Patient and Provider Attitudes and Preferences Regarding Early Palliative Care Delivery for Patients with Advanced Gastrointestinal Cancers: A Prospective Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Survey Development
2.3. Recruitment and Data Collection
2.4. Outcomes and Analysis
2.5. Sample Size and Feasibility
3. Results
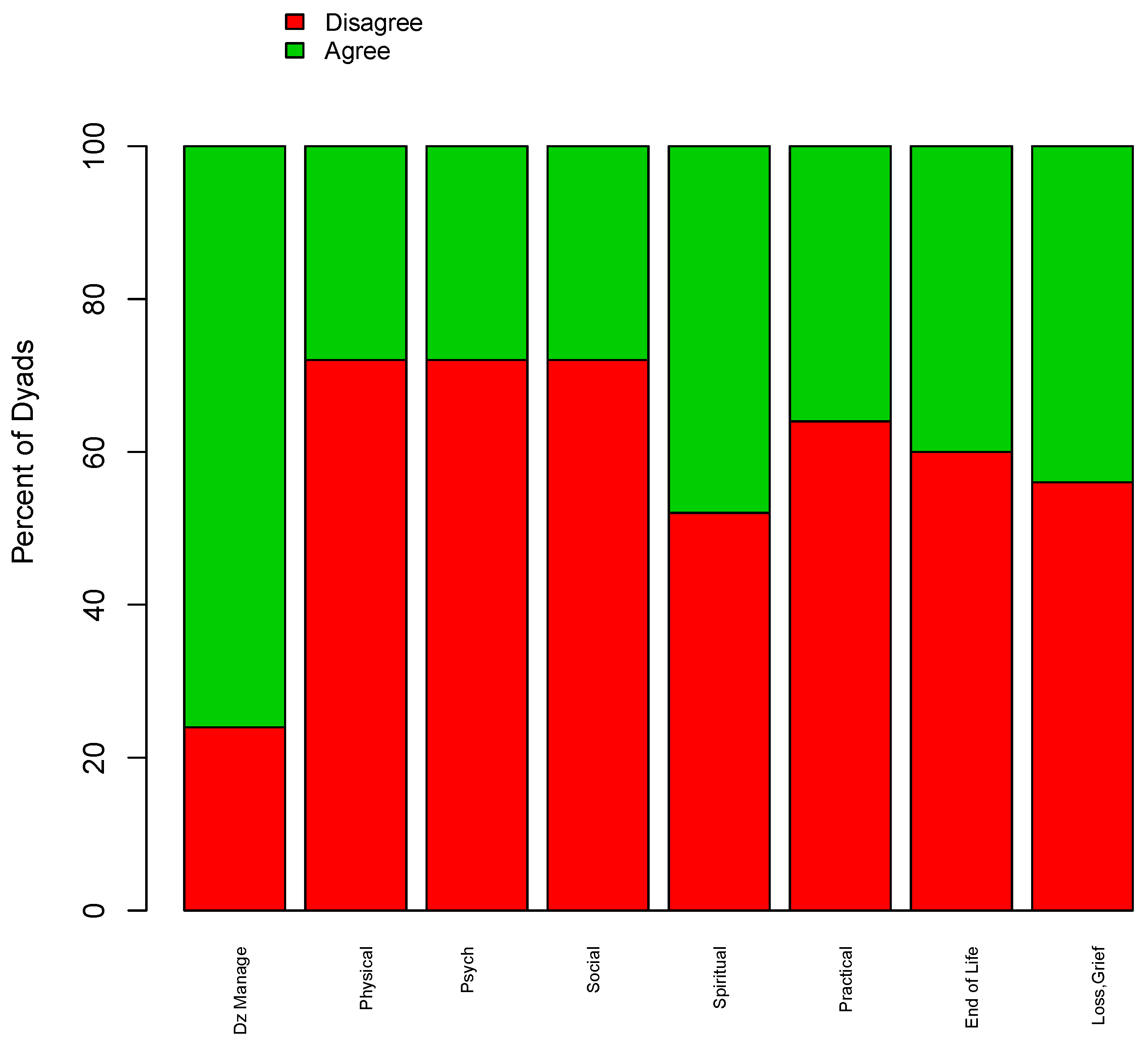
3.1. Perceived Importance of Palliative Care Domains among Patients and Providers
3.2. Patient and Provider Perception of Responsibility for Care Delivery across Domains of Palliative Care
3.3. Perceptions and Agreement among Providers Regarding Responsibility for Domains of Palliative Care
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Definition of Palliative Care. 2016. Available online: http://www.who.int/cancer/palliative/definition/en/ (accessed on 29 April 2024).
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R.; et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009, 302, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T.; Azuero, A.; et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I.; et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; et al. Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J. Clin. Oncol. 2017, 35, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. Guidelines for Palliative Care. 2016. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/guidelines/full/pebc18-3f.pdf (accessed on 29 April 2024).
- Weissman, D.E.; Meier, D.E. Identifying patients in need of a palliative care assessment in the hospital setting: A consensus report from the Center to Advance Palliative Care. J. Palliat. Med. 2011, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Bickel, K.E.; McNiff, K.; Buss, M.K.; Kamal, A.; Lupu, D.; Abernethy, A.P.; Broder, M.S.; Shapiro, C.L.; Acheson, A.K.; Malin, J.; et al. Defining high-quality palliative care in oncology practice: An American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J. Oncol. Pract. 2016, 12, e828–e838. [Google Scholar] [CrossRef]
- Cheng, M.J.; King, L.M.; Alesi, E.R.; Smith, T.J. Doing palliative care in the oncology office. J. Oncol. Pract. 2013, 9, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Bruera, E. Models of integration of oncology and palliative care. Ann. Palliat. Med. 2015, 4, 89–98. [Google Scholar] [CrossRef]
- Touzel, M.; Shadd, J. Content validity of a conceptual model of a palliative approach. J. Palliat. Med. 2018, 21, 1627–1635. [Google Scholar] [CrossRef]
- Caglayan, A.; Redmond, S.; Rai, S.; Rabbani, R.D.; Ghose, A.; Sanchez, E.; Sheriff, M.; Carrim, J.; Boussios, S. The integration of palliative care with oncology: The path ahead. Ann. Palliat. Med. 2023, 12, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.; Scalzitti, D.A.; Padrone, L.; Martins-Welch, D. From evidence to practice: Early integration of palliative care in a comprehensive cancer center. Support. Care Cancer 2023, 31, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.I.; Smith, T.J. Early integration of palliative care into oncology: Evidence, challenges and barriers. Ann. Palliat. Med. 2015, 4, 12231. [Google Scholar]
- Parajuli, J.; Hupcey, J.E. A systematic review on barriers to palliative care in oncology. Am. J. Hosp. Palliat. Med. 2021, 38, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Association CHPC. A Model to Guide Hospice Palliative Care; Association CHPC: Ottawa, ON, Canada, 2013. [Google Scholar]
- Dillman, D.A.; Smyth, J.D.; Christian, L.M. Internet, Phone, Mail, and Mixed Mode Surveys: The Tailored Design Method, 4th ed.; John Wiley & Sons Inc.: Indianapolis, Indiana, 2014. [Google Scholar]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.; Toohey, K.; Bacon, R.; Kavanagh, P.S.; Roberts, C. What are the unmet supportive care needs of people affected by cancer: An umbrella systematic review. Semin. Oncol. Nurs. 2022, 39, 151353. [Google Scholar] [CrossRef]
- Okediji, P.T.; Salako, O.; Fatiregun, O.O. Pattern and predictors of unmet supportive care needs in cancer patients. Cureus 2017, 9, e1234. [Google Scholar] [CrossRef]
- Hart, N.H.; Crawford-Williams, F.; Crichton, M.; Yee, J.; Smith, T.J.; Koczwara, B.; Fitch, M.I.; Crawford, G.B.; Mukhopadhyay, S.; Mahony, J. Unmet supportive care needs of people with advanced cancer and their caregivers: A systematic scoping review. Crit. Rev. Oncol./Hematol. 2022, 176, 103728. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.W.; Weeks, J.C.; Wright, A.A.; Block, S.D.; Prigerson, H.G. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J. Clin. Oncol. 2010, 28, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Zhukovsky, D.S.; Bruera, E. Serious illness conversations: Paving the road with metaphors. Oncologist 2018, 23, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Jackson, V.A.; Meier, D.E.; Temel, J.S. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA A Cancer J. Clin. 2013, 63, 349–363. [Google Scholar] [CrossRef] [PubMed]
- George, L.S.; Matsoukas, K.; McFarland, D.C.; Bowers, J.M.; Doherty, M.J.; Kwon, Y.S.; Atkinson, T.M.; Kozlov, E.; Saraiya, B.; Prigerson, H.G. Interventions to improve prognostic understanding in advanced stages of life-limiting illness: A systematic review. J. Pain Symptom Manag. 2022, 63, e212–e223. [Google Scholar] [CrossRef]
- Weeks, J.C.; Catalano, P.J.; Cronin, A.; Finkelman, M.D.; Mack, J.W.; Keating, N.L.; Schrag, D. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 2012, 367, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psycho-Oncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.A. “Our best hope is a cure.” Hope in the context of advance care planning. Palliat. Support. Care 2012, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bernacki, R.; Paladino, J.; Neville, B.A.; Hutchings, M.; Kavanagh, J.; Geerse, O.P.; Lakin, J.; Sanders, J.J.; Miller, K.; Lipsitz, S.; et al. Effect of the Serious Illness Care Program in outpatient oncology: A cluster randomized clinical trial. JAMA Intern. Med. 2019, 179, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Paladino, J.; Bernacki, R.; Neville, B.A.; Kavanagh, J.; Miranda, S.P.; Palmor, M.; Lakin, J.; Desai, M.; Lamas, D.; Sanders, J.J.; et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: A cluster randomized clinical trial of the Serious Illness Care Program. JAMA Oncol. 2019, 5, 801–809. [Google Scholar] [CrossRef]
- Sussman, J.; Bainbridge, D.; Evans, W.K. Towards integrating primary care with cancer care: A regional study of current gaps and opportunities in Canada. Healthc. Policy 2017, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Easley, J.; Miedema, B.; O’Brien, M.A.; Carroll, J.; Manca, D.; Webster, F.; Grunfeld, E. The role of family physicians in cancer care: Perspectives of primary and specialty care providers. Curr. Oncol. 2017, 24, 75–80. [Google Scholar] [CrossRef]
- Anvik, T.; Holtedahl, K.A.; Mikalsen, H. “When patients have cancer, they stop seeing me”–the role of the general practitioner in early follow-up of patients with cancer–A qualitative study. BMC Fam. Pract. 2006, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; McLoone, J.K.; Wakefield, C.E.; Cohn, R.J. Primary care physicians’ perspectives of their role in cancer care: A systematic review. J. Gen. Intern. Med. 2016, 31, 1222–1236. [Google Scholar] [CrossRef]
- Urquhart, R.; Kotecha, J.; Kendell, C.; Martin, M.; Han, H.; Lawson, B.; Tschupruk, C.; Marshall, E.G.; Bennett, C.; Burge, F. Stakeholders’ views on identifying patients in primary care at risk of dying: A qualitative descriptive study using focus groups and interviews. Br. J. Gen. Pract. 2018, 68, e612–e620. [Google Scholar] [CrossRef]
- Seow, H.; Bainbridge, D.; Winemaker, S.; Stajduhar, K.; Pond, G.; Kortes-Miller, K.; Marshall, D.; Kilbertus, F.; Myers, J.; Steinberg, L. Increasing palliative care capacity in primary care: Study protocol of a cluster randomized controlled trial of the CAPACITI training program. BMC Palliat. Care 2023, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Aubin, M.; Vézina, L.; Verreault, R.; Simard, S.; Hudon, É.; Desbiens, J.F.; Fillion, L.; Dumont, S.; Tourigny, A.; Daneault, S. Continuity of cancer care and collaboration between family physicians and oncologists: Results of a randomized clinical trial. Ann. Fam. Med. 2021, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Shadd, J.D.; Burge, F.; Stajduhar, K.I.; Cohen, S.R.; Kelley, M.L.; Pesut, B. Defining and measuring a palliative approach in primary care. Can. Fam. Physician 2013, 59, 1149–1150. [Google Scholar]
- Pereira, J.; Chasen, M.R. Early palliative care: Taking ownership and creating the conditions. Curr. Oncol. 2016, 23, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.; Baldwin, L.-M. The interface of primary and oncology specialty care: From diagnosis through primary treatment. J. Natl. Cancer Inst. Monogr. 2010, 2010, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, C.; Carron, T.; Cohidon, C.; Arditi, C.; Hong, Q.N.; Pluye, P.; Peytremann-Bridevaux, I.; Gilles, I. An overview of reviews on interprofessional collaboration in primary care: Barriers and facilitators. Int. J. Integr. Care 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Lisy, K.; Kent, J.; Piper, A.; Jefford, M. Facilitators and barriers to shared primary and specialist cancer care: A systematic review. Support. Care Cancer 2021, 29, 85–96. [Google Scholar] [CrossRef]
- Easley, J.; Miedema, B.; Carroll, J.C.; Manca, D.P.; O’Brien, M.A.; Webster, F.; Grunfeld, E. Coordination of cancer care between family physicians and cancer specialists: Importance of communication. Can. Fam. Physician 2016, 62, e608–e615. [Google Scholar] [PubMed]
- Brazil, K.; Sussman, J.; Bainbridge, D.; Whelan, T. Who is responsible? The role of family physicians in the provision of supportive cancer care. J. Oncol. Pract. 2010, 6, 19–24. [Google Scholar] [CrossRef]
- Aubin, M.; Vézina, L.; Verreault, R.; Fillion, L.; Hudon, E.; Lehmann, F.; Leduc, Y.; Bergeron, R.; Reinharz, D.; Morin, D. Family physician involvement in cancer care follow-up: The experience of a cohort of patients with lung cancer. Ann. Fam. Med. 2010, 8, 526–532. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Merom, H.; Sikron, F.; Livneh, J.; Sadetzki, S.; Wolf, I. Involvement of the family physician in the care of chemotherapy-treated patients with cancer: Patients’ perspectives. J. Oncol. Pract. 2014, 10, 298–305. [Google Scholar] [CrossRef]
- Reeves, E.; Liebig, B.; Schweighoffer, R. Care coordination in palliative home care: Who plays the key role? Int. J. Integr. Care 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Cherry, M.; Kennedy, R.; Weeden, K.; Burridge, L.; Clavarino, A.; O’Rourke, P.; Del Mar, C. General practitioner, specialist providers case conferences in palliative care—Lessons learned from 56 case conferences. Aust. Fam. Physician 2005, 34, 389–392. [Google Scholar] [PubMed]
- Abernethy, A.P.; Currow, D.C.; Shelby-James, T.; Rowett, D.; May, F.; Samsa, G.P.; Hunt, R.; Williams, H.; Esterman, A.; Phillips, P.A. Delivery strategies to optimize resource utilization and performance status for patients with advanced life-limiting illness: Results from the “palliative care trial” [ISRCTN 81117481]. J. Pain Symptom Manag. 2013, 45, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Hoerger, M.; Greer, J.A.; Jackson, V.A.; Park, E.R.; Pirl, W.F.; El-Jawahri, A.; Gallagher, E.R.; Hagan, T.; Jacobsen, J.; Perry, L.M.; et al. Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. J. Clin. Oncol. 2018, 36, 1096–1102. [Google Scholar] [CrossRef]
- Barbera, L.; Hwee, J.; Klinger, C.; Jembere, N.; Seow, H.; Pereira, J. Identification of the physician workforce providing palliative care in Ontario using administrative claims data. CMAJ Open 2015, 3, E292–E298. [Google Scholar] [CrossRef]
| Patient Characteristics, n = 66 | No. (%) | |
|---|---|---|
| Age (years) | Median (min, max) | 68.5 (43, 87) |
| Gender | n (%) Female | 23 (34.9) |
| Diagnosis | Oesophagus | 15 (22.7) |
| Colon | 12 (18.2) | |
| Pancreas | 10 (15.2) | |
| Stomach | 9 (13.6) | |
| Rectum | 6 (9.1) | |
| Liver | 5 (7.6) | |
| Other * | 9 (13.6) | |
| Employment Status | Currently Working | 3 (4.6) |
| Currently on Sick Leave | 13 (19.7) | |
| Retired | 48 (72.7) | |
| None of the Above | 2 (3.0) | |
| Relationship Status | Married | 43 (65.2) |
| Not Married, but in a Relationship | 3 (4.6) | |
| Single | 20 (30.3) | |
| Children | n (%) Yes | 56 (84.9) |
| Drug Coverage | No, I have no coverage | 1 (1.5) |
| Yes, I have coverage from government | 34 (51.5) | |
| Yes, I have private insurance | 31 (47.0) | |
| Members of Healthcare Team † | Medical Oncologist | 59 (89.4) |
| Radiation Oncologist | 17 (25.8) | |
| Family Doctor | 27 (40.9) | |
| Specialized Palliative Care Provider | 8 (12.1) | |
| Homecare Nurse | 23 (34.9) | |
| Other | 25 (37.9) | |
| Do you have a Family Doctor | n (%) Yes | 64 (97.0) |
| Time from prescreening to survey completion (days) | Median (range) Days | 35 (0–193) |
| Provider Characteristics †, n = 95 | ||
| Provider Type | Family Physician | 21 (22.1) |
| Medical Oncologist | 65 (68.4) | |
| Specialist Palliative Care Provider | 6 (6.3) | |
| Radiation Oncologist | 3 (3.2) | |
| Sex | n (%) Female | 44 (46.3) |
| Years Practicing | <1 | 4 (4.2) |
| 1–5 | 31 (32.6) | |
| 6–10 | 15 (15.8) | |
| 11–20 | 29 (30.5) | |
| >20 | 16 (16.8) | |
| Specific Training in Palliative Care, n (%) Yes | Family Physician | 6/20 (30.0) |
| Medical Oncologist | 39/65 (60.0) | |
| Specialist Palliative Care Provider | 6/6 (100) | |
| Radiation Oncologist | 1/3 (33.3) | |
| Domain | Agreement between Patients and Physicians Who Deem Domain Most Important or Very Relevant † | Agreement between Patients and Physicians Who Deem Domain as Somewhat or Less Relevant ‡ | Total Agreement |
|---|---|---|---|
| Disease Management | 64/75 (85.3) | 3/18 (16.7) | 67/93 (72.0) |
| Physical Concerns | 53/60 (88.3) | 8/29 (27.6) | 61/89 (68.5) |
| Psychological Concerns | 16/25 (64.0) | 15/66 (22.7) | 31/91 (34.1) |
| Social Concerns | 18/33 (54.6) | 25/55 (45.5) | 43/88 (48.9) |
| Spiritual Concerns | 6/27 (22.2) | 47/64 (73.4) | 53/91 (58.2) |
| Practical Concerns | 17/34 (50.0) | 33/57 (57.9) | 50/91 (54.9) |
| Making Plans in Case Your Health Worsens | 25/47 (53.2) | 25/46 (54.4) | 50/93 (53.8) |
| Loss and Grief | 13/25 (52.0) | 50/65 (76.9) | 63/90 (70.0) |
| Provider Type * | ||||||
|---|---|---|---|---|---|---|
| Domain | Medical Oncologist | Radiation Oncologist | Family Doctor | Specialist Palliative Care Providers | Other | Total Agreement |
| Disease Management | 38/45 (84.4) | 1/1 (100) | 1/3 (33.3) | 1/2 (50.0) | 8/15 (53.3) | 49/66 (74.2) |
| Physical Concerns | 19/26 (73.1) | 1/2 (50.0) | 2/3 (66.6) | 7/9 (77.7) | 24/26 (92.3) | 53/66 (80.3) |
| Psychological Concerns | 0/5 (0) | 0/0 | 1/3 (33.3) | 0/0 | 55/58 (94.8) | 56/66 (84.8) |
| Social Concerns | 0/0 | 0/0 | 3/3 (100) | 1/1 (100) | 62/62 (100) | 66/66 (100) |
| Spiritual Concerns | 1/1 (100) | 0/0 | 0/0 | 1/1 (100) | 64/64 (100) | 66/66 (100) |
| Practical Concerns | 0/0 | 0/0 | 0/0 | 0/0 | 65/66 (98.5) | 65/66 (98.5) |
| Making Plans in Case Your Health Worsens | 3/3 (100) | 0/0 | 3/4 (75.0) | 2/2 (100) | 57/57 (100) | 65/66 (98.5) |
| Loss and Grief | 1/1 (100) | 0/0 | 1/3 (33.3) | 1/1 (100) | 61/61 (100) | 64/66 (97.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levine, O.; Bainbridge, D.; Pond, G.R.; Slaven, M.; Dhesy-Thind, S.; Sussman, J.; Meyer, R.M. Patient and Provider Attitudes and Preferences Regarding Early Palliative Care Delivery for Patients with Advanced Gastrointestinal Cancers: A Prospective Survey. Curr. Oncol. 2024, 31, 3329-3341. https://doi.org/10.3390/curroncol31060253
Levine O, Bainbridge D, Pond GR, Slaven M, Dhesy-Thind S, Sussman J, Meyer RM. Patient and Provider Attitudes and Preferences Regarding Early Palliative Care Delivery for Patients with Advanced Gastrointestinal Cancers: A Prospective Survey. Current Oncology. 2024; 31(6):3329-3341. https://doi.org/10.3390/curroncol31060253
Chicago/Turabian StyleLevine, Oren, Daryl Bainbridge, Gregory R. Pond, Marissa Slaven, Sukhbinder Dhesy-Thind, Jonathan Sussman, and Ralph M. Meyer. 2024. "Patient and Provider Attitudes and Preferences Regarding Early Palliative Care Delivery for Patients with Advanced Gastrointestinal Cancers: A Prospective Survey" Current Oncology 31, no. 6: 3329-3341. https://doi.org/10.3390/curroncol31060253
APA StyleLevine, O., Bainbridge, D., Pond, G. R., Slaven, M., Dhesy-Thind, S., Sussman, J., & Meyer, R. M. (2024). Patient and Provider Attitudes and Preferences Regarding Early Palliative Care Delivery for Patients with Advanced Gastrointestinal Cancers: A Prospective Survey. Current Oncology, 31(6), 3329-3341. https://doi.org/10.3390/curroncol31060253






