A Locally Advanced NSCLC Patient Harboring a Rare KIF13A-RET Fusion Benefited from Pralsetinib: A Case Report
Abstract
:1. Introduction
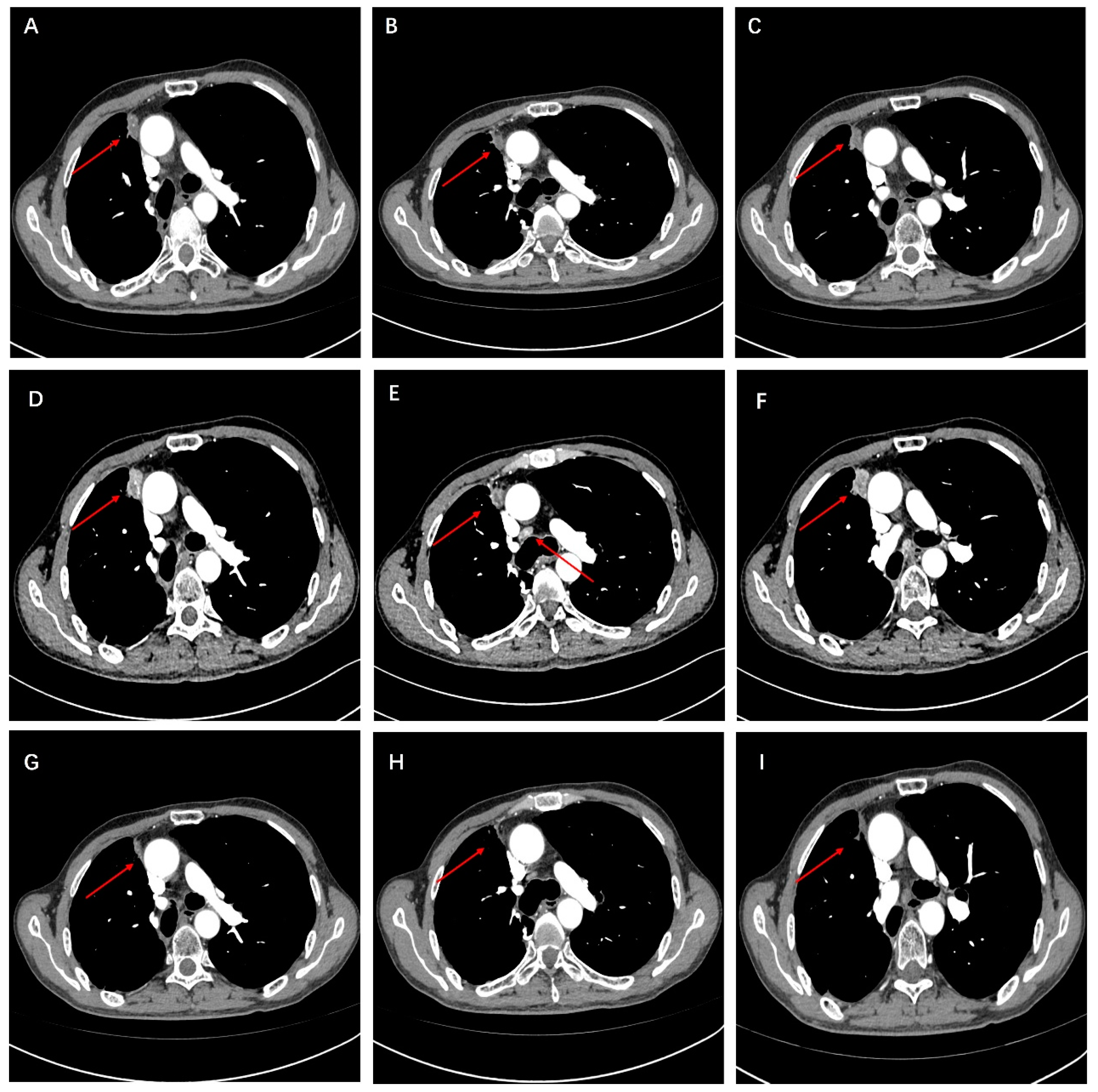
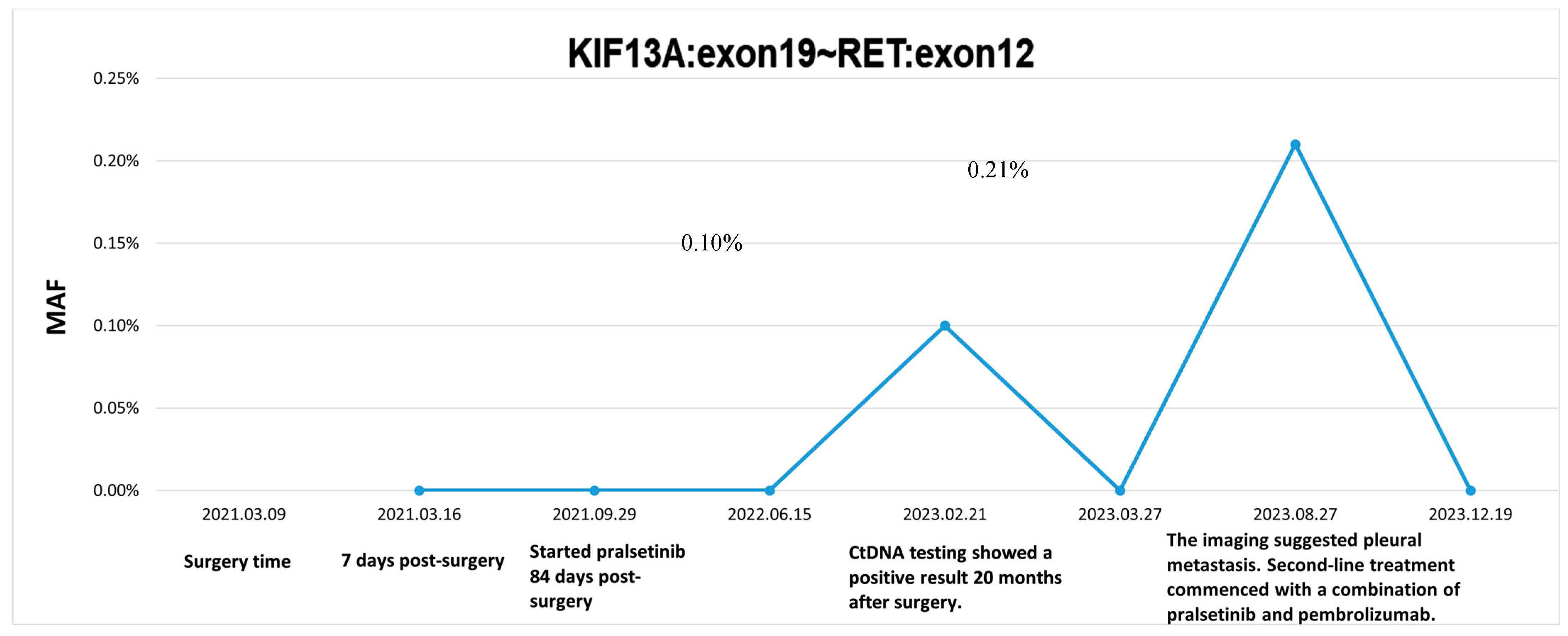
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, B.; Hong, M.; Song, J.-Y.; Jung, K.; E Lira, M.; Mao, M.; Han, J.; Kim, J.; Choi, Y.-L. Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma. Mod. Pathol. 2015, 28, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; A Cassier, P.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.; Tan, D.; Lee, D.; Misch, D.; Garralda, E.; Kim, D.-W.; et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Thein, K.Z.; Velcheti, V.; Mooers, B.H.; Wu, J.; Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021, 7, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2017, 15, 151–167. [Google Scholar] [CrossRef]
- Offin, M.; Guo, R.; Wu, S.L.; Sabari, J.; Land, J.D.; Ni, A.; Montecalvo, J.; Halpenny, D.F.; Buie, L.W.; Pak, T.; et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.B.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non–Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Wu, Y.-L.; John, T.; Grohe, C.; Majem, M.; Wang, J.; Kato, T.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non–Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J. Clin. Oncol. 2023, 41, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Mei, J.; Kang, R.; Deng, S.; Chen, Y.; Yang, Y.; Feng, G.; Deng, Y.; Gan, F.; Lin, Y.; et al. Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin. Cancer Res. 2022, 28, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, B.; Shao, Y.; Li, M.; Li, J.; Kuang, P.; Liu, Z.; Sun, T.; Wu, H.; Ou, W.; et al. Perioperative circulating tumor DNA as a potential prognostic marker for operable stage I to IIIA non–small cell lung cancer. Cancer 2021, 128, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Pu, Q.; Kang, R.; Mei, J.; Li, L.; Yang, Y.; Deng, S.; Feng, G.; Ma, L.; Lin, F.; et al. Dynamic ctDNA to inform the precise management of resected NSCLC: LUNGCA-2 study. J. Clin. Oncol. 2023, 41, 8528. [Google Scholar] [CrossRef]
- Mardinian, K.; Okamura, R.; Kato, S.; Kurzrock, R. Temporal and spatial effects and survival outcomes associated with concordance between tissue and blood KRAS alterations in the pan-cancer setting. Int. J. Cancer 2019, 146, 566–576. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.; Zhu, T.; Jiang, H.; Ou, W.; Wang, S. A Locally Advanced NSCLC Patient Harboring a Rare KIF13A-RET Fusion Benefited from Pralsetinib: A Case Report. Curr. Oncol. 2024, 31, 3808-3814. https://doi.org/10.3390/curroncol31070281
Chang Z, Zhu T, Jiang H, Ou W, Wang S. A Locally Advanced NSCLC Patient Harboring a Rare KIF13A-RET Fusion Benefited from Pralsetinib: A Case Report. Current Oncology. 2024; 31(7):3808-3814. https://doi.org/10.3390/curroncol31070281
Chicago/Turabian StyleChang, Zenghao, Tengfei Zhu, Hao Jiang, Wei Ou, and Siyu Wang. 2024. "A Locally Advanced NSCLC Patient Harboring a Rare KIF13A-RET Fusion Benefited from Pralsetinib: A Case Report" Current Oncology 31, no. 7: 3808-3814. https://doi.org/10.3390/curroncol31070281



