C1=CC=C2C3=C4C(=CC=C3)C5=CC=CC=C5C4=CC2=C1 | 0.27 | C20D12 |
| 7 | Benzo(g,h,i)perylene | C1=CC2=C3C(=C1)C4=CC=CC5=C4C6=C(C=C5)C=CC(=C36)C=C2 | 0.25 | C22H12 |
| 8 | Benzo(k)fluoranthene | C1=CC=C2C=C3C4=CC=CC5=C4C(=CC=C5)C3=CC2=C1 | 0.27 | C20H12 |
| 9 | Chrysene | C1=CC=C2C(=C1)C=CC3=C2C=CC4=CC=CC=C43 | 0.28 | C18H12 |
| 10 | Dibenz(a,h)anthracene | C1=CC=C2C(=C1)C=CC3=CC4=C(C=CC5=CC=CC=C54)C=C32 | 0.24 | C22H14 |
| 11 | Fluoranthene | C1=CC=C2C(=C1)C3=CC=CC4=C3C2=CC=C4 | 0.31 | C16H10 |
| 12 | Fluorene | C1C2=CC=CC=C2C3=CC=CC=C31 | 0.34 | C13H10 |
| 13 | pyrene | C1=CC2=C3C(=C1)C=CC4=CC=CC(=C43)C=C2 | 0.31 | C16H10 |
| 14 | Indeno(1,2,3-cd)pyrene | C1=CC=C2C(=C1)C3=C4C2=CC5=CC=CC6=C5C4=C(C=C6)C=C3 | 0.25 | C22H12 |
| 15 | m-Xylene | CC1=CC(=CC=C1)C | 0.56 | C8H10 |
| 16 | Naphthalene | C1=CC=C2C=CC=CC2=C1 | 0.37 | C10H8 |
| 17 | Acetone | CC(=O)C | 0.44 | C3H6O |
| 18 | Acrolein | C=CC=O | 0.8 | C3H4O |
| 19 | Benzene | C1=CC=CC=C1 | 0.6 | C6H6 |
| 20 | Carbon Disulfide | C(=S)=S | 0.45 | CS2 |
| 21 | Chlorodifluoromethane | C(F)(F)Cl | 0.77 | CHClF2 |
| 22 | Ethylbenzene | CCC1=CC=CC=C1 | 0.56 | C8H10 |
| 23 | Hexane | CCCCCC | 0.39 | C6H14 |
| 24 | Hexachlorobutadiene | C(=C(Cl)Cl)(C(=C(Cl)Cl)Cl)Cl | 0.34 | C4Cl6 |
| 25 | p-Xylene | CC1=CC=C(C=C1)C | 0.56 | C8H10 |
| 26 | Methylene Chloride | C(Cl)Cl | 0.76 | CH2Cl2 |
| 27 | Pentane | CCCCC | 0.41 | C5H12 |
| 28 | Propylene | CC=C | 0.45 | C3H6 |
| 29 | Styrene | C=CC1=CC=CC=C1 | 0.68 | C8H8 |
| 30 | Toluene | CC1=CC=CC=C1 | 0.58 | C6H5CH3 |
| 31 | Hexachlorobutadiene | C(=C(Cl)Cl)(C(=C(Cl)Cl)Cl)Cl | 0.34 | C4Cl6 |
| 32 | Octachlorodibenzodioxin | C12=C(C(=C(C(=C1Cl)Cl)Cl)Cl)OC3=C(O2)C(=C(C(=C3Cl)Cl)Cl)Cl | 0.23 | C12H4Cl4O2 |
| 33 | Octachlorodibenzofuran | C12=C(C(=C(C(=C1Cl)Cl)Cl)Cl)OC3=C2C(=C(C(=C3Cl)Cl)Cl)Cl | 0.23 | C12Cl8O |
| 34 | 1,2,3,4,6,7,8 HPCDD | C1=C2C(=C(C(=C1Cl)Cl)Cl)OC3=C(O2)C(=C(C(=C3Cl)Cl)Cl)Cl | 0.24 | C12HCl7O2 |
| 35 | 1,2,3,4,6,7,8 HPCDF | C1=C2C3=C(C(=C(C(=C3Cl)Cl)Cl)Cl)OC2=C(C(=C1Cl)Cl)Cl | 0.25 | C12HCl7O |
| 36 | 1,2,3,4,7,8,9 HPCDF | C1=C2C(=C(C(=C1Cl)Cl)Cl)C3=C(O2)C(=C(C(=C3Cl)Cl)Cl)Cl | 0.25 | C12HCl7O |
| 37 | 1,2,3,4,7,8 HXCDD | C1=C2C(=CC(=C1Cl)Cl)OC3=C(O2)C(=C(C(=C3Cl)Cl)Cl)Cl | 0.25 | C12H2Cl6O2 |
| 38 | 1,2,3,4,7,8 HXCDF | C1=C2C(=CC(=C1Cl)Cl)OC3=C2C(=C(C(=C3Cl)Cl)Cl)Cl | 0.26 | C12H2Cl6O |
| 39 | 1,2,3,6,7,8 HXCDD | C1=C2C(=C(C(=C1Cl)Cl)Cl)OC3=CC(=C(C(=C3O2)Cl)Cl)Cl | 0.25 | C12H2Cl6O2 |
| 40 | 1,2,3,6,7,8 HXCDF | C1=C2C3=C(C(=C(C=C3OC2=C(C(=C1Cl)Cl)Cl)Cl)Cl)Cl | 0.26 | C12H2Cl6O |
| 41 | 1,2,3,7,8,9 HXCDD | C1=C2C(=C(C(=C1Cl)Cl)Cl)OC3=C(C(=C(C=C3O2)Cl)Cl)Cl | 0.25 | C12H2Cl6O2 |
| 42 | 1,2,3,7,8,9 HXCDF | C1=C2C(=C(C(=C1Cl)Cl)Cl)C3=C(C(=C(C=C3O2)Cl)Cl)Cl | 0.26 | C12H2Cl6O |
| 43 | 1,2,3,7,8 PECDD | C1=C2C(=CC(=C1Cl)Cl)OC3=C(C(=C(C=C3O2)Cl)Cl)Cl | 0.27 | C12H3Cl5O2 |
| 44 | 1,2,3,7,8 PECDF | C1=C2C(=CC(=C1Cl)Cl)OC3=CC(=C(C(=C23)Cl)Cl)Cl | 0.27 | C12H3Cl5O |
| 45 | 2,3,4,6,7,8 HXCDF | C1=C2C3=CC(=C(C(=C3OC2=C(C(=C1Cl)Cl)Cl)Cl)Cl)Cl | 0.26 | C12H2Cl6O |
| 46 | 2,3,4,7,8 PECDF | C1=C2C3=CC(=C(C(=C3OC2=CC(=C1Cl)Cl)Cl)Cl)Cl | 0.27 | C12H3Cl5O |
| 47 | 2,3,7,8 TCDD | C1=C2C(=CC(=C1Cl)Cl)OC3=CC(=C(C=C3O2)Cl)Cl | 0.28 | C12H4Cl4O2 |
| 48 | 2,3,7,8 TCDF | C1=C2C3=CC(=C(C=C3OC2=CC(=C1Cl)Cl)Cl)Cl | 0.29 | C12H4Cl4O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauchman, S.H.; Kasselman, L.J.; Srivastava, A.; De Leon, J.; Reiss, A.B. An Assessment of the Ocular Toxicity of Two Major Sources of Environmental Exposure. Int. J. Environ. Res. Public Health 2024, 21, 780. https://doi.org/10.3390/ijerph21060780
Rauchman SH, Kasselman LJ, Srivastava A, De Leon J, Reiss AB. An Assessment of the Ocular Toxicity of Two Major Sources of Environmental Exposure. International Journal of Environmental Research and Public Health. 2024; 21(6):780. https://doi.org/10.3390/ijerph21060780
Chicago/Turabian StyleRauchman, Steven H., Lora J. Kasselman, Ankita Srivastava, Joshua De Leon, and Allison B. Reiss. 2024. "An Assessment of the Ocular Toxicity of Two Major Sources of Environmental Exposure" International Journal of Environmental Research and Public Health 21, no. 6: 780. https://doi.org/10.3390/ijerph21060780






