Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Pretreatment of Lentinus Edodes
2.3. Preparation of DESs
2.4. SWE Assisted with DES for Extracting Polysaccharides
2.5. Single Factor Design of Experiments
2.6. BBD Design of Experiments
2.7. Comparison between Different Extraction Methods
2.7.1. Hot Water Extraction
2.7.2. The Extraction of Subcritical Water
2.7.3. The Extraction of SWE Assisted with DES
2.8. Structural Characteristics
2.8.1. Monosaccharide Composition
2.8.2. Molecular Weight
2.8.3. Ultraviolet Spectroscopy
2.8.4. FT-IR Spectroscopy
2.8.5. SEM Analysis
2.9. Antioxidant Activities
2.9.1. DPPH Radical Scavenging Activity
2.9.2. Hydroxyl Radical Scavenging Activity
2.9.3. H2O2 Scavenging Activity
2.10. Statistical Analysis
3. Results and Discussion
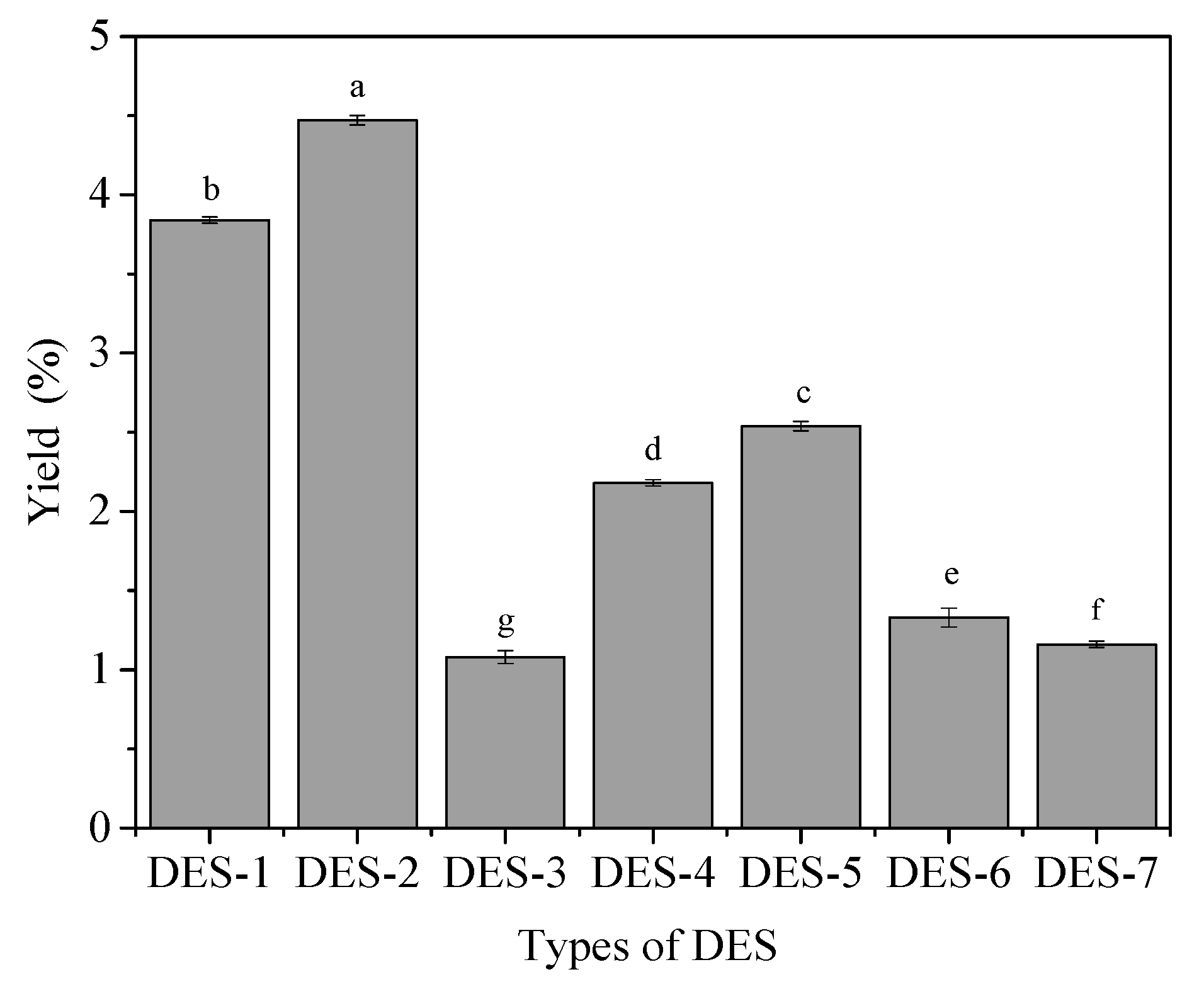
3.1. Different Types of DES Selection
3.2. Single-Factor Experiment
3.2.1. The Factor of Extraction Temperature
3.2.2. The Factor of Extraction Time
3.2.3. The Factor of the Liquid–Solid Ratio
3.2.4. The Factor of Water Content
3.3. The Extraction of LEPD Optimized by BBD
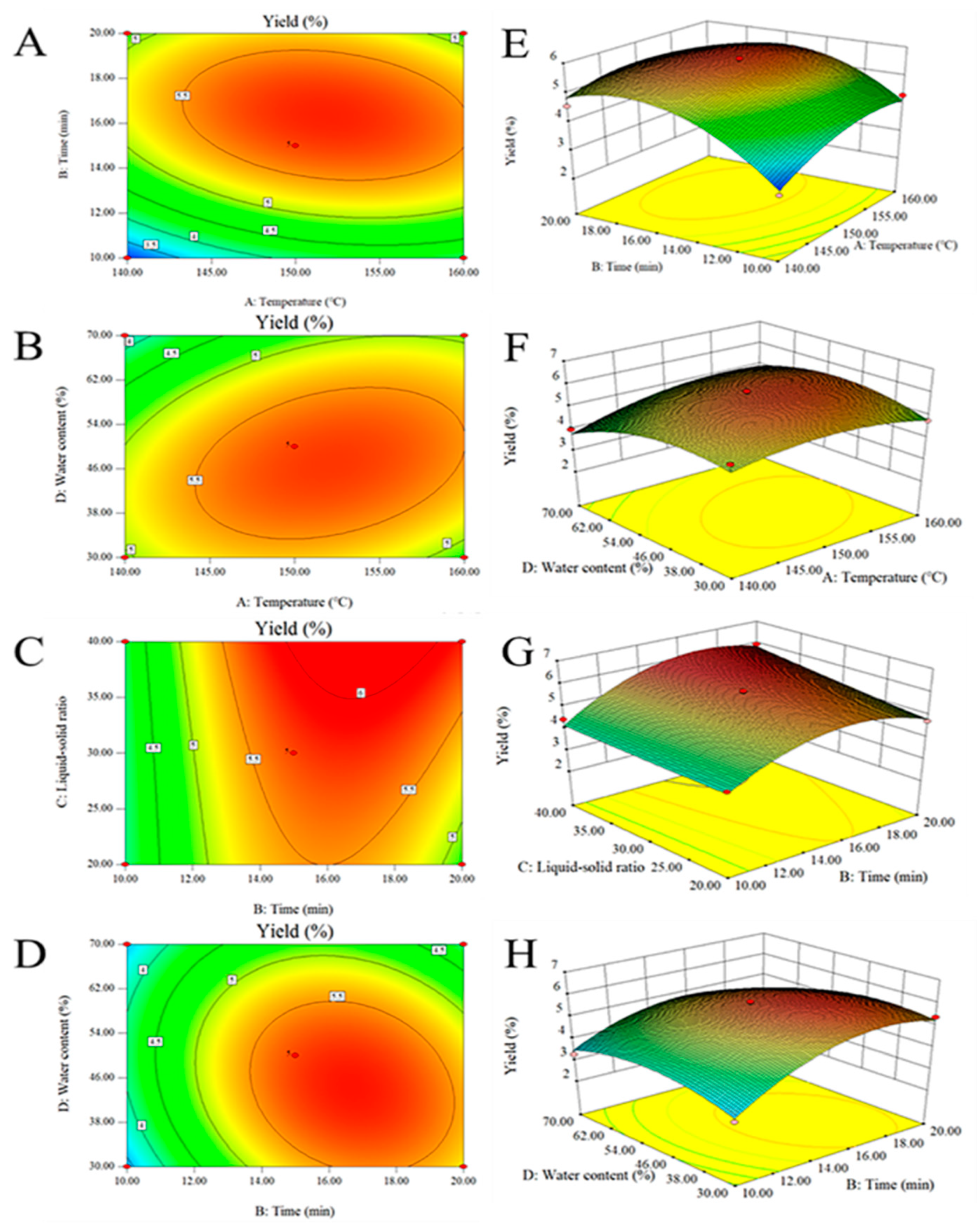
3.3.1. Statistical Analysis and Model Fitting
3.3.2. Verification of the Model
3.4. Comparison of LEPD with Other Samples
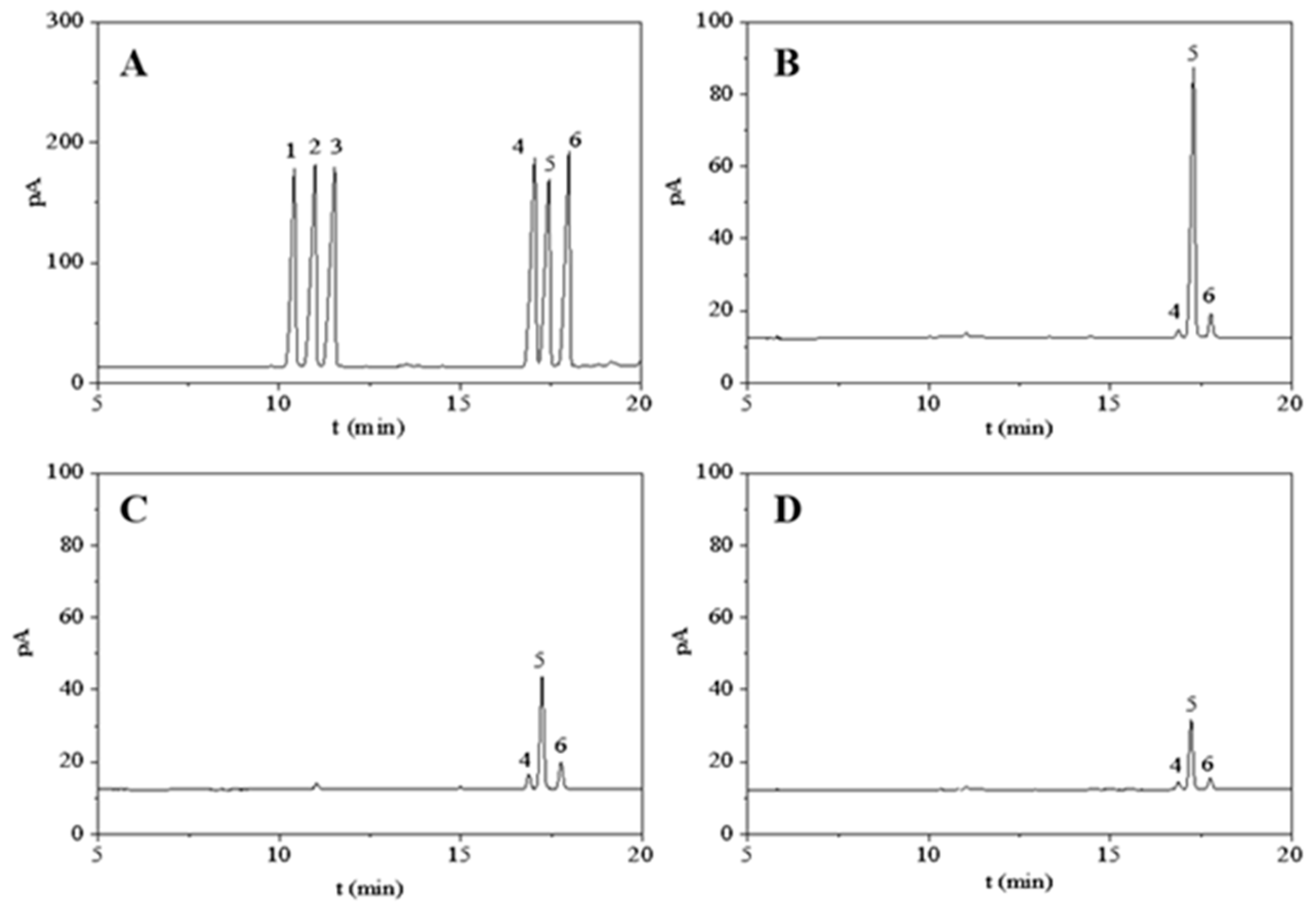
3.4.1. Monosaccharide Composition Analysis
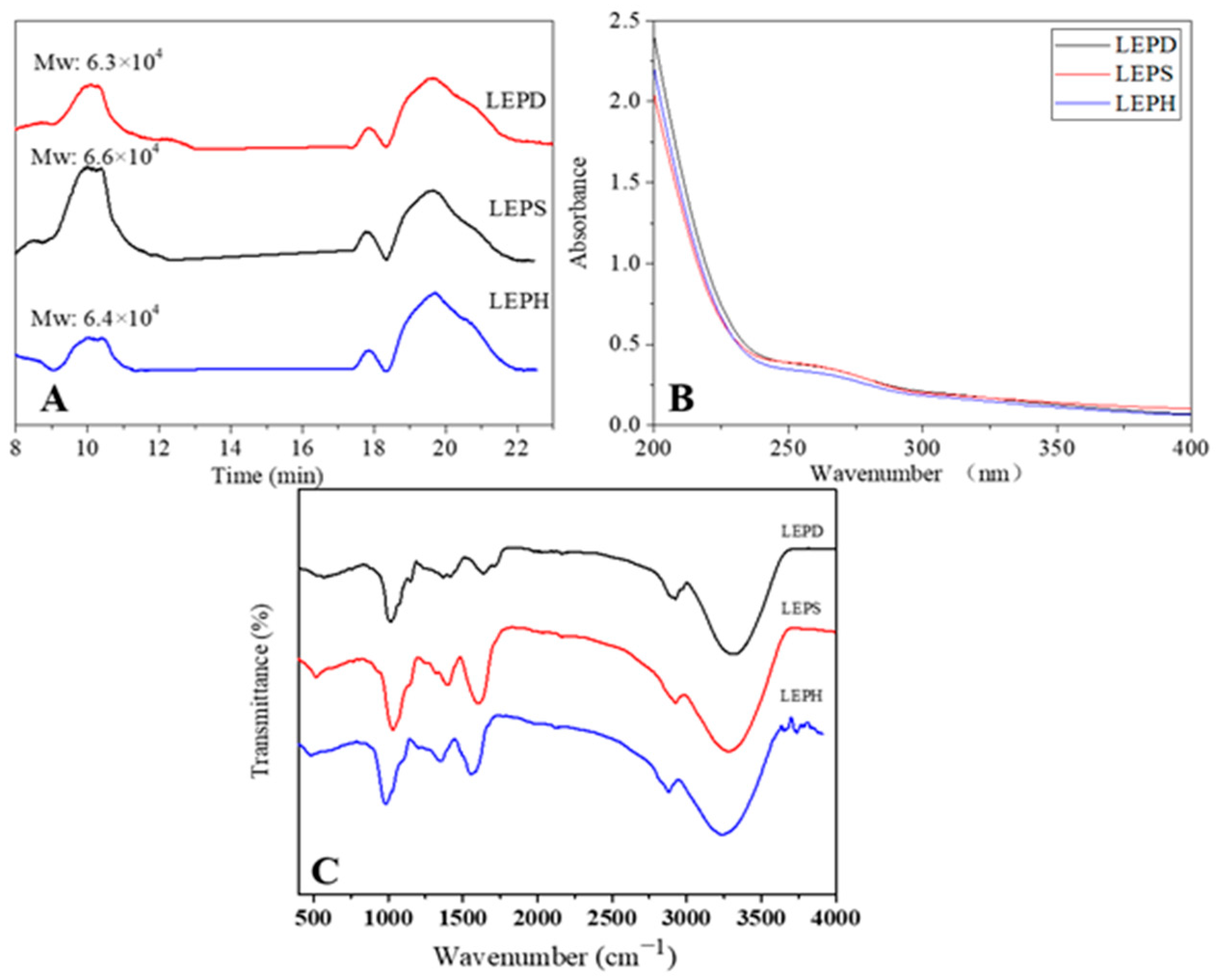
3.4.2. Analysis of Molecular Weight
3.4.3. UV Analysis
3.4.4. FT-IR Analysis
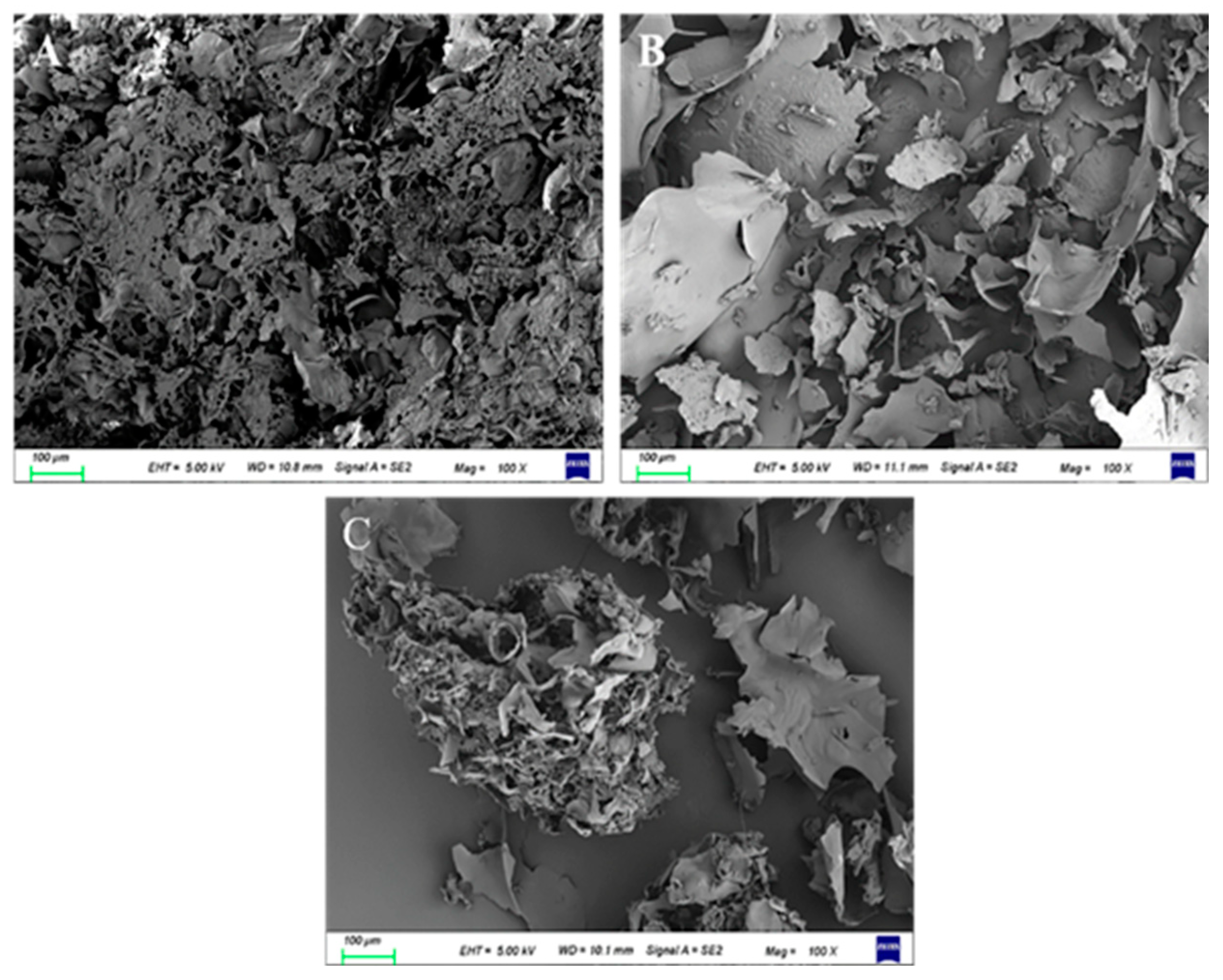
3.4.5. SEM Analysis
3.5. Antioxidant Activities of LEPs
3.5.1. DPPH Scavenging Activity
3.5.2. Hydroxyl Radical Scavenging Activity
3.5.3. Hydrogen Peroxide Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Attia, S.M.; Harisa, G.I.; Abd-Allah, A.R.; Ahmad, S.F.; Bakheet, S.A. The Influence of Lentinan on the Capacity of Repair of DNA Damage and Apoptosis Induced by Paclitaxel in Mouse Bone Marrow Cells. J. Biochem. Mol. Toxicol. 2013, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.M.; Zhang, G.X.; Kuai, J.J.; Fan, P.; Wang, X.X.; Zhou, P.; Yang, D.; Zheng, X.C.; Liu, X.M.; Wu, Q.L.; et al. Lentinan inhibits tumor angiogenesis via interferon gamma and in a T cell independent manner. J. Exp. Clin. Can. Res. 2018, 37, 260. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Shi, Y.L.; Sun, M.J. Effects of lentinan on the function o phagocyte in long-term heavy-duty exercising mice. Eur. J. Inflamm. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Ajith, T.A.; Janardhanan, K.K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Finimundy, T.C.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J.A.P.; Dillon, A.J.P.; Roesch-Ely, M. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr. Res. 2013, 33, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fu, X.; You, L.; Abbasi, A.M.; Meng, H.; Liu, D.; Aadil, R.M. Antioxidant, antitumor and immunomodulatory activities of water-soluble polysaccharides in Abrus cantoniensis. Int. J. Biol. Macromol. 2016, 89, 707–716. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antitumor Activity of Polysaccharides: An Overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Polyethylene glycol-based ultrasound-assisted extraction and ultrafiltration separation of polysaccharides from Tremella fuciformis (snow fungus). Food Bioprod. Process. 2016, 100, 464–468. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Hromadkova, Z.; Ebringerova, A.; Valachovic, P. Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.). Ultrason. Sonochem. 2002, 9, 37–44. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Rahman, A.; Li, J.H.; Wei, C.Y.; Chen, J.L.; Linhardt, R.J.; Ye, X.Q.; Chen, S.G. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.-K.; Zhang, W.-J.; Cao, Y.; Wang, S.; Kang, C.-Z.; Zhou, L.; Yang, Q.; Zhou, L.-Y.; Guo, L.-P. Extraction and separation, structure analysis and biological activity of polysaccharides from Atractylodis Rhizoma. China J. Chin. Mater. Med. 2021, 46, 2133–2141. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.; Liu, M.; Chen, F.; Yang, W.; Chang, X.; Liu, N.; Zhao, Y.; Wang, J.; Chen, X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohyd. Res. 2020, 494, 108037. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.-S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Luo, X.P.; Duan, Y.Q.; Yang, W.Y.; Zhang, H.H.; Li, C.Z.; Zhang, J.X. Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohyd. Polym. 2017, 157, 794–802. [Google Scholar] [CrossRef]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of polysaccharides extraction from Pacific oyster (Crassostrea gigas) using subcritical water: Structural characterization and biological activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Ritta, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids as Green Solvents: Progress and Prospects. American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Huber, V.; Muller, L.; Degot, P.; Touraud, D.; Kunz, W. NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa. Food Chem. 2021, 355, 129624. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Chen, M.; Lahaye, M. Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocolloid. 2021, 115, 106601. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, T.T.; Chen, Y.Y.; Wang, S.Y.; Kang, Y.F. Analysis of the polysaccharide fractions isolated from pea (Pisum sativum L.) at different levels of purification. J. Food Biochem. 2020, 44, e13248. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F.J. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yan, J.K.; Li, L.; Wang, Z.M.; Wu, J.Y. Structural elucidation of an exopolysaccharide from mycelial fermentation of a Tolypocladium sp fungus isolated from wild Cordyceps sinensis. Carbohyd. Polym. 2010, 79, 125–130. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhang, J.; Liang, L.; Miao, F.; Ji, T.; Ye, Z.; Chu, M.; Ren, J.; Xu, X. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2021, 179, 418–428. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.J.; Liu, R.H.; You, L.J. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef]
- Cao, C.; Li, C.; Chen, Q.; Huang, Q.; Pérez, M.E.M.; Fu, X. Physicochemical characterization, potential antioxidant and hypoglycemic activity of polysaccharide from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 139, 1009–1017. [Google Scholar] [CrossRef]
- Yan, S.; Pan, C.; Yang, X.; Chen, S.; Qi, B.; Huang, H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe(2+)-ultrasonic treatment: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 182, 129–135. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-N.; Woo, H.-C.; Chun, B.-S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef]
- Martin-Ramos, P.; Martin-Gil, J.; Gomez-Garcia, D.; Cuchi-Oterino, J.A. On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth. Plants 2020, 9, 1180. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, X.; Zhang, Z.; Su, X.; Shi, M. Thermal Processing Effects on the Chemical Constituent and Antioxidant Activity of Okara Extracts Using Subcritical Water Extraction. J. Chem. 2018, 2018, 6823789. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.-J.; Qian, L.-S.; Sun, W.-J.; Zhang, J.-S.; Yang, Y.; Li, N.; Zhuang, H.-N.; Wu, D. Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization. Molecules 2018, 23, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Liu, X.; Liu, J.; Fan, W.; Li, Y.; et al. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochemistry 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Liu, X.; Zhang, X.; Xu, Y.; Leng, F.; Avwenagbiku, M.O. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 2019, 128, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Chu, D.; Zhang, J.; Zheng, Y.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Li, Q.; Zhang, Q.; You, R.; Cheng, Y.; Luo, L.; Zhang, Y. Structural differences and conformational characterization of five bioactive polysaccharides from Lentinus edodes. Food Res. Int. 2014, 62, 223–232. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocolloid. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Liu, G.; Ye, J.; Li, W.; Zhang, J.; Wang, Q.; Zhu, X.A.; Miao, J.Y.; Huang, Y.H.; Chen, Y.J.; Cao, Y. Extraction, structural characterization, and immunobiological activity of ABP Ia polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 162, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, S.; Wang, X.H.; Zhang, L.N.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocolloid. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Liu, Z.H.; Xu, M.Y.; Dongye, G.Z. Extraction and analysis of sargassum hemiphyllum polysaccharides. Spectrosc. Spectr. Anal. 2004, 24, 1560–1562. [Google Scholar]
- Zhu, K.; Zhang, Y.; Nie, S.; Xu, F.; He, S.; Gong, D.; Wu, G.; Tan, L. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. pulp. Carbohyd. Polym. 2017, 155, 354–361. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohyd. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Li, Q.; Yu, N.; Wang, Y.; Sun, Y.; Lu, K.; Guan, W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohyd. Polym. 2013, 96, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Regenstein, J.M.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Liu, J.; Ke, C.; Sun, Y.; Ye, H.; Zeng, X. Structural characterization of polysaccharides from Hyriopsis cumingii. Carbohyd. Polym. 2010, 82, 1184–1190. [Google Scholar] [CrossRef]
- Ren, G.M.; Xu, L.M.; Lu, T.Y.; Yin, J.S. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Qin, W.; Qin, P.; Zhang, H.; Duan, Y. Ultrasonic-enhanced subcritical water extraction of polysaccharides by two steps and its characterization from Lentinus edodes. Int. J. Biol. Macromol. 2018, 118, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Feng, S.; Jia, X.; Li, Q.; Zhou, Y.; Ding, C. Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohyd. Polym. 2013, 92, 63–68. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tu, Z.C.; Jiang, Y.; Xiao, H.; Zhang, Q.T.; Wang, H. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of lentinan. Int. J. Biol. Macromol. 2012, 51, 926–932. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Yuan, J.-F.; Zhang, Z.-Q.; Fan, Z.-C.; Yang, J.-X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohyd. Polym. 2008, 74, 822–827. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. The antioxidant activities of carboxymethylated cushaw polysaccharide. Int. J. Biol. Macromol. 2019, 121, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Liu, W.; Qi, Q.; Hu, X.; Li, S.; Lei, J.; Rong, L. Extraction of Polysaccharide from Dendrobium nobile Lindl. by Subcritical Water Extraction. ACS Omega 2019, 4, 20586–20594. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zeng, Y.; Wang, H.; Lou, W. Extraction, purification and antioxidant activity of novel polysaccharides from Dendrobium officinale by deep eutectic solvents. Nat. Prod. Res. 2019, 33, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohyd. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]


| Number | Component 1 | Component 2 | Molar Ratio |
|---|---|---|---|
| DES-1 | Choline chloride | Urea | 1:2 |
| DES-2 | Choline chloride | Malonate | 1:2 |
| DES-3 | Choline chloride | 1,2-Propanediol | 1:2 |
| DES-4 | Choline chloride | Glycerol | 1:2 |
| DES-5 | Choline chloride | Ethylene glycol | 1:2 |
| DES-6 | Choline chloride | 1,3-Butanediol | 1:2 |
| DES-7 | Choline chloride | 1,4-Butanediol | 1:2 |
| Variable | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Temperature (°C)/X1 | 140 | 150 | 160 |
| Extraction time (min)/X2 | 10 | 15 | 20 |
| Liquid–solid solvent (mL/g)/X3 | 20 | 30 | 40 |
| Water content (%)/X4 | 30 | 50 | 70 |
| Std. | No. | X1 | X2 | X3 | X4 | Y (%) |
|---|---|---|---|---|---|---|
| 5 | 1 | 150 | 15 | 20 | 30 | 4.80 ± 0.13 |
| 6 | 2 | 150 | 15 | 40 | 30 | 5.68 ± 0.06 |
| 26 | 3 | 150 | 15 | 30 | 50 | 5.88 ± 0.08 |
| 29 | 4 | 150 | 15 | 30 | 50 | 5.69 ± 0.12 |
| 23 | 5 | 150 | 10 | 30 | 70 | 3.27 ± 0.05 |
| 15 | 6 | 150 | 10 | 40 | 50 | 4.43 ± 0.16 |
| 13 | 7 | 150 | 10 | 20 | 50 | 4.13 ± 0.08 |
| 7 | 8 | 150 | 15 | 20 | 70 | 4.72 ± 0.06 |
| 11 | 9 | 140 | 15 | 30 | 70 | 3.99 ± 0.14 |
| 25 | 10 | 150 | 15 | 30 | 50 | 5.86 ± 0.11 |
| 17 | 11 | 140 | 15 | 20 | 50 | 4.60 ± 0.10 |
| 12 | 12 | 160 | 15 | 30 | 70 | 4.80 ± 0.08 |
| 1 | 13 | 140 | 10 | 30 | 50 | 2.93 ± 0.04 |
| 18 | 14 | 160 | 15 | 20 | 50 | 5.50 ± 0.16 |
| 3 | 15 | 140 | 20 | 30 | 50 | 4.56 ± 0.12 |
| 28 | 16 | 150 | 15 | 30 | 50 | 5.61 ± 0.17 |
| 2 | 17 | 160 | 10 | 30 | 50 | 4.30 ± 0.07 |
| 27 | 18 | 150 | 15 | 30 | 50 | 5.60 ± 0.09 |
| 20 | 19 | 160 | 15 | 40 | 50 | 5.48 ± 0.13 |
| 9 | 20 | 140 | 15 | 30 | 30 | 5.26 ± 0.07 |
| 19 | 21 | 140 | 15 | 40 | 50 | 5.40 ± 0.05 |
| 16 | 22 | 150 | 20 | 40 | 50 | 5.94 ± 0.12 |
| 21 | 23 | 150 | 10 | 30 | 30 | 3.23 ± 0.05 |
| 24 | 24 | 150 | 20 | 30 | 70 | 4.18 ± 0.09 |
| 10 | 25 | 160 | 15 | 30 | 30 | 4.77 ± 0.06 |
| 22 | 26 | 150 | 20 | 30 | 30 | 5.37 ± 0.11 |
| 8 | 27 | 150 | 15 | 40 | 70 | 5.01 ± 0.05 |
| 4 | 28 | 160 | 20 | 30 | 50 | 4.86 ± 0.16 |
| 14 | 29 | 150 | 20 | 20 | 50 | 4.74 ± 0.07 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 17.93 | 14 | 1.28 | 28.08 | <0.0001 a | significant |
| X1 | 0.74 | 1 | 0.74 | 16.11 | 0.0013 a | |
| X2 | 4.51 | 1 | 4.51 | 98.96 | <0.0001 a | |
| X3 | 0.99 | 1 | 0.99 | 21.74 | 0.0004 a | |
| X4 | 0.82 | 1 | 0.82 | 18.01 | 0.0008 a | |
| X1 X2 | 0.29 | 1 | 0.29 | 6.27 | 0.0252 a | |
| X1 X3 | 0.17 | 1 | 0.17 | 3.69 | 0.0755 b | |
| X1 X4 | 0.42 | 1 | 0.42 | 9.26 | 0.0088 a | |
| X2 X3 | 0.20 | 1 | 0.20 | 4.44 | 0.0536 b | |
| X2 X4 | 0.38 | 1 | 0.38 | 8.29 | 0.0121 a | |
| X3 X4 | 0.087 | 1 | 0.087 | 1.91 | 0.1889 b | |
| X12 | 1.45 | 1 | 1.45 | 31.72 | <0.0001 a | |
| X22 | 6.96 | 1 | 6.96 | 152.65 | <0.0001 a | |
| X32 | 4.108 × 10−3 | 1 | 4.108 × 10−3 | 0.090 | 0.7685 b | |
| X42 | 2.69 | 1 | 2.69 | 58.90 | <0.0001 a | |
| Residual | 0.64 | 14 | 0.046 | |||
| Lack of Fit | 0.57 | 10 | 0.057 | 3.13 | 0.1411 b | Not significant |
| Pure Error | 0.072 | 4 | 0.018 | |||
| Cor Total R2 R2adj R2pred Adeq precision C.V.% | 18.57 0.9656 0.9312 0.8182 17.914 4.41 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ye, Z.; Liu, G.; Liang, L.; Wen, C.; Liu, X.; Li, Y.; Ji, T.; Liu, D.; Ren, J.; et al. Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities. Molecules 2022, 27, 3612. https://doi.org/10.3390/molecules27113612
Zhang J, Ye Z, Liu G, Liang L, Wen C, Liu X, Li Y, Ji T, Liu D, Ren J, et al. Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities. Molecules. 2022; 27(11):3612. https://doi.org/10.3390/molecules27113612
Chicago/Turabian StyleZhang, Jixian, Zhiqiang Ye, Guoyan Liu, Li Liang, Chaoting Wen, Xiaofang Liu, Youdong Li, Tao Ji, Dongming Liu, Jiaoyan Ren, and et al. 2022. "Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities" Molecules 27, no. 11: 3612. https://doi.org/10.3390/molecules27113612
APA StyleZhang, J., Ye, Z., Liu, G., Liang, L., Wen, C., Liu, X., Li, Y., Ji, T., Liu, D., Ren, J., & Xu, X. (2022). Subcritical Water Enhanced with Deep Eutectic Solvent for Extracting Polysaccharides from Lentinus edodes and Their Antioxidant Activities. Molecules, 27(11), 3612. https://doi.org/10.3390/molecules27113612






