Application of an Adsorption Process on Selected Materials, Including Waste, as a Barrier to the Pesticide Penetration into the Environment
Abstract
:1. Introduction
2. Materials and Methods
- (a)
- Sorbate
- (b)
- Sorbent
- (c)
- Analytical procedure
- (d)
- Sorption procedure
- (e)
- Computer calculations
- (f)
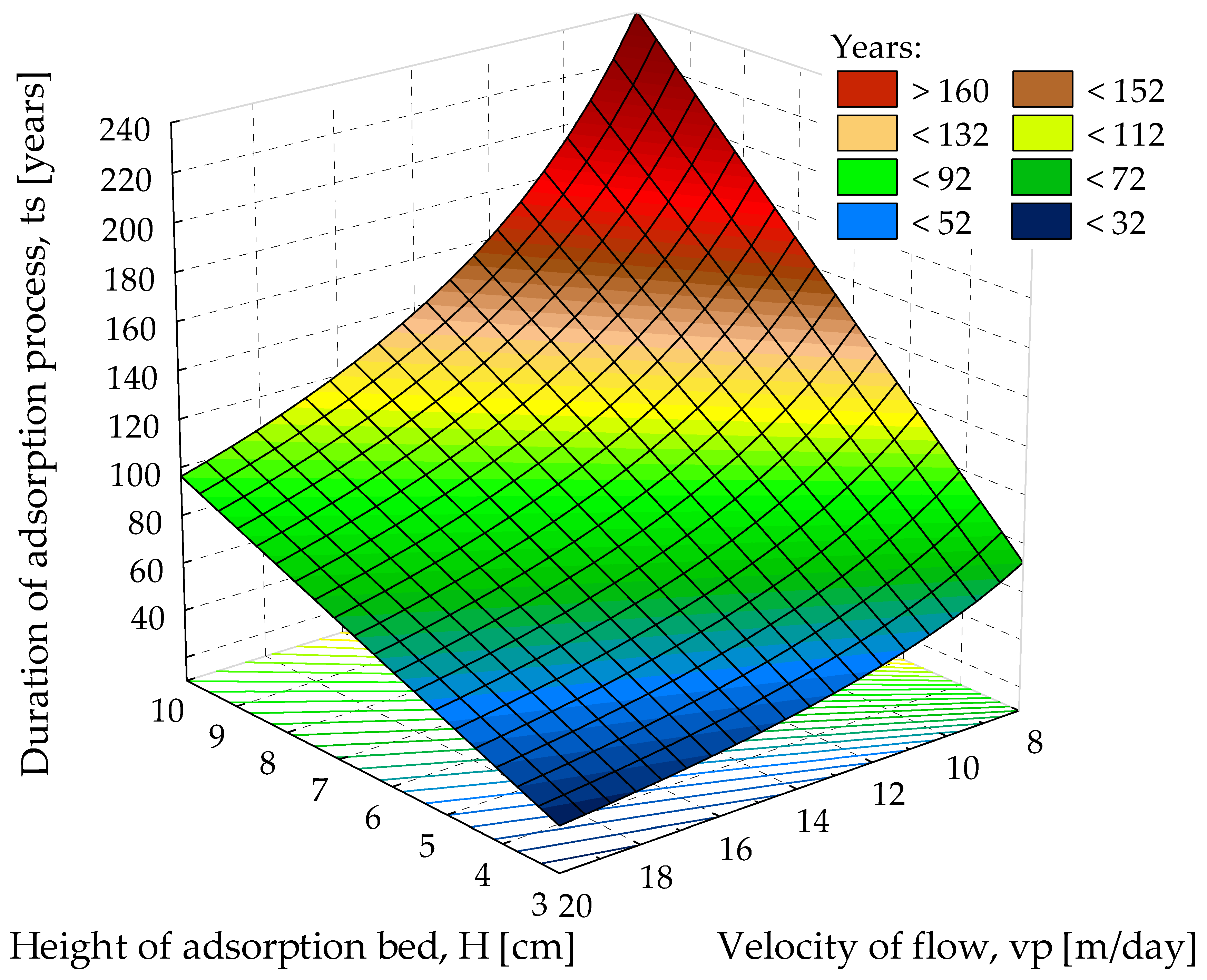
- Adsorption bed working time
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ignatowicz, K. Pesticide Waste Burials in the Area of Podlaskie Province. Rocz. Ochr. Środowiska 2008, 10, 545–555. [Google Scholar]
- Ignatowicz, K. Occurrence Study of Agrochemical Pollutants in Waters of Suprasl Catchment. Arch. Environ. Prot. 2009, 35, 69–77. [Google Scholar]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. Pesticides Removal from Waste Water by Activated Carbon Prepared from Waste Rubber Tire. Water Res. 2011, 45, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Ignatowicz, K. Sorption Process for Migration Reduction of Pesticides from Graveyards. Arch. Environ. Prot. 2008, 34, 143–149. [Google Scholar]
- Ignatowicz, K. Selection of Sorbent for Removing Pesticides during Water Treatment. J. Hazard. Mater. 2009, 169, 953–957. [Google Scholar] [CrossRef]
- Ignatowicz, K. Metals Content Chosen for Environmental Component Monitoring in Graveyards. Fresenius Environ. Bull. 2011, 20, 270–273. [Google Scholar]
- Kogut, P.; Piekarski, J.; Ignatowicz, K. Start-up of Biogas Plant with Inoculating Sludge Application. Rocz. Ochr. Środowiska 2014, 16, 534–545. [Google Scholar]
- Hassan, A.M.; El-Moteleb, M.A.; Ismael, A. Removal of Lindane and Malathion from Wastewater by Activated Carbon Prepared from Apricot Stone. Assiut Univ. Bull. Environ. 2009, 12, 56–62. [Google Scholar]
- Chin-Pampillo, J.S.; Masís-Mora, M.; Ruiz-Hidalgo, K.; Carazo-Rojas, E.; Rodríguez-Rodríguez, C.E. Removal of Carbofuran Is Not Affected by Co-Application of Chlorpyrifos in a Coconut Fiber/Compost Based Biomixture after Aging or Pre-Exposure. J. Environ. Sci. 2016, 46, 182–189. [Google Scholar] [CrossRef]
- Puchlik, M.; Ignatowicz, K.; Dąbrowski, W. Influence of Bio-Preparation on Wastewater Purtification Process in Constructed Wetlands. J. Ecol. Eng. 2015, 16, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Mudhoo, A.; Ramasamy, D.L.; Bhatnagar, A.; Usman, M.; Sillanpää, M. An Analysis of the Versatility and Effectiveness of Composts for Sequestering Heavy Metal Ions, Dyes and Xenobiotics from Soils and Aqueous Milieus. Ecotoxicol. Environ. Saf. 2020, 197, 110587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Y.; Hu, D.; Fan, J.; Shen, M.; Zeng, G. Is Vermicompost the Possible in Situ Sorbent? Immobilization of Pb, Cd and Cr in Sediment with Sludge Derived Vermicompost, a Column Study. J. Hazard. Mater. 2019, 367, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Lin, W.; Sun, S.; Wu, C.; Yang, F.; Ziwei, Y.; Chen, N.; Ren, J.; Zheng, S. Calcium Oxide Modification of Activated Sludge as a Low-Cost Adsorbent: Preparation and Application in Cd(II) Removal. Ecotoxicol. Environ. Saf. 2021, 209, 111760. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dou, J.; Xu, H. Removal of Cr(VI) Ions by Sewage Sludge Compost Biomass from Aqueous Solutions: Reduction to Cr(III) and Biosorption. Appl. Surf. Sci. 2017, 425, 728–735. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Comparison of the Sorption Capacity of Basic, Acid, Direct and Reactive Dyes by Compost in Batch Conditions. J. Environ. Manag. 2021, 294, 113005. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of Herbicides after Repeated Application in Soils Amended with Green Compost and Sewage Sludge. J. Environ. Manag. 2018, 223, 1068–1077. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Gambín, M.; Navarro, S. Leaching Behaviour Appraisal of Eight Persistent Herbicides on a Loam Soil Amended with Different Composted Organic Wastes Using Screening Indices. J. Environ. Manag. 2020, 273, 111179. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Ordax, J.M.; Draoui, K.; Azejjel, H.; Rodríguez-Cruz, M.S. Organic Sorbents as Barriers to Decrease the Mobility of Herbicides in Soils. Modelling of the Leaching Process. Geoderma 2018, 313, 205–216. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Varshney, S. Removal of Reactofix Golden Yellow 3 RFN from Aqueous Solution Using Wheat Husk—An Agricultural Waste. J. Hazard. Mater. 2007, 142, 443–448. [Google Scholar] [CrossRef]
- Ignatowicz, K.; Piekarski, J.; Skoczko, I.; Piekutin, J. Analysis of Simplified Equations of Adsorption Dynamics of HCH. Desalin. Water Treat. 2016, 57, 1420–1428. [Google Scholar] [CrossRef]
- Ninković, M.B.; Petrović, R.D.; Laušević, M.D. Removal of Organochlorine Pesticides from Water Using Virgin and Regenerated Granular Activated Carbon. J. Serb. Chem. Soc. 2010, 75, 565–573. [Google Scholar] [CrossRef]
- Piekarski, J.; Dąbrowski, T. Analysis of simplified equations of the dynamics of adsorption. J. Ecol. Eng. 2011, 24, 32–39. [Google Scholar]
- Valičková, M.; Derco, J.; Šimovičová, K. Removal of Selected Pesticides by Adsorption. Acta Chim. Slovaca 2013, 6, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Piekarski, J.; Ignatowicz, K.; Dąbrowski, T. Analysis of Selected Methods Use for Calculation of the Coefficients of Adsorption Isotherms and Simplified Equations of Adsorption Dynamics with the Use of IZO Application. Materials 2021, 14, 4192. [Google Scholar] [CrossRef]
- Ludvík, J.; Zuman, P. Adsorption of 1,2,4-Triazine Pesticides Metamitron and Metribuzin on Lignin. Microchem. J. 2000, 64, 15–20. [Google Scholar] [CrossRef]
- Tiwari, A.; Bind, A. Effective Removal of Pesticide (Dichlorvos) by Adsorption onto Super Paramagnetic Poly (Styrene-Co-Acrylic Acid) Hydrogel from Water. Int. Res. J. Environ. Sci. 2014, 3, 41–46. [Google Scholar]
- Dąbrowski, W.; Żyłka, R.; Malinowski, P. Evaluation of Energy Consumption during Aerobic Sewage Sludge Treatment in Dairy Wastewater Treatment Plant. Environ. Res. 2017, 153, 135–139. [Google Scholar] [CrossRef]
- Moawed, E.A.; Radwan, A.M. Application of Acid Modified Polyurethane Foam Surface for Detection and Removing of Organochlorine Pesticides from Wastewater. J. Chromatogr. B 2017, 1044–1045, 95–102. [Google Scholar] [CrossRef]
- Zuhra Memon, G.; Bhanger, M.I.; Akhtar, M. The Removal Efficiency of Chestnut Shells for Selected Pesticides from Aqueous Solutions. J. Colloid Interface Sci. 2007, 315, 33–40. [Google Scholar] [CrossRef]
- Chen, X. Modeling of Experimental Adsorption Isotherm Data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.; Chen, X.G.; Jayaseelan, S. The Potential Application of Activated Carbon from Sewage Sludge to Organic Dyes Removal. Water Sci. Technol. 2001, 43, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.V.; Safonov, A.V.; Konevnik, Y.V.; Teterin, Y.A.; Maslakov, K.I.; Teterin, A.Y.; Karaseva, Y.Y.; German, K.E.; Zakharova, E.V. Activated Carbon Additives for Technetium Immobilization in Bentonite-Based Engineered Barriers for Radioactive Waste Repositories. J. Hazard. Mater. 2021, 401, 123436. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Gupta, S.; Gajbhiye, V.T.; Manjaiah, K.M. Optimization of Isotherm Models for Pesticide Sorption on Biopolymer-Nanoclay Composite by Error Analysis. Chemosphere 2017, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Li, C.; Cai, Z.; Zhang, W.; Gao, H.; Chen, L.; Zeng, G.; Shu, X.; Zhao, Y. Study on Activated Carbon Derived from Sewage Sludge for Adsorption of Gaseous Formaldehyde. Bioresour. Technol. 2011, 102, 942–947. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M.; Sobek, S. Water Solution Purification by Phenol Adsorption on Solid Fraction from Thermal Treatment of Waste Biomass—Occurrences of Unfavourable Phenomenon. Desalin. Water Treat. 2020, 186, 72–77. [Google Scholar] [CrossRef]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the Adsorption and Desorption Behaviors of Antibiotics by Degradable MPs with or without UV Ageing Process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
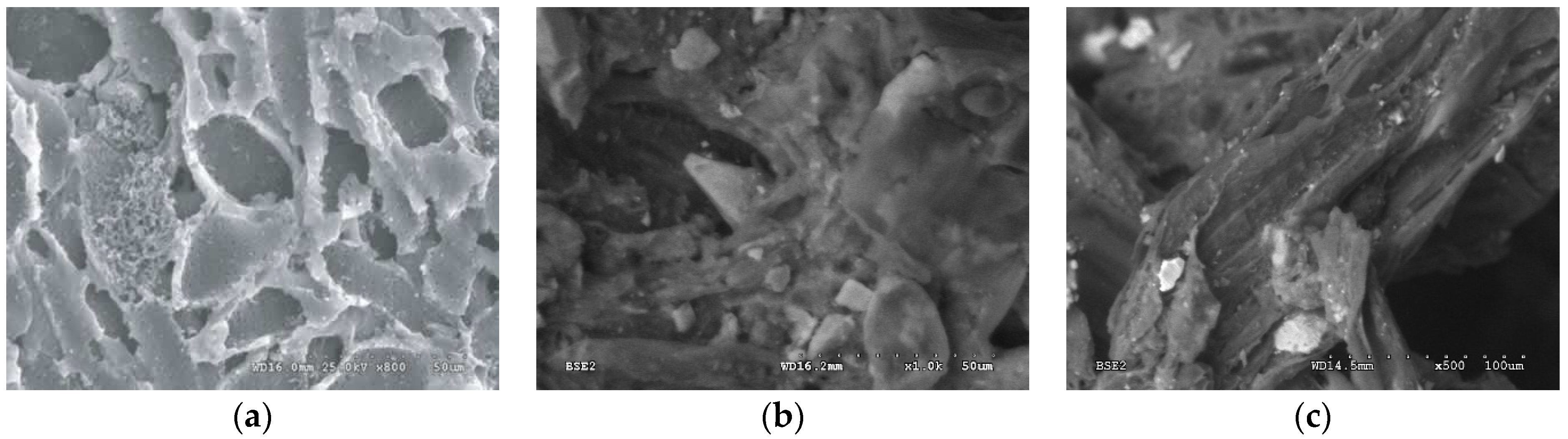

| Parameter | Unit | WG-12 | NP-5 | Compost |
|---|---|---|---|---|
| Surface area | m2/g | 900–1000 | 1300–1500 | - |
| Total pore volume | cm3/g | 0.8–0.9 | min. of 0.7 | - |
| Granulation | mm | 1.2–2.0 | 0.75–1.2 | - |
| Dechlorination | cm | 5–7 | 5–8 | - |
| Methylene blue | cm3 | min. of 18 | min. of 40 | - |
| Iodine number | mg/g | 850–950 | 1390 | 95 |
| Hardness | % | 90 | 95–97 | - |
| Grindability | % | 3.0 | 0.3 | - |
| Fertilizing components (mg/kgdm) | ||||||
| Ca | Mg | Nog | N-NH4+ | Pog | C | K |
| 5.61 | 0.46 | 1.39 | 0.009 | 1.47 | 0.45 | 5.61 |
| Metals content (mg/kgdm) | ||||||
| Pb | Cu | Cd | Cr | Ni | Zn | Hg |
| 7.0 | 22.7 | 0.63 | 9.9 | 5.8 | 210 | 2.5 |
| Other (%) | ||||||
| pH | Water content | Dry mass | Organic matter | |||
| 6.7 | 67.5 | 32.5 | 67.5 | |||
| Parameter | Sample No. | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Initial concentration, C0 (mg/dm3) | 5.00 | |||||
| Adsorbent dose, m (g) | 0.002 | 0.004 | 0.006 | 0.010 | 0.015 | 0.025 |
| Equilibrium concentration, Cr (mg/dm3) | ||||||
| NP5 activated carbon | 0.2466 | 0.1196 | 0.0801 | 0.0450 | 0.0317 | 0.0188 |
| WG-12 activated carbon | 0.0451 | 0.0210 | 0.0129 | 0.0065 | 0.0044 | 0.0027 |
| Compost from sewage sludge | 0.6300 | 0.3560 | 0.2590 | 0.0989 | 0.0807 | 0.0556 |
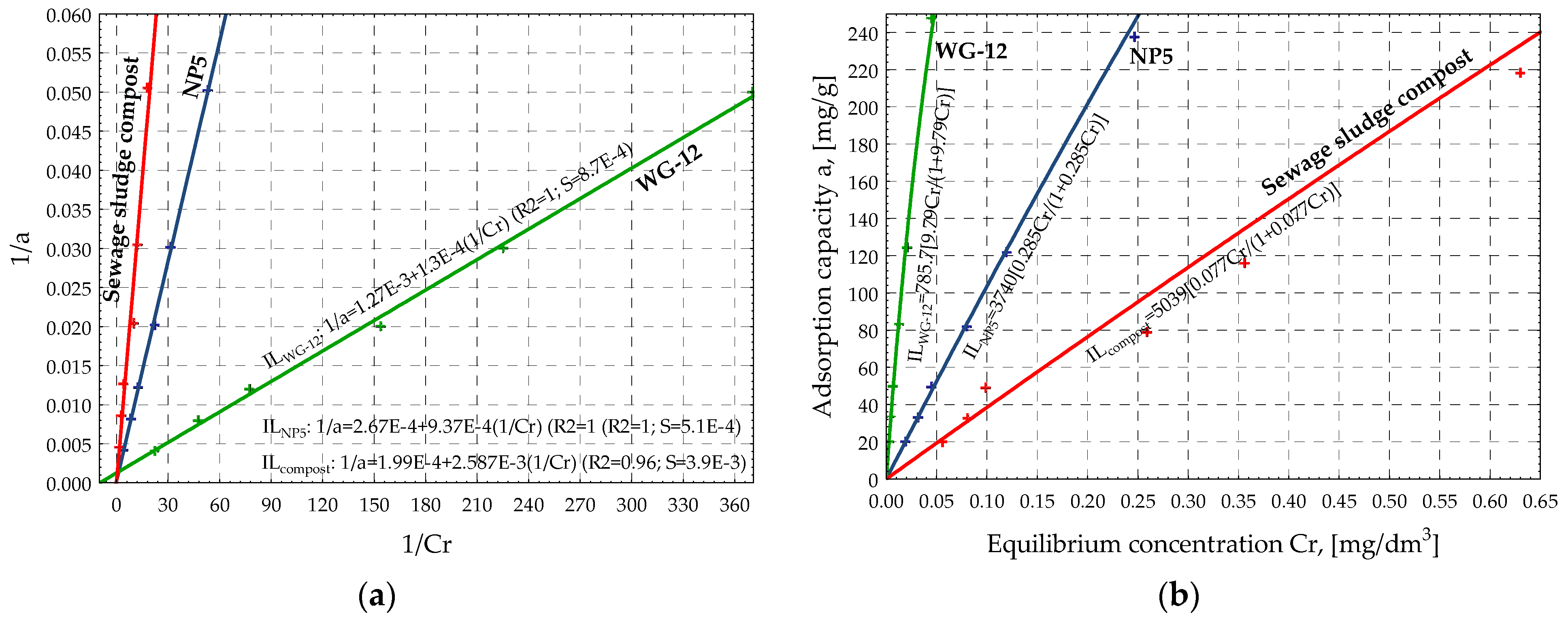
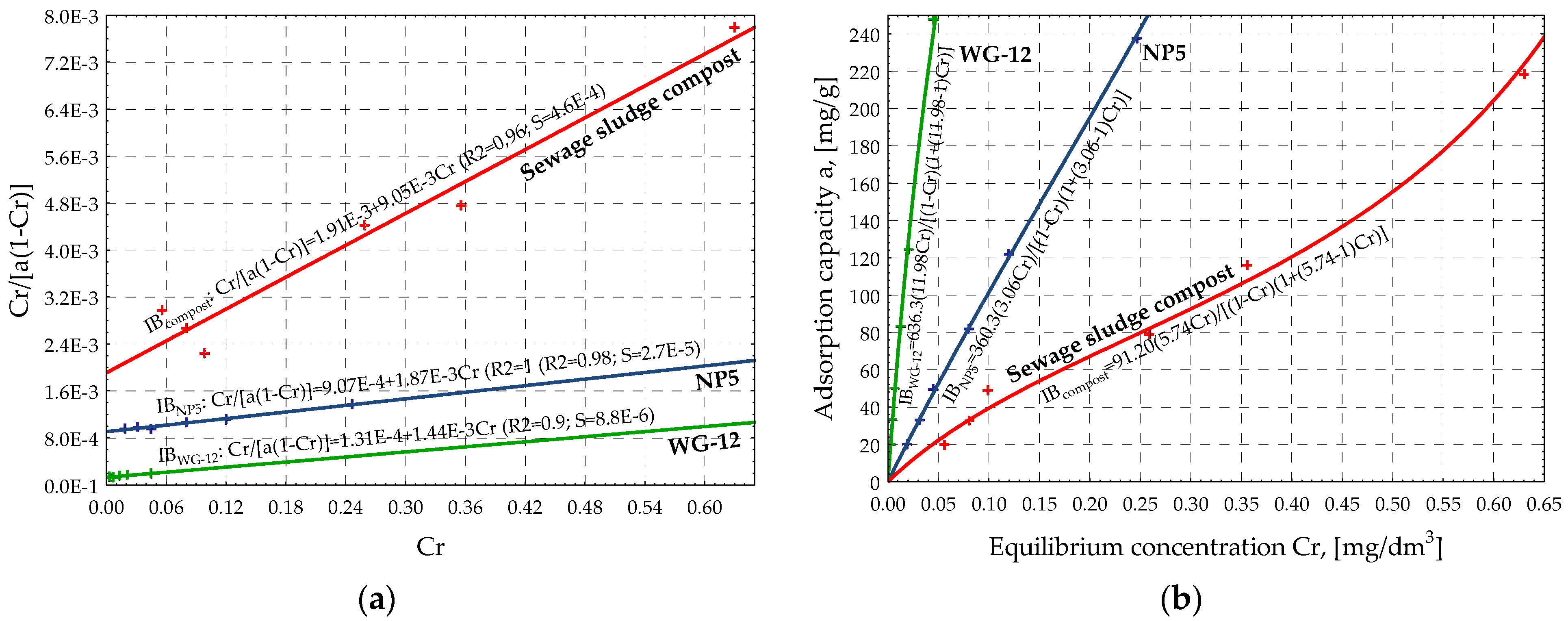
| Adsorbent | Isotherms | Linear Equation | Classical Equation | ||||
|---|---|---|---|---|---|---|---|
| Coefficient | Value | R2 | S | Coefficient | Value | ||
| NP-5 | Freundlich | a | 6.840 | 1.00 | 3.0 × 10−2 | k | 932.5 |
| b | 0.962 | n | 1.039 | ||||
| Langmuir | a | 2.67 × 10−4 | 1.00 | 5.1 × 10−5 | xm | 3740 | |
| b | 9.37 × 10−4 | KL | 0.285 | ||||
| BET | a | 9.07 × 10−4 | 0.98 | 2.7 × 10−6 | xm | 360.3 | |
| b | 1.87 × 10−3 | KB | 3.06 | ||||
| WG-12 | Freundlich | a | 8.230 | 1.00 | 5.5 × 10−2 | k | 3754 |
| b | 0.875 | n | 1.143 | ||||
| Langmuir | a | 1.27 × 10−3 | 1.00 | 8.7 × 10−4 | xm | 785.7 | |
| b | 1.30 × 10−4 | KL | 9.79 | ||||
| BET | a | 1.31 × 10−4 | 0.90 | 8.8 × 10−6 | xm | 636.3 | |
| b | 1.44 × 10−3 | KB | 11.98 | ||||
| sewage sludge compost | Freundlich | a | 5.740 | 0.97 | 0.17 | k | 309.6 |
| b | 0.902 | n | 1.108 | ||||
| Langmuir | a | 1.99 × 10−4 | 0.96 | 3.9 × 10−3 | xm | 5039 | |
| b | 2.59 × 10−3 | KL | 0.077 | ||||
| BET | a | 1.91 × 10−3 | 0.96 | 4.6 × 10−4 | xm | 91.20 | |
| b | 9.05 × 10−3 | KB | 5.737 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarski, J.; Ignatowicz, K.; Dąbrowski, T. Application of an Adsorption Process on Selected Materials, Including Waste, as a Barrier to the Pesticide Penetration into the Environment. Materials 2022, 15, 4680. https://doi.org/10.3390/ma15134680
Piekarski J, Ignatowicz K, Dąbrowski T. Application of an Adsorption Process on Selected Materials, Including Waste, as a Barrier to the Pesticide Penetration into the Environment. Materials. 2022; 15(13):4680. https://doi.org/10.3390/ma15134680
Chicago/Turabian StylePiekarski, Jacek, Katarzyna Ignatowicz, and Tomasz Dąbrowski. 2022. "Application of an Adsorption Process on Selected Materials, Including Waste, as a Barrier to the Pesticide Penetration into the Environment" Materials 15, no. 13: 4680. https://doi.org/10.3390/ma15134680
APA StylePiekarski, J., Ignatowicz, K., & Dąbrowski, T. (2022). Application of an Adsorption Process on Selected Materials, Including Waste, as a Barrier to the Pesticide Penetration into the Environment. Materials, 15(13), 4680. https://doi.org/10.3390/ma15134680






