Malaria Severity in the Elimination Continuum: A Retrospective Cohort Study between Beitbridge and Lupane Districts in Zimbabwe, 2021–2023
Abstract
:1. Introduction
- Contrast variations in socio-demographic characteristics that affect malaria severity;
- Compare the role of malaria prevention practices on malaria severity;
- Assess the association between travel history and malaria severity using multivariate logistic regression models.
Operational Definitions
2. Materials and Methods
2.1. Study Design and Sampling
- (i)
- Exposed group: Individuals in this group were traced to have contracted malaria from a known malarious area outside the elimination district including areas beyond the country’s borders. The DHIS2 tracker electronic database names the data element “Malaria cases imported”.
- (ii)
- Unexposed: Individuals in this group were traced to have contracted malaria in the reporting district. The DHIS2 tracker electronic database names the data element “Malaria cases local”.
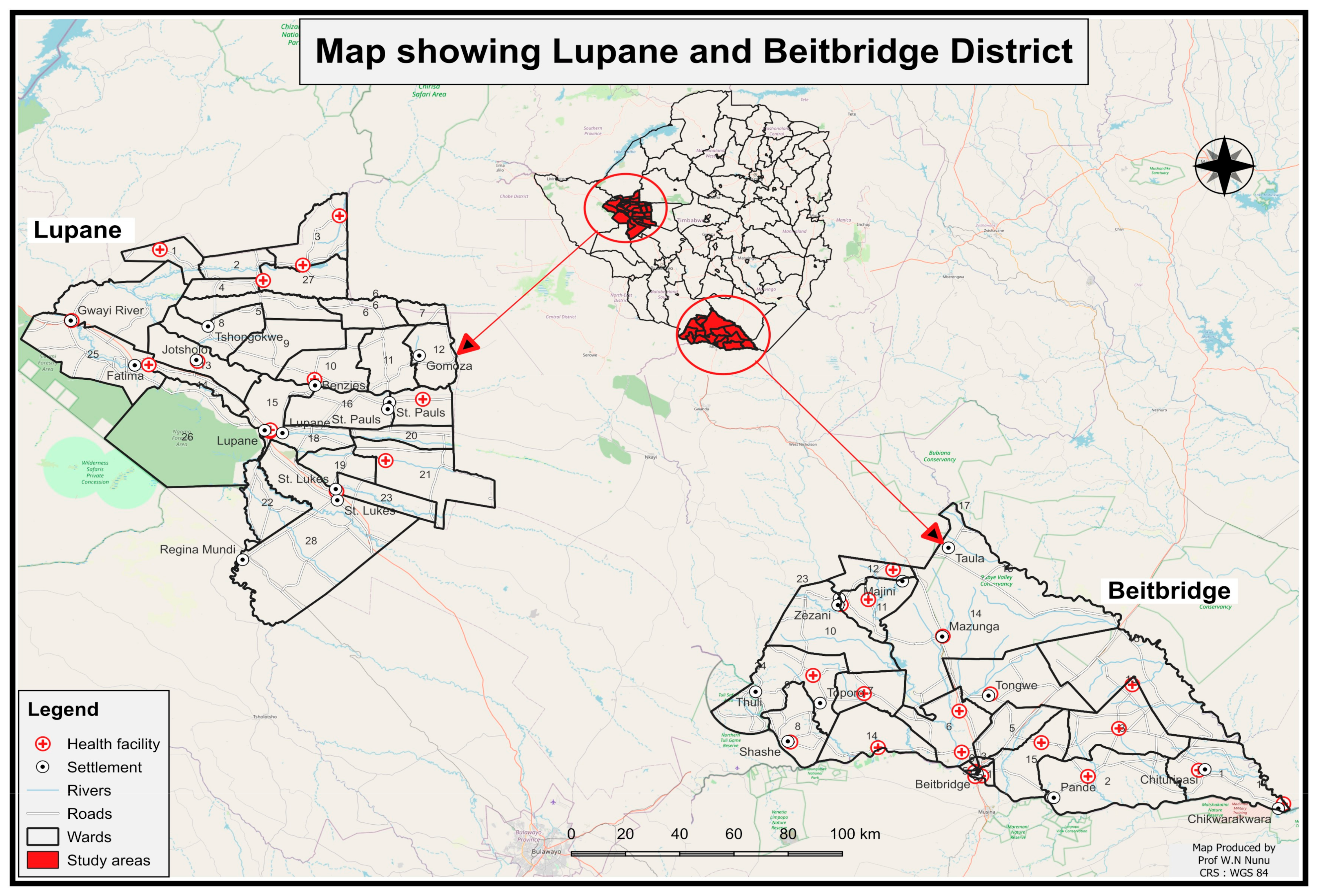
2.2. Study Sites
2.3. Ethics and Data Collection
2.4. Validity and Reliability
2.5. Statistical Analysis
2.5.1. Univariate Analysis
2.5.2. Bivariate Analysis
2.5.3. Multivariate Binary Logistic Regression Analysis
- (i)
- Bivariate selection: The chi-squared test was employed from the omnibus test of the model coefficients to assess the significance of each independent variable. If an independent variable was obtained (p-value ≤ 0.25), the present study considered that the variable contributed significantly (effect) to the model in explaining variability in malaria severity and proceeded to the multivariate modelling. However, the study considered important variables with a p-value >0.25 for the multivariate logistic analysis based on the literature.
- (ii)
- Backward stepwise selection (full model): Our study used backward stepwise selection using the primary independent variable (travel history exposure with no (0) and yes (1)), the dependent variable (malaria severity with binary outcomes for uncomplicated malaria (0) and severe malaria (1)), and all the confounder variables regardless of their significance. The study considered a variable to be a confounder and returned it to the model if its removal caused a change in the estimated RR value of the remaining variables in the full model of more than ten percent (>10%). As the prevalence of malaria is less than 10% within the elimination districts in Zimbabwe [21], the multivariate regression analyses presented results in the form of odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs), and statistical significance was determined by a p-value < 0.05.
- (iii)
- Interaction tests among independent variables were conducted by multiplying the values of the two independent variables involved in the interaction (x1.x2) and assessing whether the effect of one predictor variable on the outcome variable depended on the level of another predictor variable. The Wald test was used to determine the significance of the interaction term (p < 0.05, implying an interaction effect).
- (iv)
- Model evaluation: Our study utilised the omnibus, pseudo-parameters of the Nagelkerke R-squared, and Hosmer–Lemeshow tests for the model evaluation. The omnibus test assessed the overall fit of the logistic regression model by testing the null hypothesis that all regression coefficients were equal to zero. A significant omnibus test (p < 0.05) indicated a good overall fit for the data. Nagelkerke R-squared pseudo-parameters quantified the variation explained by the model, with values closer to one (1) indicating a stronger relationship between the predictors and malaria severity. The Hosmer–Lemeshow test assessed the model’s goodness of fit, with a non-significant result (p-value > 0.05) suggesting good calibration and fit to the data. These tests collectively helped us assess the adequacy, explanatory power, and predictive accuracy of the logistic regression model for malaria severity, based on the given predictors.
3. Results
4. Discussion
4.1. Demographic Characteristics
4.2. Malaria Prevention Practices
4.3. Evidence to Malaria Resurgence due to Local Transmission
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasir, S.M.I.; Amarasekara, S.; Wickremasinghe, R.; Fernando, D.; Udagama, P. Prevention of re-establishment of malaria: Historical perspective and future prospects. Malar. J. 2020, 19, 4–16. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Report Part Title: Malaria Elimination and Prevention of Re-Establishment Report Title: WHO Technical Brief for Countries Preparing Malaria Funding Requests for the Global Fund (2020–2022); World Health Organization: Geneva, Switzerland, 2020; Available online: https://about.jstor.org/terms (accessed on 27 May 2024).
- Das, A.M.; Hetzel, M.W.; Yukich, J.O.; Stuck, L.; Fakih, B.S.; Al-Mafazy, A.-W.H.; Ali, A.; Chitnis, N. Modelling the impact of interventions on imported, introduced and indigenous malaria infections in Zanzibar, Tanzania. Nat. Commun. 2023, 14, 2750. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, A.; Dhingra, N.; Sharma, S.N. Transition of Malaria Control to Malaria Elimination in India. J. Commun. Dis. 2022, 54, 124–140. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Q.; Liu, Z.; Li, Z.; Dong, X. Impact of the COVID-19 Pandemic on Malaria Control in Africa: A Preliminary Analysis. Trop. Med. Infect. Dis. 2023, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Ahmad, S.; Sher, A.; Al-Awadhi, M. Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries. Microorganisms 2021, 9, 2–24. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/teams/global-malaria-programme (accessed on 27 February 2024).
- Guth, J.; Lamy, M.; Murali, N.; Pankaj, P.; Yuthavong, Y. Meeting malaria elimination targets and remaining challenges: Qualitative research on perceptions of stakeholders in India and Southeast Asia. Asia Pacific Policy Stud. 2022, 9, 178–195. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Malaria, 16 October 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: http://apps.who.int/bookorders (accessed on 23 March 2024).
- Singh, A.; Kanaujia, A.; Singh, V.K. Research on Sustainable Development Goals: How has Indian Scientific Community Responded? J. Sci. Ind. Res. 2022, 81, 1147–1161. [Google Scholar] [CrossRef]
- Azizi, H.; Majdzadeh, R.; Ahmadi, A.; Raeisi, A.; Nazemipour, M.; Mansournia, M.A.; Schapira, A. Development and validation of an online tool for assessment of health care providers’ management of suspected malaria in an area, where transmission has been interrupted. Malar. J. 2022, 21, 2–3. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, L.; Yin, J.; Zhou, S.; Xia, Z. 1-3-7 surveillance and response approach in malaria elimination: China’s practice and global adaptions. Malar. J. 2023, 22, 152. [Google Scholar] [CrossRef]
- Mbunge, E.; Millham, R.; Sibiya, N.; Takavarasha, S. Is malaria elimination a distant dream? Reconsidering malaria elimination strategies in Zimbabwe. Public Health Pract. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Bharti, P.K.; Rajvanshi, H.; Nisar, S.; Jayswar, H.; Saha, K.B.; Shukla, M.M.; Mishra, A.K.; Sharma, R.K.; Das, A.; Kaur, H.; et al. Demonstration of indigenous malaria elimination through Track-Test-Treat-Track (T4) strategy in a Malaria Elimination Demonstration Project in Mandla, Madhya Pradesh. Malar. J. 2020, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Hasyim, H.; Ihram, M.A.; Fakhriyatiningrum; Misnaniarti Idris, H.; Liberty, I.A.; Flora, R.; Zulkifli, H.; Tessema, Z.T.; Maharani, F.E.; Syafrudin, D.; et al. Environmental determinants and risk behaviour in the case of indigenous malaria in Muara Enim Regency, Indonesia: A case-control design. PLoS ONE 2023, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Mugarisi, V. Zimbabwe Ramps up Efforts to Eliminate Malaria. World Health Organization. Published September 28, 2023. Available online: https://www.afro.who.int/sites/default/les/2023-09/IMG_9588.jpg (accessed on 15 March 2024).
- Gavi, S.; Tapera, O.; Mberikunashe, J.; Kanyangarara, M. Malaria incidence and mortality in Zimbabwe during the COVID-19 pandemic: Analysis of routine surveillance data. Malar. J. 2021, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- United States (US) Presidents Malaria Initiative. President’s Malaria Initiative Zimbabwe Malaria Operational Plan FY 2023. Available online: https://www.pmi.gov (accessed on 7 April 2024).
- Government of Zimbabwe. National Health Strategy for Zimbabwem 2021–2025. 2020. Available online: https://faolex.fao.org/docs/pdf/zim225019.pdf (accessed on 6 August 2023).
- National Malaria Control Program. 2022 Annual Malaria Report: National Malaria Control Program (NMCP) Ministry of Health & Child Care. Unpublished report.
- Mundagowa, P.T.; Chimberengwa, P.T. Malaria outbreak investigation in a rural area south of Zimbabwe: A case-control study. Malar. J. 2020, 19, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Roll Back Malaria. RBM Partnership Strategic Plan 2021–2025. 2020. Available online: https://endmalaria.org/sites/default/files/RBM%20Partnership%20Strategic%20Plan%202021-2025_web.pdf (accessed on 23 October 2023).
- World Health Organization. Malaria Surveillance, Monitoring & Evaluation: A Reference Manual. 2018. Available online: https://www.who.int/docs/default-source/documents/publications/gmp/malaria-surveillance-monitoring-and-evaluation---a-reference-manual.pdf?sfvrsn=46489b3b_2 (accessed on 4 January 2024).
- MoHCC/NMCP. National Malaria Control and Elimination Strategy, 2021–2025, 2020; Unpublished report.
- Dube, B.; Mberikunashe, J.; Dhliwayo, P.; Tangwena, A.; Shambira, G.; Chimusoro, A.; Madinga, M.; Gambinga, B. How far is the journey before malaria is knocked out malaria in Zimbabwe: Results of the malaria indicator survey 2016. Malar. J. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Dhliwayo, I.; Muboko, N.; Gandiwa, E. Local perceptions on poverty and conservation in a community-based natural resource program area: A case study of Beitbridge district, southern Zimbabwe. Front. Conserv. Sci. 2023, 4, 1232613. [Google Scholar] [CrossRef]
- Muchena, G.; Dube, B.; Chikodzore, R.; Pasipamire, J.; Murugasampillay, S.; Mberikunashe, J. A review of progress towards sub-national malaria elimination in Matabeleland South Province, Zimbabwe (2011–2015): A qualitative study. Malar. J. 2018, 17, 2–7. [Google Scholar] [CrossRef] [PubMed]
- ZIMSTAT. Population and Housing Census (Volume 1). 2022. Available online: https://www.zimstat.co.zw/wp-content/uploads/Demography/Census/2022_PHC_Report_27012023_Final.pdf (accessed on 6 August 2023).
- Mukungurutse, C.; Nyapwere, N.; Manyanga, A.; Mhaka, L. Pedological Characterization and Classification of Typical Soils of Lupane District, Zimbabwe. Int. J. Plant Soil. Sci. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Maseko, A.; Nunu, W.N. Risk factors associated with high malaria incidence among communities in selected wards in Binga district, Zimbabwe: A case-control study. Sci. African. 2020, 9, e00473. [Google Scholar] [CrossRef]
- Hassen, J.; Dinka, H. Magnitude of urban malaria and its associated risk factors: The case of Batu town, Oromia Regional State, Ethiopia. J. Int. Med. Res. 2022, 50, 03000605221080686. [Google Scholar] [CrossRef]
- Siziba, A.; Nunu, W.; Mudonhi, N.; Ndlovu, V.; Ndlovu, B.; Sanganyado, E. Risk factors associated with a high incidence of sexually transmitted infections in Beitbridge, Zimbabwe. In Curationis Aosis.; 2022; 45, p. a2191. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9350539/pdf/CUR-45-2191.pdf (accessed on 14 June 2024). [CrossRef] [PubMed]
- Yin, J.-H.; Zhang, L.; Yi, B.-Y.; Zhou, S.-S.; Xia, Z.-G. Imported malaria from land bordering countries in China: A challenge in preventing the reestablishment of malaria transmission. Travel. Med. Infect. Dis. 2023, 53, 102575. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, A.; Ntasumumuyange, D.; Small, M.; Rulisa, S.; Moscovitz, A.N.; Magriples, U. Assessment of perinatal outcomes of pregnant women with severe versus simple malaria. PLoS ONE 2021, 16, e0247053. [Google Scholar] [CrossRef] [PubMed]
- Oyegoke, O.O.; Adewumi, T.S.; Aderoju, S.A.; Tsundzukani, N.; Mabunda, E.; Adeleke, M.A.; Maharaj, R.; Okpeku, M. Towards malaria elimination: Analysis of travel history and case forecasting using the SARIMA model in Limpopo Province. Parasitol. Res. 2023, 122, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Teshome, T. Prevalence and Associated Factors of Malaria Infection among Outpatients Visiting Shewa Robit Health Center, Northcentral Ethiopia. J. Trop. Med. 2022, 2022, 1784012. [Google Scholar] [CrossRef]
- Wubishet, M.K.; Berhe, G.; Adissu, A.; Tafa, M.S. Effectiveness of long-lasting insecticidal nets in prevention of malaria among individuals visiting health centres in Ziway-Dugda District, Ethiopia: Matched case–control study. Malar. J. 2021, 20, 301. [Google Scholar] [CrossRef]
- Ahmed, S.; Reithinger, R.; Kaptoge, S.K.; Ngondi, J.M. Travel is a key risk factor for malaria transmission in pre-elimination settings in Sub-Saharan Africa: A review of the literature and meta-analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1380–1387. [Google Scholar] [CrossRef]
| Variables | Category | n (%) | Beitbridge District | n (%) | Lupane District | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UM | SM | RR; 95% CI | p-Value | UM | SV | RR; 95% CI | p-Value | ||||
| Travel History | No | 1056 (87) | 937 (86) | 119 (98) | 1 | 1056 (87) | 808 (85) | 248 (99) | 1 | ||
| Yes | 151 (13) | 149 (14) | 2 (2) | 0.04 (0.01;0.38) | 0.004 | 151 (13) | 148 (15) | 3 (1) | 0.03 (0.01;0.11) | <0.001 | |
| Age group | <5 years | 77 (6) | 61 (6) | 16 (13) | 1 | 122 (10) | 58 (6) | 64 (25) | 1 | ||
| 5 years + | 1130 (94) | 1025 (94) | 105 (87) | 0.18 (0.06;0.62) | 0.006 | 1085 (90) | 898 (94) | 187 (75) | 0.22 (0.13;0.36) | <0.001 | |
| 23% (>10%) * | 47% (>10%) * | ||||||||||
| Sex | Female | 670 (55) | 688 (62) | 2 (2) | 1 | 279 (23) | 196 (21) | 83 (33) | 1 | ||
| Male | 537 (45) | 418 (38) | 119 (98) | 126 (24.1;660.4) | <0.001 | 928 (77) | 760 (79) | 168 (67) | 0.79 (0.54;1.16) | 0.227 | |
| 75% (>10%) * | 26 (>10%) * | ||||||||||
| Occupation | Minor | 371 (31) | 335 (31) | 36 (30) | 1 | 425 (35) | 320 (33) | 105 (42) | 1 | ||
| Student | 132 (11) | 116 (11) | 16 (13) | 2.23 (0.75;6.75) | 0.148 | 161 (13) | 128 (13) | 33 (13) | 1.13 (0.65;1.95) | 0.664 | |
| Unemployed | 286 (24) | 266 (24) | 20 (17) | 0.61 (0.20;1.91) | 0.400 | 161 (13) | 111 (12) | 50 (20) | 2.02 (1.22;3.33) | 0.007 | |
| Employed | 418 (34) | 369 (34) | 49 (40) | 2.23 (0.94;5.38) | 0.069 | 460 (39) | 397 (42) | 63 (25) | 1.00 (0.64;1.56) | 0.990 | |
| 55% (>10%) * | 27% (>10%) * | ||||||||||
| Had visitor(s) | No | 232 (19) | 213 (20) | 19 (16) | 1 | 580 (48) | 534 (56) | 46 (18) | 1 | ||
| Yes | 975 (81) | 873 (80) | 102 (84) | 0.81 (0.27;2.44) | 0.708 | 627 (52) | 422 (44) | 205 (82) | 6.19 (4.16;9.22) | <0.001 | |
| 31% (>10%) * | 75% (>10%) * | ||||||||||
| Residence | Urban | 491 (41) | 444 (41) | 47 (39) | 1 | 609 (51) | 534 (56) | 75 (30) | 1 | ||
| Rural | 716 (59) | 642 (59) | 74 (61) | 0.83 (0.43;1.62) | 0.587 | 598 (49) | 422 (44) | 176 (70) | 1.94 (1.35;2.79) | <0.001 | |
| 35% (>10%) * | 27% (>10%) * | ||||||||||
| Prompt treatment | Within 24 hrs | 848 (70) | 813 (75) | 35 (29) | 1 | 720 (60) | 564 (59) | 156 (62) | 1 | ||
| After 24 h | 359 (30) | 273 (25) | 86 (71) | 6.78 (3.34;13.8) | <0.001 | 487 (40) | 392 (41) | 95 (38) | 1.01 (0.70;1.43) | 0.973 | |
| Mean ± SD | 2.2 ± 2.2 | 47% (>10%) * | Mean ± SD = 2.6 ± 2.4 | 32% (>10%) * | |||||||
| Malaria parasite | Other | 26 (2) | 26 (3) | 0 (0) | -- | -- | 30 (3) | 29 (3) | 1 (0.4) | -- | -- |
| Malariae | 103 (9) | 100 (9) | 3 (2) | -- | -- | 159 (13) | 141 (15) | 18 (7) | -- | -- | |
| Falciparum | 1078 (89) | 960 (88) | 118 (98) | -- | -- | 1018 (84) | 786 (82) | 232 (93) | -- | -- | |
| Malaria contact | Asymptomatic | 12 (1) | 12 (1) | 0 (0) | -- | -- | 2 (0.2) | 2 (0.2) | 0 (0) | -- | -- |
| Symptomatic | 228 (19) | 228 (21) | 0 (0) | -- | -- | 185 (15) | 170 (18) | 15 (6) | -- | -- | |
| Index | 967 (80) | 846 (78) | 121 (100) | -- | -- | 1020 (85) | 784 (82) | 236 (94) | -- | -- | |
| LLIN use | Owned used | 369 (30) | 357 (33) | 12 (10) | 1 | 429 (35) | 412 (43) | 17 (8) | 1 | ||
| Owned unused | 347 (29) | 318 (29) | 29 (24) | 24.87 (8.21;75.4) | <0.001 | 238 (20) | 169 (18) | 69 (27) | 7.83 (4.29;14.3) | <0.001 | |
| None | 491 (41) | 411 (38) | 80 (66) | 47.4 (16.4;137.2) | <0.001 | 540 (45) | 375 (39) | 165 (65) | 12.3 (7.02;21.4) | <0.001 | |
| 178% (>10%) * | 135% (>10%) * | ||||||||||
| Slept outdoors | No | 954 (79) | 933 (86) | 21 (17) | 1 | 598 (49) | 488 (51) | 110 (44) | 1 | ||
| Yes | 253 (21) | 153 (14) | 100 (83) | 84.4 (36.1;197.4) | <0.001 | 609 (51) | 468 (49) | 141 (56) | 1.93 (1.36;2.74) | <0.001 | |
| 88% (>10%) * | 51% (>10%) * | ||||||||||
| Variables | Model I: Overall | Model II: Beitbridge | Model III: Lupane | |||
|---|---|---|---|---|---|---|
| β (p-Value) | RR; 95% CI | β (p-Value) | RR; 95% CI | β (p-Value) | RR; 95% CI | |
| Travel History | −1.70 (0.013) | 0.18 (0.05;0.70) | −3.12 (0.004) | 0.04 (0.01;0.38) | −3.39 (<0.001) | 0.03 (0.01;0.11) |
| District | −1.00 (<0.001) | 0.37 (0.28;0.51) | ||||
| Age group | −1.48 (<0.001) | 0.23 (0.15;0.35) | −1.70 (0.006) | 0.18 (0.06;0.62) | −1.53 (<0.001) | 0.22 (0.13;0.36) |
| Gender | 1.02 (<0.001) | 2.77 (2.01;3.81) | 4.83 (<0.001) | 126.1 (24.09;660.4) | −2.24 (0.227) | |
| Occupation 1 | 0.82 (0.148) | 0.12 (0.664) | ||||
| Occupation 2 | −0.49 (0.400) | 0.70 (0.007) | 2.02 (1.22;3.35) | |||
| Occupation 3 | 0.81 (0.069) | 0.00 (0.990) | ||||
| Had visitor (s) | 1.70 (<0.001) | 5.45 (3.81;7.80) | −0.21 (0.708) | 1.82 (<0.001) | 6.19 (4.16;9.22) | |
| Residence | 0.49 (0.001) | 1.62 (1.21;2.17) | −0.19 (0.587) | 0.66 (<0.001) | 1.94 (1.35;2.79) | |
| Prompt treatment | 0.71 (<0.001) | 2.04 (1.53;2.70) | 1.92 (<0.001) | 6.78 (3.34;13.79) | 0.01 (0.973) | |
| LLIN use 1 | 1.85 (<0.001) | 6.34 (3.95;10.2) | 3.21 (<0.001) | 24.9 (8.206;75.35) | 2.06 (<0.001) | 7.83 (4.2914.2) |
| LLIN use 2 | 2.60 (<0.001) | 13.5 (8.66;21.0) | 3.86 (<0.001) | 47.4 (16.38;137.2) | 2.51 (<0.001) | 12.3 (7.02;21.4) |
| Slept outdoors | 1.63 (<0.001) | 5.11 (3.82;6.85) | 4.44 (<0.001) | 84.4 (36.08;197.4) | 0.66 (<0.001) | 1.92 (1.36;2.74) |
| Travel × Sex | −3.35 (<0.001) | 0.02 | ||||
| Omnibus | <0.05 | <0.05 | <0.05 | |||
| Hosmer–Lemeshow | 0.12 | 0.17 | 0.08 | |||
| PAC (%) | 87.8 | 95.9 | 85.0 | |||
| Cox and Snell R-squared | 0.26 | 0.36 | 0.29 | |||
| Nagelkerke R-squared | 0.45 | 0.75 | 0.45 | |||
| −2 Log likelihood | 1341.2 | 254.2 | 822.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betera, S.; Wispriyono, B.; Nunu, W.N.; Susanna, D.; Midzi, N.; Dhliwayo, P.; Yelda, F.; Nyamukondiwa, M. Malaria Severity in the Elimination Continuum: A Retrospective Cohort Study between Beitbridge and Lupane Districts in Zimbabwe, 2021–2023. Int. J. Environ. Res. Public Health 2024, 21, 877. https://doi.org/10.3390/ijerph21070877
Betera S, Wispriyono B, Nunu WN, Susanna D, Midzi N, Dhliwayo P, Yelda F, Nyamukondiwa M. Malaria Severity in the Elimination Continuum: A Retrospective Cohort Study between Beitbridge and Lupane Districts in Zimbabwe, 2021–2023. International Journal of Environmental Research and Public Health. 2024; 21(7):877. https://doi.org/10.3390/ijerph21070877
Chicago/Turabian StyleBetera, Same, Bambang Wispriyono, Wilfred Njabulo Nunu, Dewi Susanna, Nicholas Midzi, Patience Dhliwayo, Fitra Yelda, and Melisa Nyamukondiwa. 2024. "Malaria Severity in the Elimination Continuum: A Retrospective Cohort Study between Beitbridge and Lupane Districts in Zimbabwe, 2021–2023" International Journal of Environmental Research and Public Health 21, no. 7: 877. https://doi.org/10.3390/ijerph21070877






