Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel
Abstract
:1. Introduction
2. Overview on Fuels Desulfurization Technologies
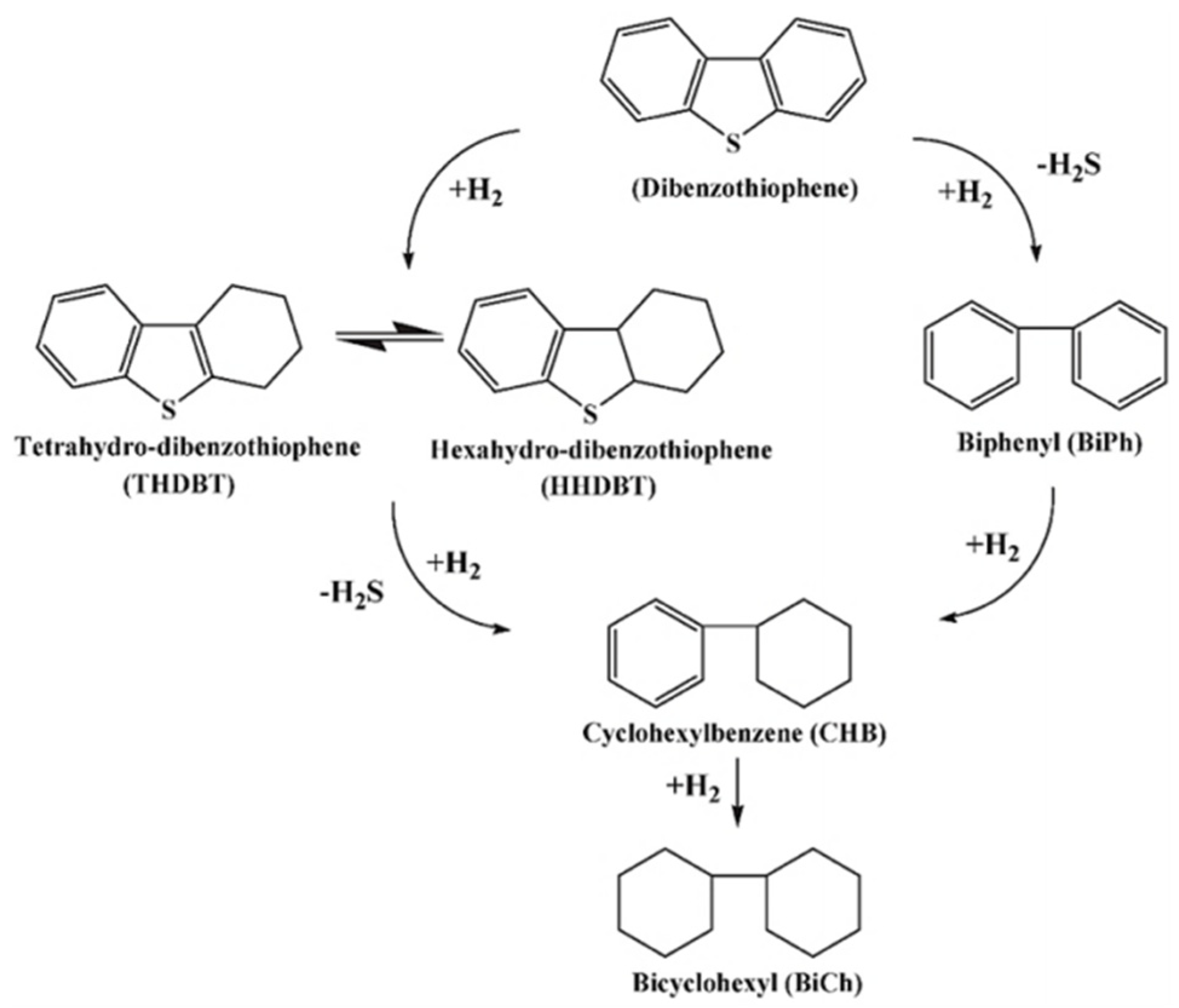
2.1. Hydrodesulfurization (HDS)
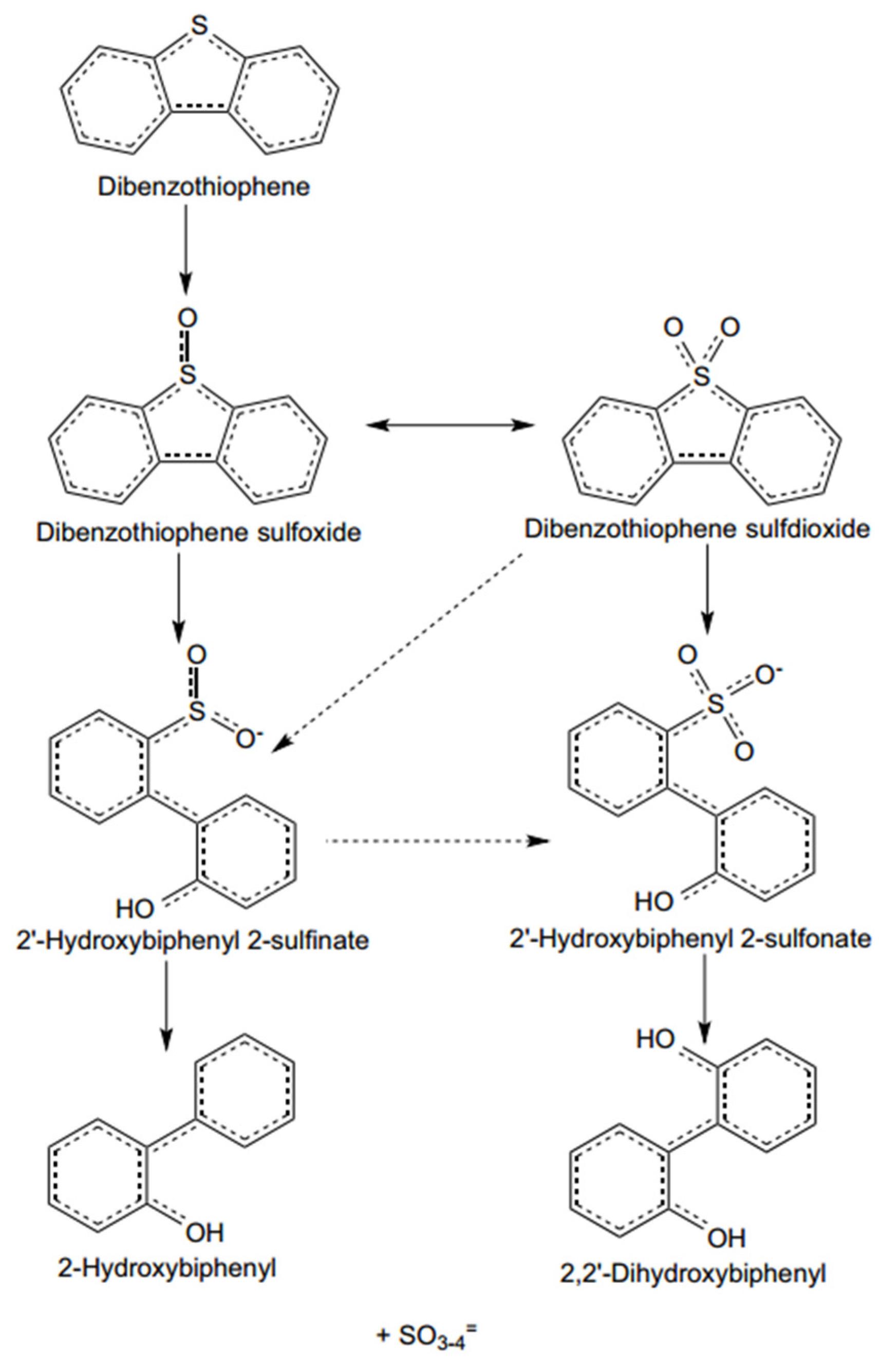
2.2. Oxidative Desulfurization (ODS)
2.3. Biodesulfurization (BDS)
2.4. Adsorption (ADS)
2.5. Extraction (EDS)

3. Ionic Liquids and Their Application in the Desulfurization Process
3.1. Ionic Liquids
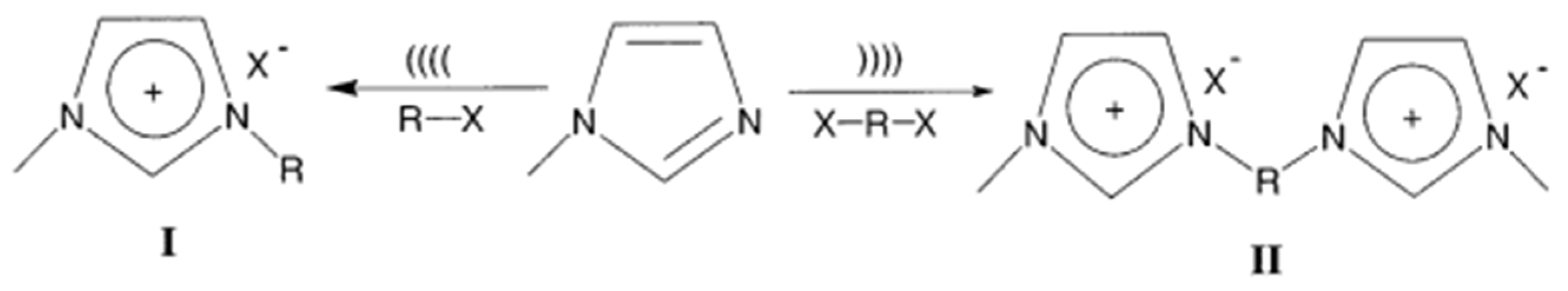
3.2. Synthesis of Ionic Liquids
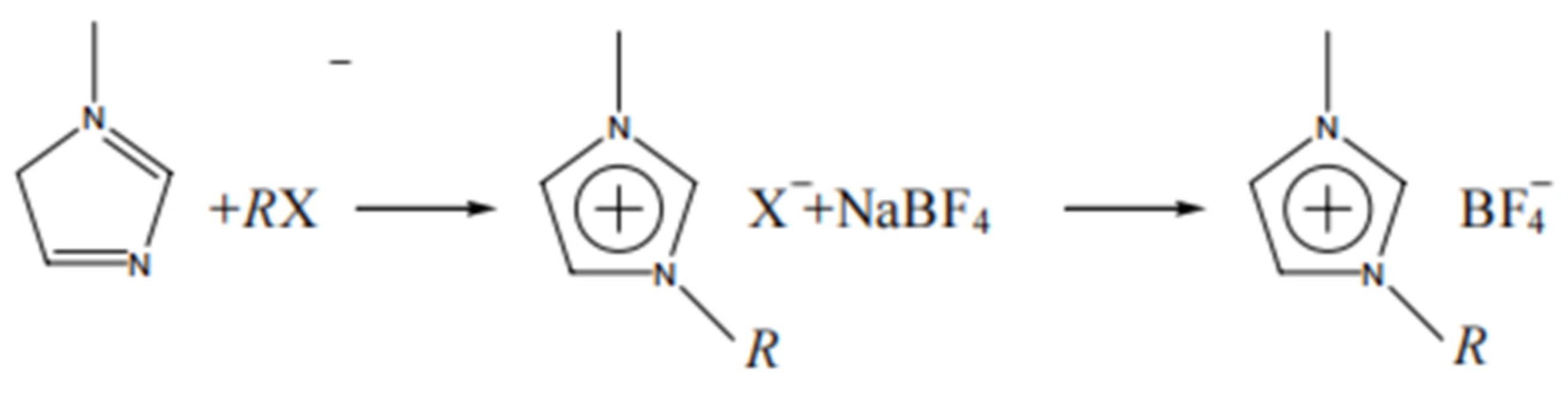
3.2.1. One-Step Synthesis of Ionic Liquids
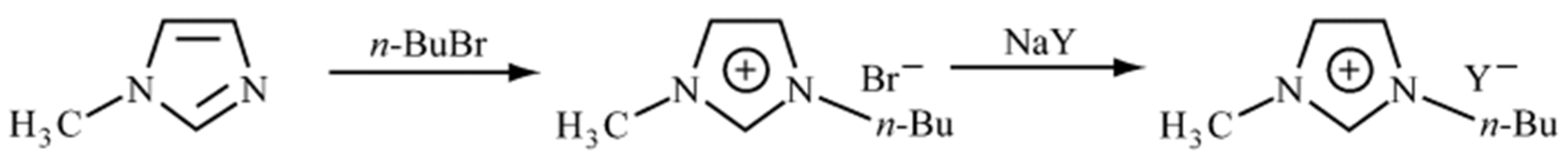
3.2.2. Two-Step Synthesis of Ionic Liquids
3.2.3. Microwave Synthesis of Ionic Liquids
3.2.4. Ultrasonic Synthesis of Ionic Liquids
3.3. Application of Ionic Liquids in Fuel Desulfurization Processes
3.3.1. Ionic Liquid Oxidative Desulfurization
3.3.2. Ionic Liquid Extraction Desulfurization
3.3.3. Ionic Liquid Extraction Oxidative Desulfurization
3.3.4. Ionic Liquid Catalytic Oxidative Desulfurization
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Wang, X.; Deng, H.; Zhao, J.; Li, Y. Molecular mechanism and experimental study of fuel oil extractive desulfurization technology based on ionic liquid. J. Energy Inst. 2024, 112, 101452. [Google Scholar] [CrossRef]
- Samet, J.M. Health effects of sulfur oxide and particulate air pollution. Trans. Am. Nucl. Soc. 1987, 55, 3–4. [Google Scholar]
- Fu, C.; Kuang, D.; Zhang, H.; Ren, J.; Chen, J. Different components of air pollutants and neurological disorders. Front. Public Health 2022, 10, 959921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G.; Zhang, Q.L.; Zhang, Z.C. Extractive desulfurization and denitrogenation of fuels using ionic liquids. Ind. Eng. Chem. Res. 2004, 43, 614–622. [Google Scholar] [CrossRef]
- Boniek, D.; Figueiredo, D.; dos Santos, A.F.B.; de Resende Stoianoff, M.A. Biodesulfurization: A mini review about the immediate search for the future technology. Clean Technol. Environ. Policy 2014, 17, 29–37. [Google Scholar] [CrossRef]
- Mendez, I.I.; Hermann, L.L.; Hazelton, P.R.; Coombs, K.M. A comparative analysis of Freon substitutes in the purification of reovirus and calicivirus. J. Virol. Methods 2000, 90, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Delaby, O.; Smith, R. Minimization of flue-gas emissions. Process Saf. Environ. Prot. 1995, 73, 21–32. [Google Scholar]
- Nazir, M.S.; Ahmad, S.; Tahir, Z.; ul Sadaf, H.; Ali, Z.; Akhtar, M.N.; Azam, K.; Abdullah, M.A. A Review on the Methods in Diesel Desulfurization. Curr. Anal. Chem. 2021, 17, 815–830. [Google Scholar] [CrossRef]
- Yao, H.; Luo, J.; Hou, D.; Chang, X.; Yang, Y. Recent advances in research and development in desulfurization of fuel oil. Spec. Petrochem. 2009, 26, 77–81. [Google Scholar]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. Rsc Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Shang, H.; Du, W.; Liu, Z.; Zhang, H. Development of microwave induced hydrodesulfurization of petroleum streams: A review. J. Ind. Eng. Chem. 2013, 19, 1061–1068. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Oxidative desulfurization of fuels using ionic liquids: A review. Front. Chem. Sci. Eng. 2015, 9, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Zolotareva, D.; Zazybin, A.; Rafikova, K.; Dembitsky, V.M.; Dauletbakov, A.; Yu, V. Ionic liquids assisted desulfurization and denitrogenation of fuels. Vietnam J. Chem. 2019, 57, 133–163. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Zhao, J.; Zhang, H.; Kang, X.; Dong, F. Research of QSPR/QSAR for Ionic Liquids. Prog. Chem. 2012, 24, 1236–1244. [Google Scholar]
- Abro, R.; Abdeltawab, A.A.; Al-Deyab, S.S.; Yu, G.; Qazi, A.B.; Gao, S.; Chen, X. A review of extractive desulfurization of fuel oils using ionic liquids. RSC Adv. 2014, 4, 35302–35317. [Google Scholar] [CrossRef]
- Grange, P. Catalytic Hydrodesulfurization. Catal. Rev. 2006, 21, 135–181. [Google Scholar] [CrossRef]
- Mittal, V.; Cai, T.; Krishnadevarajan, K.; Xu, Q. Emission-Considered Diesel Blending Optimization. Chem. Eng. Technol. 2014, 37, 293–300. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Hou, Z.; Xin, J.; Meng, Q.; Han, L.; Xiao, C.; Hu, D.; Duan, A.; Xu, C. Synthesis and characterization of Beta-FDU-12 and the hydrodesulfurization performance of FCC gasoline and diesel. Fuel Process. Technol. 2018, 172, 55–64. [Google Scholar] [CrossRef]
- Shafi, R.; Hutchings, G.J. Hydrodesulfurization of hindered dibenzothiophenes: An overview. Catal. Today 2000, 59, 423–442. [Google Scholar] [CrossRef]
- Kwak, C.; Kim, M.Y.; Choi, K.G.; Moon, S.H. Effect of phosphorus addition on the behavior of CoMoS/Al2O3 catalyst in hydrodesulfurization of dibenzothiophene and 4,6-dimethyl dibenzothiophene. Appl. Catal. A-Gen. 1999, 185, 19–27. [Google Scholar] [CrossRef]
- Sun, M.; Prins, R. Kinetic Investigation of the Effect of Nickel and Fluorine on the HDN of Methylcyclohexylamine over WS2/Al2O3 Catalysts. J. Catal. 2001, 201, 138–144. [Google Scholar] [CrossRef]
- Soled, S.L.; Miseo, S.; Baumgartner, J.E.; Nistor, I.; Nandi, P.; Guzman, J.; Levin, D.; Wilson, K.; Bergweff, J.A.; Huiberts, R.J.; et al. Hydroprocessing Catalysts and Their Production. U.S. Patent 10022712, 17 July 2018. [Google Scholar]
- Zhang, L.-L.; Yang, K.-X.; Xue, C.-L.; Li, W.-X.; Li, B.-Q.; Du, Y.-E.; Chen, Y.-Q.; Liu, Y.-J. MOF-derived CoP/C catalyst for efficient dibenzothiophene hydrodesulfurization. Fuel 2024, 369, 131778. [Google Scholar] [CrossRef]
- Broderick, D.H.; Gates, B.C. Hydrodesulfurization of dibenzothiophene catalyzed by sulfided CoO-MoO3-γ-Al2O3—Reaction-kinetics. In Abstracts of Papers of the American Chemical Society; No. MAR; American Chemical Society: Washington, DC, USA, 1980; Volume 179, p. 11-FUEL. [Google Scholar]
- Chen, H.; Li, Y.; Zhao, D.; Wang, C. Advances in Oxidative Desulfurization of Gasoline and Diesel Oil. Chem. Ind. Eng. Prog. 2004, 23, 913–916. [Google Scholar]
- Yuyan, Z.; Lijuan, F.; Sulian, Z.; Yingfei, H.O.U.; Yingmin, Y.U.; Xiaobin, W.E.I.; Chunhu, L.I. New technology of catalytic oxidative desulfurization of diesel oil. Chem. Res. Appl. 2007, 19, 1191–1198. [Google Scholar]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.H.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, C.; Liu, Y.; Wang, X. Recent Advances of Biological Desulfurization Technology. Chem. Eng. Oil Gas 2005, 34, 489–491. [Google Scholar]
- Liu, D.; Xun, S.; Yang, B.; He, M.; Liu, J.; Jiang, W.; Li, H.; Zhu, W. Phosphotungstic-Acid-Based Ionic Liquid Catalyst for Efficient Photocatalytic Oxidative Desulfurization under Visible Light. Energy Fuels 2024, 38, 6553–6559. [Google Scholar] [CrossRef]
- Khedkar, S.; Shanker, R. Isolation and classification of a soil actinomycete capable of sulphur-specific biotransformation of dibenzothiophene, benzothiophene and thianthrene. J. Appl. Microbiol. 2015, 118, 62–74. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, D.K. Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem. Eng. J. 2010, 50, 104–109. [Google Scholar] [CrossRef]
- Tang, H.; Li, Q.; Wang, Z.; Yan, D.; Xing, J. Simultaneous Removal of Thiophene and Dibenzothiophene by Immobilized Pseudomonas delafieldii R-8 cells. Chin. J. Chem. Eng. 2012, 20, 47–51. [Google Scholar] [CrossRef]
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2021, 214, 106685. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmed, O.U.; Al-Wahaibi, T.; Al-Wahaibi, Y.; AlNashef, I.M. Deep oxidative desulfurization of liquid fuels. Rev. Chem. Eng. 2014, 30, 337–378. [Google Scholar] [CrossRef]
- Chen, T.-C.; Shen, Y.-H.; Lee, W.-J.; Lin, C.-C.; Wan, M.-W. An economic analysis of the continuous ultrasound-assisted oxidative desulfurization process applied to oil recovered from waste tires. J. Clean. Prod. 2013, 39, 129–136. [Google Scholar] [CrossRef]
- Guo, Y.-H.; Pan, G.-X.; Xu, M.-H.; Wu, T.; Wang, Y.-Y. Synthesis and Adsorption Desulfurization Performance of Modified Mesoporous Silica Materials M-MCM-41 (M = Fe, Co, Zn). Clays Clay Miner. 2019, 67, 325–333. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Wu, P.; Xun, S.; Jiang, W.; Li, H.; Zhu, W. Recent progress on extractive desulfurization of fuel oils through reactions based on ionic liquids as solvents and catalysts. CIESC J. 2021, 72, 276–291. [Google Scholar]
- He, L.; He, J.; Cui, P.; Feng, Y.; Hua, M.; Zhang, J.; Wu, P.; Zhu, W.; Li, H.; Liu, Z.; et al. Microporous boron nitride-based porous ionic liquid for enhanced extractive desulfurization of fuel. Sep. Purif. Technol. 2023, 307, 122781. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Liu, S.; Cui, B.; Yang, X.; Zheng, X.; Xu, W.; Liu, Y. Application of ionic liquid in fuels desulfurization. Appl. Chem. Ind. 2011, 40, 888–891. [Google Scholar]
- Sun, N.; Zhang, S.; Zhang, X.; Li, Z. Database and QSPR of Physical Properties of Ionic Liquids. Chin. J. Process Eng. 2005, 5, 698–702. [Google Scholar]
- Zhang, Y.; Liu, Z.; Huang, C.; Gao, J.; Xu, C. Anion Existing Forms of Ionic Liquid and Property Research. Chem. World 2003, 44, 665–669. [Google Scholar]
- Zhang, Q.; Wang, R.; Li, Z.; Deng, Y. Advances of Ionic Liquid Application in Green Catalysis and Clean Synthesis. Petrochem. Technol. 2007, 36, 975–984. [Google Scholar]
- Taheri, M.; Zhu, R.; Yu, G.; Lei, Z. Ionic liquid screening for CO2 capture and H2S removal from gases: The syngas purification case. Chem. Eng. Sci. 2021, 230, 116199. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621–629. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Gardas, R.L.; Goncalves, F.; Coutinho, J.A.P. Ecotoxicological risk profile of ionic liquids: Octanol-water distribution coefficients and toxicological data. J. Chem. Technol. Biotechnol. 2011, 86, 957–963. [Google Scholar] [CrossRef]
- Wells, A.S.; Coombe, V.T. On the freshwater ecotoxicity and biodegradation properties of some common ionic liquids. Org. Process Res. Dev. 2006, 10, 794–798. [Google Scholar] [CrossRef]
- Ke, M.; Zhou, A.; Song, Z.; Jiang, Q. Toxicity of ionic liquids. Prog. Chem. 2007, 19, 671–679. [Google Scholar]
- Li, X.; Zhang, L.; Li, L.; Hu, Y.; Liu, J.; Xu, Y.; Luo, C.; Zheng, C. NO Removal from Flue Gas Using Conventional Imidazolium-Based Ionic Liquids at High Pressures. Energy Fuels 2018, 32, 6039–6048. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv. 2012, 2, 12525–12551. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Wu, T.; Zheng, Z.; Wu, Y.; Zheng, R.; Zhang, Q.; Lin, D.; Zhou, X. Research on one-step synthesis technology of quaternary ammonium salts on imidazolium type ionic liquid catalyst. Abstr. Pap. Am. Chem. Soc. 2006, 232, 981. [Google Scholar]
- Zhang, S.J.; Yuan, X.L.; Chen, Y.H.; Zhang, X.P. Solubilities of CO2 in 1-butyl-3-methylimidazolium hexafluorophosphate and 1,1,3,3-tetramethylguanidium lactate at elevated pressures. J. Chem. Eng. Data 2005, 50, 1582–1585. [Google Scholar] [CrossRef]
- Li, Z.; Gu, A.; Zhou, Q. Preparation of Gold Nanochains in 1-Butyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquids. Rare Met. Mater. Eng. 2009, 38, 1454–1457. [Google Scholar]
- Wang, Q.; Wang, S.; Qi, M.-C.; Zang, S.-L. Synthesis and Characterization of alkeyl imidazolium ionic liquids. In Proceedings of the 4th International Conference on Textile Engineering and Materials (ICTEM), Shenzhen, China, 23–24 August 2014; pp. 452–455. [Google Scholar]
- Varma, R.S.; Namboodiri, V.V. An expeditious solvent-free route to ionic liquids using microwaves. Chem. Commun. 2001, 7, 643–644. [Google Scholar] [CrossRef]
- Ming-Chien, H.; Tse-Wei, L. Microwave-assisted rapid synthesis of ionic liquids. In Proceedings of the 2011 International Conference on Consumer Electronics, Communications and Networks (CECNet), Xianning, China, 16–18 April 2011; pp. 5050–5052. [Google Scholar]
- Xuetang, X.U.; Qinghua, P.; Wengui, D.; Gang, L.A.I.; Xiongmin, L.I.U. Microwave-assisted synthesis of ionic liquid bmim PTSA. Chem. Res. Appl. 2008, 20, 71–74. [Google Scholar]
- Hsiao, M.-C.; Lin, T.-W. Ultrasound assisted rapid synthesis of ionic liquids. In Proceedings of the 2011 International Conference on Consumer Electronics, Communications and Networks (CECNet), Xianning, China, 16–18 April 2011; pp. 5461–5463. [Google Scholar]
- Namboodiri, V.V.; Varma, R.S. Solvent-free sonochemical preparation of ionic liquids. Org. Lett. 2002, 4, 3161–3163. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Chen, Y.; Wang, Z. Removal of sulfide from fuels by ionic liquids: Prospects for the future. Braz. J. Chem. Eng. 2023, 40, 929–963. [Google Scholar] [CrossRef]
- Kairbekov, Z.K.; Myltykbaeva, Z.K.; Muktaly, D.; Nysanova, B.; Anisimov, A.V.; Akopyan, A.V. Peroxide Oxidative Desulfurization of a Diesel Fuel. Theor. Found. Chem. Eng. 2018, 52, 677–680. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, R.; Zhang, G.; Zheng, Y.; Zhao, J. Oxidative desulfurization of model fuel in the presence of molecular oxygen over polyoxometalate based catalysts supported on carbon nanotubes. Fuel 2018, 224, 261–270. [Google Scholar] [CrossRef]
- Ma, C.; Dai, B.; Liu, P.; Zhou, N.; Shi, A.; Ban, L.; Chen, H. Deep oxidative desulfurization of model fuel using ozone generated by dielectric barrier discharge plasma combined with ionic liquid extraction. J. Ind. Eng. Chem. 2014, 20, 2769–2774. [Google Scholar] [CrossRef]
- Cai, Y.-Q.; Song, G.-H.; Liang, D.-N. Desulfurization using the 1,2-dimethylimidazolium ionic liquid as an adsorbent. Chin. Chem. Lett. 2015, 26, 317–319. [Google Scholar] [CrossRef]
- Xu, H.; Han, Z.; Zhang, D.; Liu, C. Theoretical elucidation of the dual role of [HMIm]BF4 ionic liquid as catalyst and extractant in the oxidative desulfurization of dibenzothiophene. J. Mol. Catal. A Chem. 2015, 398, 297–303. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, W.; Fan, X.; Zhang, M.; Wei, Y.; He, J.; Li, C.; Li, H.; Zhu, W. Synthesis of amphiphilic peroxophosphomolybdates for oxidative desulfurization of fuels in ionic liquids. Pet. Sci. 2018, 15, 890–897. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Li, T.; Wang, Y.; Hu, B. Application and research progress of extraction-oxidation desulfurization using ionic liquids. Appl. Chem. Ind. 2020, 49, 452–457. [Google Scholar]
- Songqing, H.U.; Jun, Z.; Bing, L.I.U.; Dianling, F.U. Feasibility study of extraction desulfurization with ionic liquids. Acta Pet. Sin. (Pet. Process. Sect.) 2007, 23, 100–103. [Google Scholar]
- Rodriguez-Cabo, B.; Arce, A.; Soto, A. Desulfurization of fuels by liquid-liquid extraction with 1-ethyl-3-methylimidazolium ionic liquids. Fluid Phase Equilibria 2013, 356, 126–135. [Google Scholar] [CrossRef]
- Zeng, X.; Li, D.; Zhang, X.; Wang, L.; Zhang, S. Desulfurization Process of Gasoline by Extraction with Ionic Liquid. Chin. J. Process Eng. 2007, 7, 506–509. [Google Scholar]
- Desai, K.; Dharaskar, S.; Khalid, M.; Gedam, V. Effectiveness of ionic liquids in extractive-oxidative desulfurization of liquid fuels: A review. Chem. Pap. 2022, 76, 1989–2028. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Zhang, Q. Design rules of ionic liquids tasked for highly efficient fuel desulfurization by mild oxidative extraction. Fuel 2017, 189, 334–339. [Google Scholar] [CrossRef]
- Lo, W.H.; Yang, H.Y.; Wei, G.T. One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids. Green Chem. 2003, 5, 639–642. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, Y.; Li, E.; Gan, Y. Study on amino acid-based imidazole ionic liquid oxidative extraction desulfurization. Chem. Res. Appl. 2021, 33, 1956–1963. [Google Scholar]
- Cui, Y.X.; Tang, X.D.; Hu, X.Q.; Guo, Q.X.; Yan, Y. “One pot” desulfurization of straight-run diesel oil with ionic liquid. Acta Pet. Sinica. Pet. Process. Sect. 2009, 3, 425–429. [Google Scholar]
- Jiang, W.; Zhu, W.; Chang, Y.; Chao, Y.; Yin, S.; Liu, H.; Zhu, F.; Li, H. Ionic liquid extraction and catalytic oxidative desulfurization of fuels using dialkylpiperidinium tetrachloroferrates catalysts. Chem. Eng. J. 2014, 250, 48–54. [Google Scholar] [CrossRef]
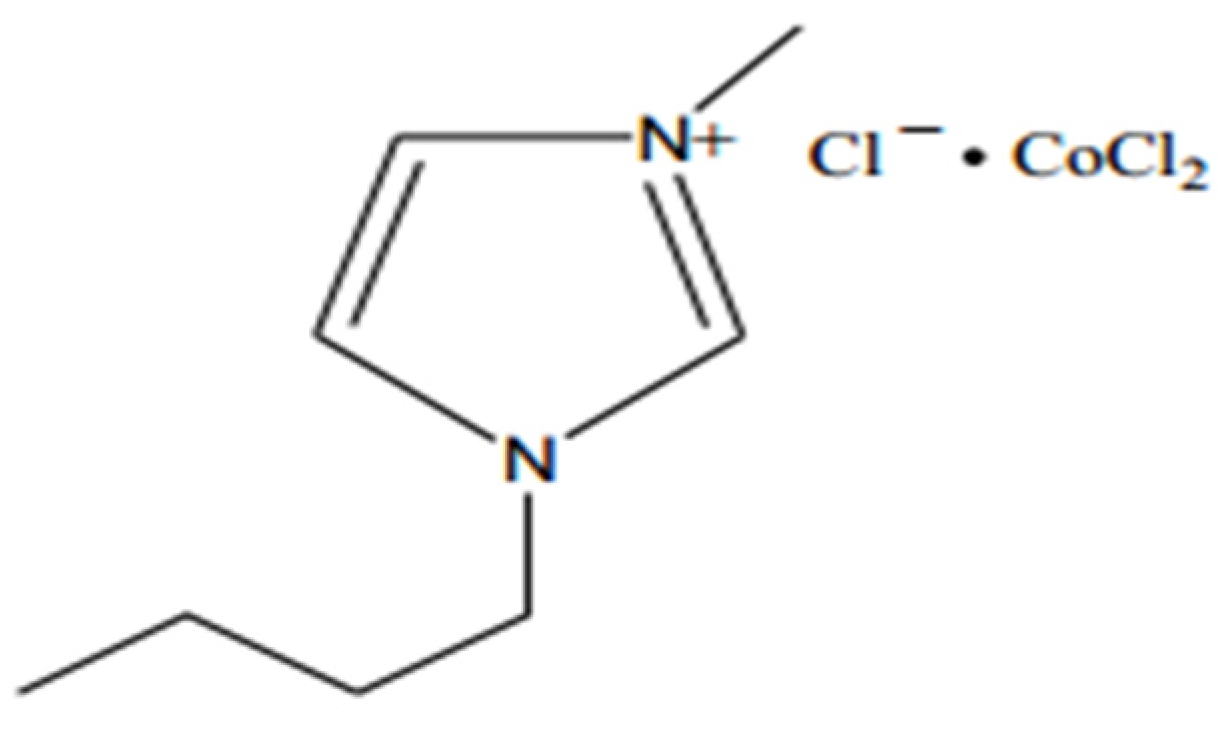
- Xu, H.; Zhang, D.; Wu, F.; Cao, R. Deep oxidative desulfurization of fuels based on C4mimCl CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Mostafa, H.Y.; Abd El-Aty, D.M.; Ashmawy, A.M. Novel Gemini ionic liquid for oxidative desulfurization of gas oil. Sci. Rep. 2023, 13, 6198. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Khoshsima, A.; Sabzi, M.; Rostam, A. Toward Application of Ionic Liquids to Desulfurization of Fuels: A Review. Energy Fuels 2022, 36, 4119–4152. [Google Scholar] [CrossRef]
- Gao, H.; Li, W.; Xing, J.; Li, Y.; Xiong, X.; Liu, H. Research Advance in Deep Desulfurization of Fuel Oil by Extraction with Ionic Liquid. Petrochem. Technol. 2007, 36, 966–970. [Google Scholar]
- An, Y.; Lu, L.; Li, C.; Cheng, S.; Gao, G. Oxidative Desulfurization Catalyzed by Molybdophosphate-Based Ionic Liquid. Chin. J. Catal. 2009, 30, 1222–1226. [Google Scholar]
- Liu, D.; Gui, J.; Liu, D.; Peng, X.; Yang, S.; Sun, Z. Deep Oxidative Desulfurization of Real Diesel Catalyzed by Na2WO4 in Ionic Liquid. Energy Sources Part A-Recovery Util. Environ. Eff. 2013, 35, 1–8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, R.; Matulis, V.E. Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel. Int. J. Environ. Res. Public Health 2024, 21, 914. https://doi.org/10.3390/ijerph21070914
Wang P, Wang R, Matulis VE. Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel. International Journal of Environmental Research and Public Health. 2024; 21(7):914. https://doi.org/10.3390/ijerph21070914
Chicago/Turabian StyleWang, Peng, Rui Wang, and Vitaly Edwardovich Matulis. 2024. "Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel" International Journal of Environmental Research and Public Health 21, no. 7: 914. https://doi.org/10.3390/ijerph21070914




