Social Wellbeing in Cancer Survivorship: A Cross-Sectional Analysis of Self-Reported Relationship Closeness and Ambivalence from a Community Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Measures
2.2.1. Impact of Cancer on Relationships
2.2.2. Demographic and Clinical Information
2.3. Data Analysis
3. Results
3.1. Sample
3.2. Exploratory Factor Analysis
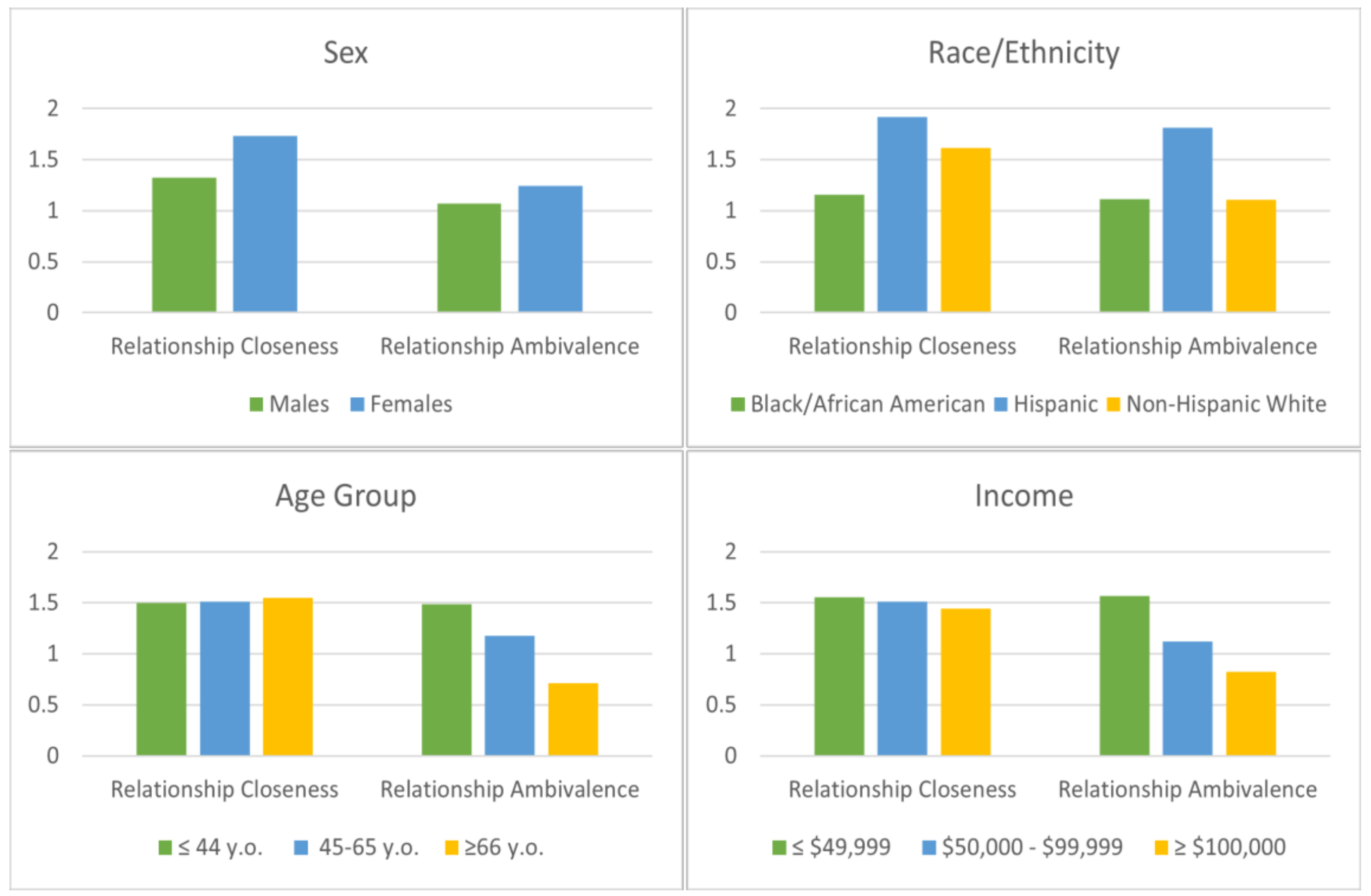
3.3. Differences in Relationship Closeness and Ambivalence by Sociodemographic Characteristics
3.4. Differences in Relationship Closeness and Ambivalence by Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- AACR. Disparities in Cancer Survivorship; AACR: Orlando, FL, USA, 2020. [Google Scholar]
- Fitch, M.I.; Nicoll, I.; Lockwood, G. Exploring the impact of physical, emotional, and practical changes following treatment on the daily lives of cancer survivors. J. Psychosoc. Oncol. 2020, 39, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.R.; Weaver, K.E.; Aziz, N.M.; Alfano, C.M.; Bellizzi, K.M.; Kent, E.E.; Forsythe, L.P.; Rowland, J.R. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. 2015, 9, 239–251. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Rowland, J.H.; Paskett, E.D.; Van Leeuwen, F.; Moskowitz, C.; Wollins, S.K.; Robison, L.L. Identification of key gaps in cancer survivorship research: Findings from the American Society of Clinical Oncology Survey. J. Oncol. Pract. 2016, 12, 190–193. [Google Scholar] [CrossRef]
- Meeker, C.R.; Wong, Y.-N.; Egleston, B.L.; Hall, M.J.; Plimack, E.R.; Martin, L.P.; von Mehren, M.; Lewis, B.R.; Geynisman, D.M. Distress and financial distress in adults with cancer: An age-based analysis. J. Natl. Compr. Cancer Netw. 2017, 15, 1224. [Google Scholar] [CrossRef] [Green Version]
- Vrontaras, N. Cancer patients’ views on the family changes and the family social support. J. Eur. Psychol. Stud. 2018, 9, 16–27. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Mehnert, A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011, 77, 109–130. [Google Scholar] [CrossRef]
- Mehnert, A.; de Boer, A.; Feurstein, M. Employment challenges for cancer survivors. Am. Cancer Soc. 2013, 119, 2151–2159. [Google Scholar] [CrossRef]
- Lange, M.; Licaj, I.; Clarisse, B.; Humbert, X.; Grellard, J.M.; Tron, L.; Joly, F. Cognitive complaints in cancer survivors and expectations for support: Results from a web–based survey. Cancer Med. 2019, 8, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.J.; Hollingdrake, O.; Bui, U.; Nekhlyudov, L.; Hart, N.H.; Lui, C.-W.; Feuerstein, M. Evolving landscape of cancer survivorship research: An analysis of the Journal of Cancer Survivorship, 2007–2020. J. Cancer Surviv. 2021, 15, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, K.I.; Wiedt, T.L.; Daniels, E.C.; Yabroff, K.R.; Guerra, C.E.; Wender, R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J. Clin. 2020, 70, 31–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and ethnic disparities in cancer survival: The Contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef]
- Howell, D.; Mayer, D.K.; Fielding, R.; Eicher, M.; Verdonck-de Leeuw, I.M.; Johansen, C.; Soto-Perez-de-Celis, E.; Foster, C.; Chan, R.; Alfano, C.M.; et al. Management of cancer and health after the clinic visit: A call to action for self-management in cancer care. JNCI J. Natl. Cancer Inst. 2020, 113, 523–531. [Google Scholar] [CrossRef]
- Lisy, K.; Kent, J.; Piper, A.; Jefford, M. Facilitators and barriers to shared primary and specialist cancer care: A systematic review. Support. Care in Cancer. 2020, 29, 85–96. [Google Scholar] [CrossRef]
- Nekhlyudov, L.; Mollica, M.A.; Jacobsen, P.B.; Mayer, D.K.; Shulman, L.N.; Geiger, A.M. Developing a Quality of Cancer Survivorship Care Framework: Implications for clinical care, research, and policy. J. Natl. Cancer Inst. 2019, 111, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- Kent, E.E.; Rowland, J.H.; Northouse, L.; Litzelman, K.; Chou, W.-Y.S.; Shelburne, N.; Timura, C.; O’Mara, A.; Huss, K. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer 2016, 122, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Alfano, C.M.; Leach, C.R.; Smith, T.G.; Miller, K.D.; Alcaraz, K.I.; Cannady, R.S.; Wender, R.C.; Brawley, O.W. Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J. Clin. 2019, 69, 35–49. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Smith, A.; Schmit, S.; Keegan, T.H.M.; Zebrack, B.; Lynch, C.F.; Deapen, D.; Shnorhavorian, M.; Tompkins, B.J.; Simon, M. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Am. Cancer Soc. 2012, 118, 5155–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keesing, S.; Rosenwax, L.; McNamara, B. A dyadic approach to understanding the impact of breast cancer on relationships between partners during early survivorship. BMC Women’s Health 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquati, C.; Kayser, K. Dyadic coping across the lifespan: A comparison between younger and middle-aged couples with breast cancer. Front. Psychol. 2019, 10, 404. [Google Scholar] [CrossRef]
- Manne, S.; Kashy, D.; Virtue, S.M.; Zaider, T. Relationship communication and the course of psychological outcomes among couples coping with localised prostate cancer. Eur. J. Cancer Care 2021, 30, e13401. [Google Scholar] [CrossRef]
- Weaver, K.E.; Forsythe, L.P.; Reeve, B.B.; Alfano, C.M.; Rodriguez, J.; Sabatino, S.A.; Hawkins, N.A.; Rowland, J.H. Mental and physical health-related quality of life among U.S. cancer survivors: Population estimates from the 2010 National Health Interview Survey. Cancer Empidemiol. Biomark. Prev. 2012, 21, 2108–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woźniak, K.; Iżycki, D. Cancer: A family at risk. Menopause Rev. 2014, 13, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Litzelman, K.; Yabroff, K.R. How are spousal depressed mood, distress and quality of life associated with risk of depressed mood in cancer survivors? Longitudinal findings from a national sample. Cancer Epidemiol. Biomark. Prev. 2016, 24, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Hawkey, A.J.; Ussher, J.M.; Perz, J.; Parton, C.; Patterson, P.; Bateson, D.; Hobbs, K.; Kirsten, L. The impact of cancer-related fertility concerns on current and future couple relationships: People with cancer and partner perspectives. Eur. J. Cancer Care 2020, 30, e13348. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Yi, J.; Wright, J.; Warner, E.L.; Smith, K.R. Marriage and divorce among young adult cancer survivors. J. Cancer Surviv. 2012, 6, 441–450. [Google Scholar] [CrossRef]
- Song, H.Y.; Kwon, J.A.; Choi, J.W.; Kim, S.J.; Park, E.C. Gender differences in marital disruption among patients with cancer: Results from the Korean National Health and Nutrition Examination Survey (KNHANES). Asian Pac. J. Cancer Prev. 2014, 15, 6547–6552. [Google Scholar] [CrossRef]
- Stephens, C.; Westmaas, J.L.; Kim, J.; Cannady, R.; Stein, K. Gender differences in associations between cancer-related problems and relationship dissolution among cancer survivors. J. Cancer Surviv. 2016, 10, 865–873. [Google Scholar] [CrossRef]
- Fugmann, D.; Boeker, M.; Holsteg, S.; Steiner, N.; Prins, J.; Karger, A. A systematic review: The effect of cancer on the divorce rate. Front. Psychol. 2022, 13, 828656. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, B.; Karger, A.; Zimmermann, T. Cancer and relationship dissolution: Perspective of partners of cancer patients. Front. Psychol. 2021, 12, 624902. [Google Scholar] [CrossRef] [PubMed]
- Bowling, A. Measuring Disease. A Review of Disease-Specific Quality of Life Measurement Scales; Open University Press: Buckingham, UK, 2001. [Google Scholar]
- Kraemer, L.M.; Stanton, A.L.; Meyerowitz, B.E.; Rowland, J.H.; Ganz, P.A. A longitudinal examination of couples’ coping strategies as predictors of adjustment to breast cancer. J. Fam. Psychol. 2011, 25, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, I.; Newell, C. Measuring Health: A Guide to Rating Scales and Questionnaires; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Cicognani, E. Social Well-Being. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 6193–6197. [Google Scholar]
- Lo, C.; Lin, J.; Gagliese, L.; Zimmermann, C.; Mikulincer, M.; Rodin, G. Age and depression in patients with metastatic cancer: The protective effects of attachment security and spiritual wellbeing. Ageing Soc. 2009, 30, 325–336. [Google Scholar] [CrossRef]
- Kadambi, S.; Soto-Perez-de-Celis, E.; Garg, T.; Loh, K.P.L.; Krok-Schoen, J.L.; Battisti, N.M.L.; Moffat, G.T.; Gil, L.J., Jr.; Mohile, S.; Hsuh, T. Social support for older adults with cancer: Young international society of geriatric oncology review paper. J. Geriatr. Oncol. 2020, 11, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Farrell, A.K.; Stanton, S.C.E. Toward a mechanistic understanding of links between close relationships and physical Health. Curr. Dir. Psychol. Sci. 2019, 28, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Regan, T.; Acquati, C.; Zimmermann, T. Interpersonal Relationships. In Handbook of Cancer Survivorship; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–284. [Google Scholar]
- Manne, S.; Badr, H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer 2008, 112, 2541–2555. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Carver, C.S.; Ting, A. Family caregivers’ unmet needs in long-term cancer survivorship. Semin. Oncol. Nurs. 2019, 35, 380–383. [Google Scholar] [CrossRef]
- Husson, O.; Mols, F.; Fransen, M.P.; Poll-Franse, L.V.; Ezendam, N.P.M. Low subjective health literacy is associated with adverse health behaviors and worse health-related quality of life among colorectal cancer survivors: Results from the profiles registry. Psychooncology 2015, 24, 478–486. [Google Scholar] [CrossRef]
- Manne, S.; Kashy, D.A.; Siegel, S.; Virtue, S.M.; Heckman, C.; Ryan, D. Unsupportive partner behaviors, social-cognitive processing, and psychological outcomes in couples coping with early stage breast cancer. J. Fam. Psychol. 2014, 28, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilstra, C.E.; Fardell, J.E.; Burns, M.A.; Ellis, S.J.; Anazodo, A.C.; Trahair, T.N.; Sansom-Daly, U.M. Determinants of social functioning among adolescents and young adults with cancer: A systematic review. Psychooncology 2021, 30, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Henshall, L.C.; Greenfield, M.S.; Gale, K.N. Typologies for restructuring relationships in cancer survivorship: Temporal changes in social support and engagement with self-management practices. Cancer Nurs. 2018, 41, E32–E40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinnott, S.M.; Park, C.L. Social well-being in Adolescent and Young Adult cancer survivors. J. Adolesc. Young Adult Oncol. 2019, 8, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kashy, D.A.; Spillers, R.L.; Evans, T.V. Needs assessment of family caregivers of cancer survivors: Three cohorts comparison. Psycho Oncol. 2010, 19, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, H.; Bakhshaie, J.; Chhabria, K. Dyadic Interventions for cancer survivors and caregivers: State of the science and new directions. Semin. Oncol. Nurs. 2019, 35, 337–341. [Google Scholar] [CrossRef]
- Luckett, T.; Goldstein, D.; Butow, P.N.; Gebski, V.; Aldridge, L.J.; McGrane, J.; Ng, W.; King, M.T. Psychological morbidity and quality of life of ethnic minority patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2011, 12, 1240–1248. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Acquati, C.; Cheung, M. Family communication and coping among racial-ethnic minority cancer patients: A systematic review. Health Soc. Care Community 2022, 30, e605–e620. [Google Scholar] [CrossRef]
- Chan, R.J.; Nekhlyudov, L.; Duijts, S.F.A.; Hudson, S.V.; Jones, J.M.; Keogh, J.; Love, B.; Lustberg, M.B.; Mehnert-Theuerkauf, A.; Nathan, P.; et al. Future research in cancer survivorship. J. Cancer Surviv. 2021, 15, 659–667. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B. Mplus User’s G. 1998–2012. Available online: file:///C:/Users/MDPI/Downloads/Mplus%20user%20guide%20Ver_7_r6_web.pdf (accessed on 6 February 2019).
- Kim, Y.; Mitchell, H.-R.; Ting, A. Application of psychological theories on the role of gender in caregiving to psycho-oncology research. Psycho Oncol. 2019, 28, 228–254. [Google Scholar] [CrossRef] [PubMed]
- Tamres, L.K.; Janicki, D.; Helgeson, V.S. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personal. Soc. Psychol. Rev. 2002, 6, 2–30. [Google Scholar] [CrossRef]
- Walsh, C.A.; Yi, J.C.; Rosenberg, A.R.; Crouch, M.V.; Leisenring, W.M.; Syrjala, K.L. Factors associated with social functioning among long-term cancer survivors treated with hematopoietic stem cell transplantation as adolescents or young adults. Psychooncology 2020, 29, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Zebrack, B.J.; Aguilar, C.; Hayes-Lattin, B.; Cole, S. Cancer in adolescents and young adults: Who remains at risk of poor social functioning over time? Cancer 2017, 123, 2743–2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquati, C.; Zebrack, B.J.; Faul, A.C.; Embry, L.; Aguilar, C.; Block, R.; Hayes-Lattin, B.; Freyer, D.R.; Cole, S. Sexual functioning among young adult cancer patients: A 2-year longitudinal study. Cancer 2018, 124, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Sopfe, J.; Gupta, A.; Appiah, L.C.; Eric, J.C.; Peterson, P.N. Sexual dysfunction in Adolescent and Young Adult survivors of childhood cancer: Presentation, risk factors, and evaluation of an underdiagnosed late effect. A Narrative Review. J. Adolesc. Young Adult Oncol. 2020, 9, 549–560. [Google Scholar] [CrossRef]
- Robin, C. Impact of Cancer on Romantic Relationships Among Young Adults: A Systematic Review. J. Clin. Psychol. Med. Settings 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Barnett, M.; McDonnell, G.; DeRosa, A.; Schuler, T.; Philip, E.; Peterson, L.; Touza, K.; Jhanwar, S.; Atkinson, T.M.; Ford, J.S. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): A systematic review. J. Cancer Surviv. 2016, 10, 814–831. [Google Scholar] [CrossRef] [Green Version]
- Warner, E.L.; Kent, E.E.; Trevino, K.M.; Parsons, H.M.; Zebrack, B.J.; Kirchhoff, A.C. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer 2016, 122, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.B.; Spencer, J.C.; Pinheiro, L.C.; Carey, L.A.; Olshan, A.F.; Reeder-Hayes, K.E. Financial impact of breast cancer in Black versus White women. J. Clin. Oncol. 2018, 36, 1695–1701. [Google Scholar] [CrossRef]
- Politi, M.C.; Yen, R.W.; Elwyn, G.; O’Malley, A.J.; Saunders, C.H.; Schubbe, D.; Forcino, R.; Durand, M.A. Women who are young, non-white, and with lower socioeconomic status report higher financial toxicity up to 1 year after breast cancer surgery: A mixed-effects regression analysis. Oncologist 2021, 26, e142–e152. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Dowling, E.C.; Guy, G.P., Jr.; Banegas, M.P.; Davidoff, A.; Han, X.; Virgo, K.S.; McNeel, T.S.; Chawla, N.; Blanch-Hartigan, D.; et al. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J. Clin. Oncol. 2016, 34, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Shankaran, V.; Jolly, S.; Blough, D.; Ramsey, S.D. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J. Clin. Oncol. 2012, 30, 1608–1614. [Google Scholar] [CrossRef]
- Chino, F.; Peppercorn, J.M.; Rushing, C.; Kamal, A.H.; Altomare, I.; Samsa, G.; Zafar, S.Y. Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol. 2017, 3, 1582–1584. [Google Scholar] [CrossRef]
- Smith, G.L.; Lopez-Olivo, M.A.; Advani, P.G.; Ning, M.S.; Geng, Y.; Giordano, S.H.; Volk, R.J. Financial burdens of cancer treatment: A systematic review of risk factors and outcomes. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 1184–1192. [Google Scholar] [CrossRef]
- Ver Hoeve, E.S.; Ali-Akbarian, L.; Price, S.N.; Lothfi, N.M.; Hamann, H.A. Patient-reported financial toxicity, quality of life, and health behaviors in insured US cancer survivors. Support. Care Cancer 2021, 29, 349–358. [Google Scholar] [CrossRef]
- Boffa, D.J.; Mallin, K.; Herrin, J.; Resio, B.; Salazar, M.C.; Palis, B.; Facktor, M.; McCabe, R.; Nelson, H.; Shulman, L.N. Survival after cancer treatment at top-ranked US cancer hospitals vs. affiliates of top-ranked cancer hospitals. J. Am. Med. Assoc. Oncol. 2020, 3, e203942. [Google Scholar] [CrossRef]
- Wolfson, J.; Sun, C.-L.; Wyatt, L.; Hurria, A.; Bhatia, S. Impact of care at comprehensive cancer centers on outcome—Results from a population-based study. Cancer 2016, 121, 3885–3893. [Google Scholar] [CrossRef] [Green Version]
- Zebrack, B.; Kayser, K.; Padgett, L.; Sundstrom, L.; Jobin, C.; Nelson, K.; Fineberg, I.C. Institutional capacity to provide psychosocial oncology support services: A report from the Association of Oncology Social Work. Cancer 2016, 122, 1937–1945. [Google Scholar] [CrossRef]

| Variable | N | % |
|---|---|---|
| Sex | ||
| Female | 238 | 47.1% |
| Male | 267 | 52.9% |
| Age (recoded in 3 groups) | ||
| ≤44 | 198 | 39.2% |
| 45–65 | 179 | 35.4% |
| ≥66 | 128 | 25.3% |
| Race/Ethnicity | ||
| Asian | 10 | 2.0% |
| Black/African American | 115 | 22.8% |
| Hispanic | 38 | 7.5% |
| Non-Hispanic white | 333 | 65.9% |
| Other | 9 | 1.8% |
| Income (USD) | ||
| ≤49,999 | 150 | 31.6% |
| 50,000–99,999 | 204 | 43.0% |
| ≥100,000 | 120 | 25.3% |
| Education | ||
| Less than high school | 6 | 1.2% |
| High-school graduate | 63 | 12.5% |
| Some college | 129 | 25.5% |
| Associate degree | 62 | 12.3% |
| Bachelor’s degree | 147 | 29.1% |
| Master’s degree | 78 | 15.4% |
| Doctorate degree | 20 | 4.0% |
| Insurance | ||
| Insurance coverage | 491 | 97.2% |
| Lack of insurance coverage | 14 | 2.8% |
| Region in the US | ||
| Midwest | 116 | 23% |
| Northeast | 129 | 25.5% |
| Southeast | 133 | 26.3% |
| Southwest/West | 127 | 25.1% |
| Cancer Type | ||
| Prostate cancer | 69 | 13.7% |
| Breast cancer (early stage) | 66 | 13.1% |
| Colorectal cancer | 41 | 8.1% |
| Endometrial, cervical, or ovarian cancer | 37 | 7.3% |
| Thyroid | 26 | 5.1% |
| Breast cancer (metastatic) | 24 | 4.8% |
| Bladder | 22 | 4.4% |
| Head and neck | 21 | 4.2% |
| Kidney cancer | 21 | 4.2% |
| Lymphoma | 19 | 3.8% |
| Leukemia | 18 | 3.6% |
| Brain tumor | 17 | 3.4% |
| Liver cancer | 16 | 3.2% |
| Melanoma | 16 | 3.2% |
| Pancreatic | 9 | 1.8% |
| Myeloma | 8 | 1.6% |
| Stomach | 5 | 1.0% |
| Other | 45 | 8.9% |
| Treatment | ||
| No treatment | 19 | 3.8% |
| Single treatment | 202 | 40.0% |
| Multiple treatment | 284 | 56.2% |
| Time Since Diagnosis | ||
| ≤12 months | 89 | 17.6% |
| 13–24 months | 126 | 25.0% |
| >2–4 years | 114 | 22.6% |
| >4 years | 176 | 34.9% |
| Treatment Facility | ||
| Academic medical center | 146 | 29.7% |
| Community cancer center | 84 | 17.1% |
| Community hospital | 151 | 30.8% |
| Private physician practice | 77 | 15.7% |
| US Department of Veterans Affairs medical lefts | 19 | 3.9% |
| Unsure | 14 | 2.9% |
| Current Cancer Status | ||
| Diagnosed but not yet treatment | 36 | 7.1% |
| Active treatment | 110 | 21.8% |
| Completed treatment and maintenance therapy | 137 | 27.1% |
| Completed treatment and not on maintenance therapy | 204 | 40.4% |
| Comfort care | 8 | 1.6% |
| Other | 10 | 2.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquati, C.; Miller-Sonet, E.; Zhang, A.; Ionescu, E. Social Wellbeing in Cancer Survivorship: A Cross-Sectional Analysis of Self-Reported Relationship Closeness and Ambivalence from a Community Sample. Curr. Oncol. 2023, 30, 1720-1732. https://doi.org/10.3390/curroncol30020133
Acquati C, Miller-Sonet E, Zhang A, Ionescu E. Social Wellbeing in Cancer Survivorship: A Cross-Sectional Analysis of Self-Reported Relationship Closeness and Ambivalence from a Community Sample. Current Oncology. 2023; 30(2):1720-1732. https://doi.org/10.3390/curroncol30020133
Chicago/Turabian StyleAcquati, Chiara, Ellen Miller-Sonet, Anao Zhang, and Elena Ionescu. 2023. "Social Wellbeing in Cancer Survivorship: A Cross-Sectional Analysis of Self-Reported Relationship Closeness and Ambivalence from a Community Sample" Current Oncology 30, no. 2: 1720-1732. https://doi.org/10.3390/curroncol30020133
APA StyleAcquati, C., Miller-Sonet, E., Zhang, A., & Ionescu, E. (2023). Social Wellbeing in Cancer Survivorship: A Cross-Sectional Analysis of Self-Reported Relationship Closeness and Ambivalence from a Community Sample. Current Oncology, 30(2), 1720-1732. https://doi.org/10.3390/curroncol30020133





