Abstract
Surgical margins following rectal cancer resection impact oncologic outcomes. We examined the relationship between margin status and race, ethnicity, region of care, and facility type. Patients undergoing resection of a stage II–III locally advanced rectal cancer (LARC) between 2004 and 2018 were identified through the National Cancer Database. Inverse probability of treatment weighting (IPTW) was performed, with margin positivity rate as the outcome of interest, and race/ethnicity and region of care as the predictors of interest. In total, 58,389 patients were included. After IPTW adjustment, non-Hispanic Black (NHB) patients were 12% (p = 0.029) more likely to have margin positivity than non-Hispanic White (NHW) patients. Patients in the northeast were 9% less likely to have margin positivity compared to those in the south. In the west, NHB patients were more likely to have positive margins than NHW patients. Care in academic/research centers was associated with lower likelihood of positive margins compared to community centers. Within academic/research centers, NHB patients were more likely to have positive margins than non-Hispanic Other patients. Our results suggest that disparity in surgical management of LARC in NHB patients exists across regions of the country and facility types. Further research aimed at identifying drivers of this disparity is warranted.
1. Introduction
Despite a reduction in colorectal cancer (CRC) morbidity and mortality in the general population, disparities persist among racial and ethnic groups [1,2,3,4,5]. Since a significant fraction (approximately 46,000 new cases in 2023) of the CRC burden is attributed to rectal cancer, a disease entity which requires a multidisciplinary approach, ample opportunities exist across the continuum of multimodality rectal cancer therapy for reducing disparity in rectal cancer care [6]. Although the current multimodality management of locally advanced rectal cancer (LARC) (T3–4, and/or N+, stage II and III) includes radiation and chemotherapy, most recently referred to as total neoadjuvant therapy (TNT), surgery remains an essential component, as a negative (R0) resection margin is required in order to obtain optimal cancer-specific outcomes [7,8]. However, despite advances in surgical techniques and multimodality therapy, rates of positive margins, such as the circumferential resection margin (CRM), which is defined as the closest distance of the tumor to the mesorectal fascia, remain high in the United States [9,10].
The CRM is a crucial prognostic factor impacting local and distant recurrence as well as disease-free survival, especially following preoperative combined modality therapy [10,11,12,13]. Factors associated with risk of obtaining a positive CRM following resection of a LARC include surgical technique, body habitus (obesity, narrow, deep male pelvis), emergency surgery, incomplete oncological preoperative staging, and lack of preoperative chemoradiation therapy utilization, when indicated [3,8,10,11,14,15]. Although previous studies have demonstrated an increased likelihood of positive CRM in non-Hispanic Black (NHB) compared to NHW patients, these studies did not specifically focus on LARC [16,17], nor adequately control for the type of operation performed or the administration of preoperative radiotherapy [17]. In order to further understand the factors driving the increased likelihood of positive CRM in NHB patients, our study focuses on LARC and rigorously controls for multiple potential covariates, such as the type of surgery and the administration of radiotherapy, and examines whether this disparity persists across different regions of the country and within different facility types.
2. Methods
The hospital-based American College of Surgeons (ACS) National Cancer Database (NCDB) was queried to identify patients diagnosed with LARC (i.e., stage II and III) who underwent surgical resection from 2004 to 2019. Patients were excluded if they (1) were under 18 years; (2) were not surgically treated or received local excision only; (3) had an unknown sequence of radiation versus surgery; and (4) had unknown pathologic grade. Patients were then stratified into the following four groups based on race and ethnicity: (1) NHW; (2) NHB; (3) Hispanic; and (4) non-Hispanic Other (NHO). The primary outcome of interest, margin positivity, was assessed as a binary outcome (Yes/No). The primary predictors of interest were race and ethnicity. Secondary predictors of interest included (1) United States (US) region and (2) facility type. Wilcoxon rank sum test and chi square tests were used to compare mean age across groups and categorical variables, respectively. To make the categories of race and ethnicity more comparable and reduce potential confounding and bias, an inverse probability of treatment weight (IPTW) [18] was generated using a multinomial logistic regression model where the outcomes were race and ethnicity. Predictors included the following confounders: age, sex, Charlson–Deyo score [19], pathologic stage, days from diagnosis to surgery, pathologic grade, type of surgery, timing of radiation therapy in relation to surgery (defined as either (1) no radiation, (2) intraoperative radiation, (3) neoadjuvant radiation, (4) adjuvant radiation, or (5) both neo- and adjuvant radiation), insurance type, high school education, and direct distance from patient’s residence to hospital. Overall mean weight was used to assure between-group balance. Once between-group balance was ascertained, binary logistic regression was used to generate odds ratios (OR), 95% confidence intervals (CI), and p-values to assess associations between margin positivity and race and ethnicity groups, and adjusted for the effect using the IPTW weight, region of the United States, defined as (1) northeast, (2) midwest, (3) south, or (4) west, and facility type, defined as (1) academic/research, (2) network cancer program, or (3) community cancer program. Supplement A lists the breakdown of states assigned to each region of the USA. Finally, interaction terms in logistic regression models were used to assess differences in margin positivity within and across regions and facility types with respect to race/ethnicity.
All tests were two-sided at alpha = 0.05. R version 4.2.2 (Comprehensive R Archive Network, Vienna, Austria) and R Studio Version 2022.12.0+353 (Posit Software, PBC, Boston, MA, USA) were used for data management. All analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC, USA).
3. Results
3.1. Baseline Demographics
Our initial query of the NCDB identified 363,557 patients diagnosed with rectal cancer between 2004 and 2019, of whom 88,406 had pathologic stage II and III and were included in the analysis. After excluding patients with missing data for our confounders of interest, 58,389 patients remained and underwent analysis. Figure 1 summarizes our inclusion and exclusion process.
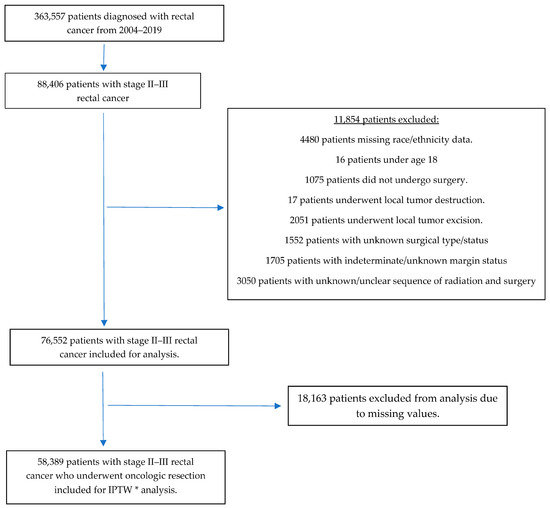
Figure 1.
Inclusion and exclusion flow chart * Inverse-probability treatment weighting.
Of the included patients, 81%, 8%, 6%, and 5% were NHW, NHB, Hispanic, and NHO, respectively, 60% were male, and the mean age was 62 years. Table 1 summarizes additional baseline characteristics.

Table 1.
Characteristics of patients with rectal adenocarcinoma in the National Cancer Database between 2004 and 2019 who had positive or negative margins (N = 58,389).
3.2. Multivariable ITPW Analysis
Weighted binary logistic regression analysis results for positive margins found that NHB patients were 12% more likely than NHW patients to have a positive margin after resection (OR 1.12, 95% CI 1.01–1.23, p = 0.029). Patients treated in the northeastern United States were 9% less likely to have positive margins compared to those treated in the south (OR 0.91, 95% CI 0.84–0.99, p = 0.023). In addition, patients treated at academic/research cancer programs were 11% less likely to have positive margins compared to those treated at community cancer programs (OR 0.89, 95% CI 0.83–0.95, p = 0.0006). Table 2 summarizes these findings.

Table 2.
Inverse probability of treatment weighted (IPTW) a estimates of odds ratios for positive margins.
3.3. Regional Variations in Positive Margins
Within the west, NHB patients were 46% more likely to have positive margins compared to NHW patients (OR: 1.46, 95% CI 1.03–2.09, p = 0.036). Similarly, NHB patients operated on in the west were 54% more likely to have positive margins compared to NHO patients (OR 1.54, 95% CI 1.03–2.29, p = 0.035). NHB patients in the northeast were 42% less likely to have positive margins compared to NHB patients operated on in the west (OR 0.58, 95% CI 0.37–0.89, p = 0.012). Similarly, NHW patients in the northeast were 11% less likely to have positive margins compared to NHW patients operated on in the west (OR 0.89, 95% CI 0.80–0.99, p = 0.037) (Table 3).

Table 3.
Significant differences in margin positivity rates between race/ethnicity across regions of the United States.
3.4. Variations in Positive Margins by Type of Facility
Within academic/research programs, NHB patients were 53% more likely than NHO patients to have positive margins (OR 1.53, 95% CI 1.12–2.07, p = 0.007). NHW patients treated in community cancer programs were 12% (OR 1.12, 95% CI 1.03–1.20, p = 0.007) more likely to have positive margins compared to NHW patients treated in academic/research programs. Similarly, NHW patients treated in integrated network cancer programs were 15% (OR 1.15, 95% CI 1.05–1.26, p = 0.002) more likely to have positive margins compared to NHW patients treated in academic/research programs. In addition, NHO patients treated in community cancer programs were 46% (OR 1.46, 95% CI 1.06–2.00, p = 0.02) more likely to have positive margins compared to NHO patients treated in academic/research programs (Table 4).

Table 4.
Significant differences in margin positivity rates between race/ethnicity across facility type.
4. Discussion
Our study, which examines a large national database representative of more than 1500 Commission on Cancer-accredited facilities, demonstrates in a study cohort of nearly 60,000 LARC patients that NHB patients had higher odds of positive CRMs compared to their NHW counterparts, suggesting the existence of a disparity in the management of NHB patients afflicted with rectal cancer. Although prior studies utilizing the NCDB have also demonstrated an increased likelihood (19% to 29%) [16,17,20,21] of positive CRM in NHB patients undergoing rectal cancer resection compared to NHW patients, our study performed an IPTW analysis, a more robust approach than the adjusted logistic regressions performed by prior studies, which demonstrated that NHB patients are 12% more likely to undergo LARC resection with positive margins compared to NHW, and that this disparity may vary between regions of the country and facility types.
Our results indicate that patients undergoing surgery in the northeast appear to have superior outcomes. Although differences in margin positivity status may be multifactorial, the fact that, relative to the rest of the country, there are more National Cancer Institute (NCI) designated cancer centers in the northeast suggests that “cutting edge cancer treatments” may be more common in the northeast [22]. This is consistent with studies reporting adherence to the National Accreditation Program for Rectal Cancer (NAPRC) standards, which demonstrate that, relative to the northeast (New England), performance measure achievement (defined as negative proximal, distal, and circumferential margins and >12 lymph nodes harvested during resection) was inferior in other regions of the country [23].
Although our study controls for multiple potentially confounding variables such as age, sex, Charlson–Deyo score, pathologic stage, days from diagnosis to surgery, pathologic grade, type of surgery, timing of radiation therapy in relation to surgery, insurance type, high school education, and direct distance from patient’s residence to hospital recorded in the NCDB, the persistence of a relative increased likelihood of a positive margin in NHB compared to NHW patients following rectal cancer resection suggests that factors not reported in the NCDB or controlled for in our study may be potential drivers of this disparity. Such factors may include, but are not limited to, (1) hospital volume; (2) surgeon specialization; (3) patient body mass index (BMI); (4) anatomic pelvic variations; and (5) implicit bias.
Hospital volume, a factor that we did not control for, is known to impact margin status [16] as well as mitigate 5-year overall survival rate differences between NHB and NHW patients [24]. These results would suggest that rectal cancer patients be preferentially operated on at high volume centers (HVC). However, since this may not be currently feasible and yet surgeon-specific variables such as certification in colorectal surgery have been associated with a decreased likelihood of obtaining positive CRM [25], triaging rectal cancer patients preferentially to sub-specialists, may be a viable alternative and should thereby minimize disparity in rectal cancer management.
In addition to treatment-related variables, patient-related variables, such as BMI and pelvic anatomic variation, not routinely captured by the NCDB may also be contributing to the disparity in the surgical management of NHB relative to NHW patients. Given that obesity is more prevalent among NHB compared to any other ethnic group and that it can add complexity to the surgical resection of rectal cancers, it is possible that differences in BMI between NHB and NHW patients may account, in part, for the noted disparity [26,27,28]. Similarly, previous studies have also demonstrated differences in pelvic anatomy by race. For example, white women have a wider pelvic inlet and outlet and shallower anterior–posterior outlet than African American women [29]. Since certain pelvimetric variables may be predictive of poor rectal cancer resection and positive CRM [30,31,32], it is possible that anatomical differences between NHB and NHW patients may also contribute to the observed differences in LARC resection margin status.
It is possible that implicit bias, defined as “a preference for a social group that is both unconscious and automatic” [33], may also contribute to the noted disparity in the surgical management of LARC. According to some studies, implicit bias is prevalent among surgeons [34,35] and may therefore impact overall management. In addition, the fact that the disparity persisted even within academic centers suggests that implicit bias may be differentially impacting the extent of attending supervision and engagement during the surgical management of NHB LARC patients.
We acknowledge several limitations in our study. First, the data used were retrospectively collected and therefore subject to bias on the part of data collectors and causality cannot be inferred. An observational prospective study would be needed to confirm whether causality exists. However, the IPTW multivariable analysis that we employed was able to control for the numerous confounding variables collected by the NCDB and allowed us to compare margin positivity rates among racial/ethnic groups in the NCDB. Our ability to control for other potential confounding demographic factors such as higher education/employment status, and access to routine healthcare was also limited by the data captured by the NCDB.
In conclusion, our study demonstrates that racial disparities in the management of LARC, defined by resection margin status, vary between regions of the country and facility type, even when multiple potential confounders are controlled for. Insofar as subspecialty training and specialization in rectal cancer surgical management influence resection margin status, efforts to increase subspecialty training as well as adherence to programs aimed at optimizing the multidisciplinary and multimodality management of LARC should help reduce disparities in rectal cancer care. Similarly, since BMI and pelvic anatomy impact margin positivity and differ between individuals of varying race and ethnicity, efforts to increase diversity in clinical trial participation are warranted. Such efforts may provide opportunities for improving the surgical approach in individuals with elevated BMI and challenging pelvic anatomy and thereby improve the overall results of rectal cancer management. Lastly, ongoing efforts to educate healthcare providers on minimizing implicit bias may further reduce disparity in rectal cancer management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol31070280/s1, Supplement A. Breakdown of states assigned to each region of the United States of America
Author Contributions
W.Y.L., D.N.V., C.B.A., L.H.C., P.S., T.W., M.R.K., J.M.S., and J.G.G.: Writing—Original Draft; Writing—Review and Editing; C.B.A.: Methodology; Data Curation; J.G.G.: Conceptualization; Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was exempted from review by the Institutional Review Board at the University of North Carolina at Chapel Hill (IRB# 20-1493).
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from the American College of Surgeons (ACS) National Cancer Database (NCDB) and are available with the permission of ACS.
Acknowledgments
This work was presented in part at the 2023 American Society of Clinical Oncology Gastrointestinal Cancers Symposium and the 2023 American Society of Colon and Rectal Surgeons Annual Scientific Meeting. The NCDB is a joint project of the Commission on Cancer (CoC) of the ACS and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein. They have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Conflicts of Interest
J.G.G.: Consultant for Intuitive Surgical. Other authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sharma, I.; Kim, S.; Sridhar, S.; Basha, R. Colorectal Cancer: An Emphasis on Factors Influencing Racial/Ethnic Disparities. Crit. Rev. Oncog. 2020, 25, 151–160. [Google Scholar] [CrossRef]
- Howard, R.; Hendren, S.; Patel, M.; Gunaseelan, V.M.; Wixson, M.; Waljee, J.; Englesbe, M.; Bicket, M.C. Racial and Ethnic Differences in Elective Versus Emergency Surgery for Colorectal Cancer. Ann. Surg. 2023, 278, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- May, F.P.; Almario, C.V.; Ponce, N.; Spiegel, B.M.R. Racial Minorities Are More Likely Than Whites to Report Lack of Provider Recommendation for Colon Cancer Screening. Am. J. Gastroenterol. 2015, 110, 1388–1394. [Google Scholar] [CrossRef]
- Snyder, R.A.; Hu, C.Y.; Zafar, S.N.; Francescatti, A.; Chang, G.J. Racial Disparities in Recurrence and Overall Survival in Patients with Locoregional Colorectal Cancer. J. Natl. Cancer Inst. 2021, 113, 770–777. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Quirke, P.; Dixon, M.F.; Durdey, P.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 2, 996–999. [Google Scholar] [CrossRef]
- Mukkai Krishnamurty, D.; Wise, P.E. Importance of surgical margins in rectal cancer. J. Surg. Oncol. 2016, 113, 323–332. [Google Scholar] [CrossRef] [PubMed]
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006, 333, 779. [Google Scholar] [CrossRef] [PubMed]
- Rickles, A.S.; Dietz, D.W.; Chang, G.J.; Wexner, S.D.; Berho, M.E.; Remzi, F.H.; Greene, F.L.; Fleshman, J.W.; Abbas, M.A.; Peters, W.; et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann. Surg. 2015, 262, 891–898. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What Is the Role for the Circumferential Margin in the Modern Treatment of Rectal Cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef]
- Trakarnsanga, A.; Gonen, M.; Shia, J.; Goodman, K.A.; Nash, G.M.; Temple, L.K.; Guillem, J.G.; Paty, P.B.; Garcia-Aguilar, J.; Weiser, M.R. What is the Significance of the Circumferential Margin in Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiotherapy? Ann. Surg. Oncol. 2013, 20, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Detering, R.; Rutgers, M.L.W.; Bemelman, W.A.; Hompes, R.; Tanis, P.J. Prognostic importance of circumferential resection margin in the era of evolving surgical and multidisciplinary treatment of rectal cancer: A systematic review and meta-analysis. Surgery 2021, 170, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Teng, A.; Pedersen, R.C.; Tavangari, F.R.; Attaluri, V.; McLemore, E.C.; Stern, S.L.; Bilchik, A.J.; Goldfarb, M.R. Racial and Socioeconomic Treatment Disparities in Adolescents and Young Adults with Stage II–III Rectal Cancer. Ann. Surg. Oncol. 2017, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bocca, G.; Mastoridis, S.; Yeung, T.; James, D.R.C.; Cunningham, C. Visceral-to-subcutaneous fat ratio exhibits strongest association with early post-operative outcomes in patients undergoing surgery for advanced rectal cancer. Int. J. Color. Dis. 2022, 37, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.C.; You, Y.N.; Hu, C.-Y.; Cormier, J.N.; Feig, B.W.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Nelson, H.; Chang, G.J. A Novel Risk-Adjusted Nomogram for Rectal Cancer Surgery Outcomes. JAMA Surg. 2013, 148, 769. [Google Scholar] [CrossRef] [PubMed]
- Bakkila, B.F.; Kerekes, D.; Nunez-Smith, M.; Billingsley, K.G.; Ahuja, N.; Wang, K.; Oladele, C.; Johnson, C.H.; Khan, S.A. Evaluation of Racial Disparities in Quality of Care for Patients with Gastrointestinal Tract Cancer Treated with Surgery. JAMA Netw. Open 2022, 5, e225664. [Google Scholar] [CrossRef] [PubMed]
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2022, 15, 14–20. [Google Scholar] [CrossRef]
- Deyo, R.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Naffouje, S.A.; Ali, M.A.; Kamarajah, S.K.; White, B.; Salti, G.I.; Dahdaleh, F. Assessment of Textbook Oncologic Outcomes Following Proctectomy for Rectal Cancer. J. Gastrointest. Surg. 2022, 26, 1286–1297. [Google Scholar] [CrossRef]
- Simon, H.L.; De Paula, T.R.; Profeta Da Luz, M.M.; Kiran, R.P.; Keller, D.S. Predictors of Positive Circumferential Resection Margin in Rectal Cancer: A Current Audit of the National Cancer Database. Dis. Colon Rectum. 2021, 64, 1096–1105. [Google Scholar] [CrossRef]
- Alimena, S.; Davis, M.; Pelletier, A.; Terry, K.; King, M.; Feldman, S. Regional Variation in Access to Oncologic Care and Racial Disparities Among Cervical Cancer Patients. Am. J. Clin. Oncol. 2022, 45, 415–421. [Google Scholar] [CrossRef]
- Brady, J.T.; Xu, Z.; Scarberry, K.B.; Saad, A.; Fleming, F.J.; Remzi, F.H.; Wexner, S.D.; Winchester, D.P.; Monson, J.R.; Lee, L.; et al. Evaluating the Current Status of Rectal Cancer Care in the US: Where We Stand at the Start of the Commission on Cancer’s National Accreditation Program for Rectal Cancer. J. Am. Coll. Surg. 2018, 226, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.B.; Straker, R.J.; Keele, L.; Kelz, R.R.; Fraker, D.L.; Roses, R.E.; Miura, J.T.; Karakousis, G.C. The impact of hospital volume on racial disparities in resected rectal cancer. J. Surg. Oncol. 2022, 125, 465–474. [Google Scholar] [CrossRef]
- Justiniano, C.F.; Aquina, C.T.; Fleming, F.J.; Xu, Z.; Boscoe, F.P.; Schymura, M.J.; Temple, L.K.; Becerra, A.Z. Hospital and surgeon variation in positive circumferential resection margin among rectal cancer patients. Am. J. Surg. 2019, 218, 881–886. [Google Scholar] [CrossRef]
- Chern, H.; Chou, J.; Donkor, C.; Shia, J.; Guillem, J.G.; Nash, G.M.; Paty, P.B.; Temple, L.K.; Wong, D.W.; Weiser, M.R. Effects of Obesity in Rectal Cancer Surgery. J. Am. Coll. Surg. 2010, 211, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yuval, J.B.; Patil, S.; Gangai, N.; Omer, D.M.; Akselrod, D.G.; Fung, A.; Harmath, C.B.; Kampalath, R.; Krehbiel, K.; Lee, S.; et al. MRI assessment of rectal cancer response to neoadjuvant therapy: A multireader study. Eur. Radiol. 2023, 33, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2022. Available online: https://www.cdc.gov/obesity/php/data-research/adult-obesity-facts.html (accessed on 12 April 2024).
- Handa, V.L.; Lockhart, M.E.; Fielding, J.R.; Bradley, C.S.M.; Brubaker, L.; Cundiff, G.W.; Ye, W.; Richter, H.E. Racial Differences in Pelvic Anatomy by Magnetic Resonance Imaging. Obstet. Gynecol. 2008, 111, 914–920. [Google Scholar] [CrossRef]
- Chau, J.; Solomon, J.; Liberman, A.S.; Charlebois, P.; Stein, B.; Lee, L. Pelvic dimensions on preoperative imaging can identify poor-quality resections after laparoscopic low anterior resection for mid- and low rectal cancer. Surg. Endosc. 2020, 34, 4609–4615. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kim, N.K.; Lee, K.Y.; Sohn, S.K.; Cho, C.H.; Kim, M.J.; Kim, H.; Shinn, R.K. Factors Influencing Pathologic Results after Total Mesorectal Excision for Rectal Cancer: Analysis of Consecutive 100 Cases. Ann. Surg. Oncol. 2008, 15, 721–728. [Google Scholar] [CrossRef]
- Boyle, K.M.; Petty, D.; Chalmers, A.G.; Quirke, P.; Cairns, A.; Finan, P.J.; Sagar, P.M.; Burke, D. MRI assessment of the bony pelvis may help predict resectability of rectal cancer. Colorectal Dis. 2005, 7, 232–240. [Google Scholar] [CrossRef]
- Greenwald, A.G.; Banaji, M.R. Implicit social cognition: Attitudes, self-esteem, and stereotypes. Psychol. Rev. 1995, 102, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Zebib, L.; Strong, B.; Moore, G.; Ruiz, G.; Rattan, R.; Zakrison, T.L. Association of Racial and Socioeconomic Diversity With Implicit Bias in Acute Care Surgery. JAMA Surg. 2019, 154, 459. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.H.; Schneider, E.B.; Sriram, N.; Dossick, D.S.; Scott, V.K.; Swoboda, S.M.; Losonczy, L.; Haut, E.R.; Efron, D.T.; Pronovost, P.J.; et al. Unconscious race and class bias: Its association with decision making by trauma and acute care surgeons. J. Trauma Acute Care Surg. 2014, 77, 409–416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).