24-Epibrassinolide and 2,6-Dichlorobenzonitrile Promoted Celery Petioles and Hypocotyl Elongation by Altering Cellulose Accumulation and Cell Length
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Exogenous 24-EBL, PCZ and DCB Treatments
2.3. Anatomical Structure Analysis
2.4. Measurement of Cellulose Content
2.5. Total RNA Extraction, cDNA Synthesis
2.6. RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
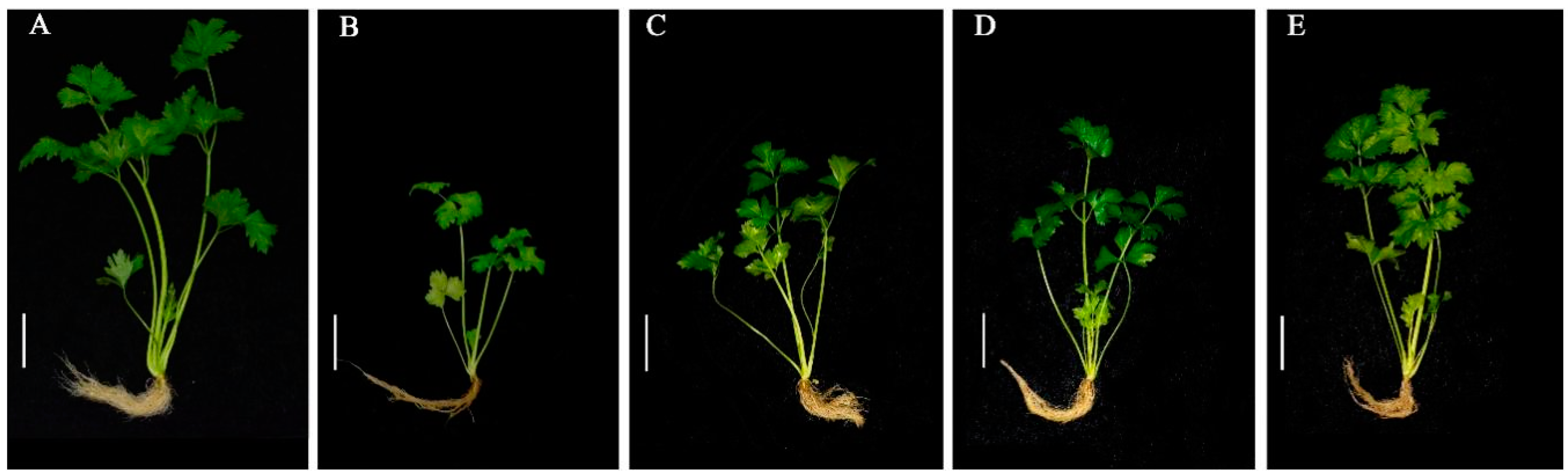
3.1. Effects of Exogenous 24-EBL, PCZ and DCB Treatments on the Growth and Development of Celery
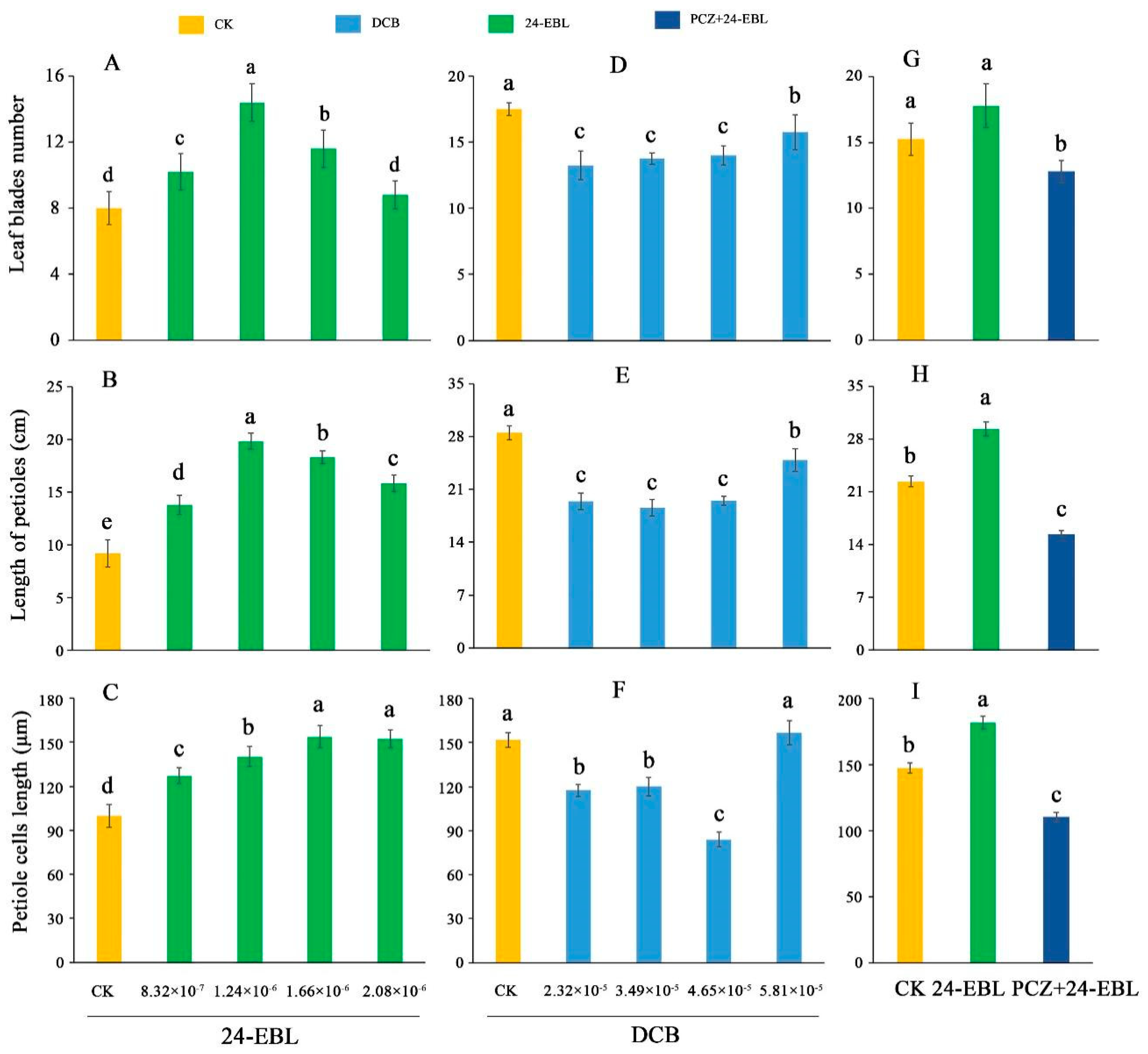
3.2. Effects of 24-EBL, PCZ and DCB Treatments on Length of Petioles, Number of Leaf Blades and Cell Length of Petioles in Celery
3.3. Effects of 24-EBL and DCB Treatments on Length of Hypocotyl and Hypocotyl Cells
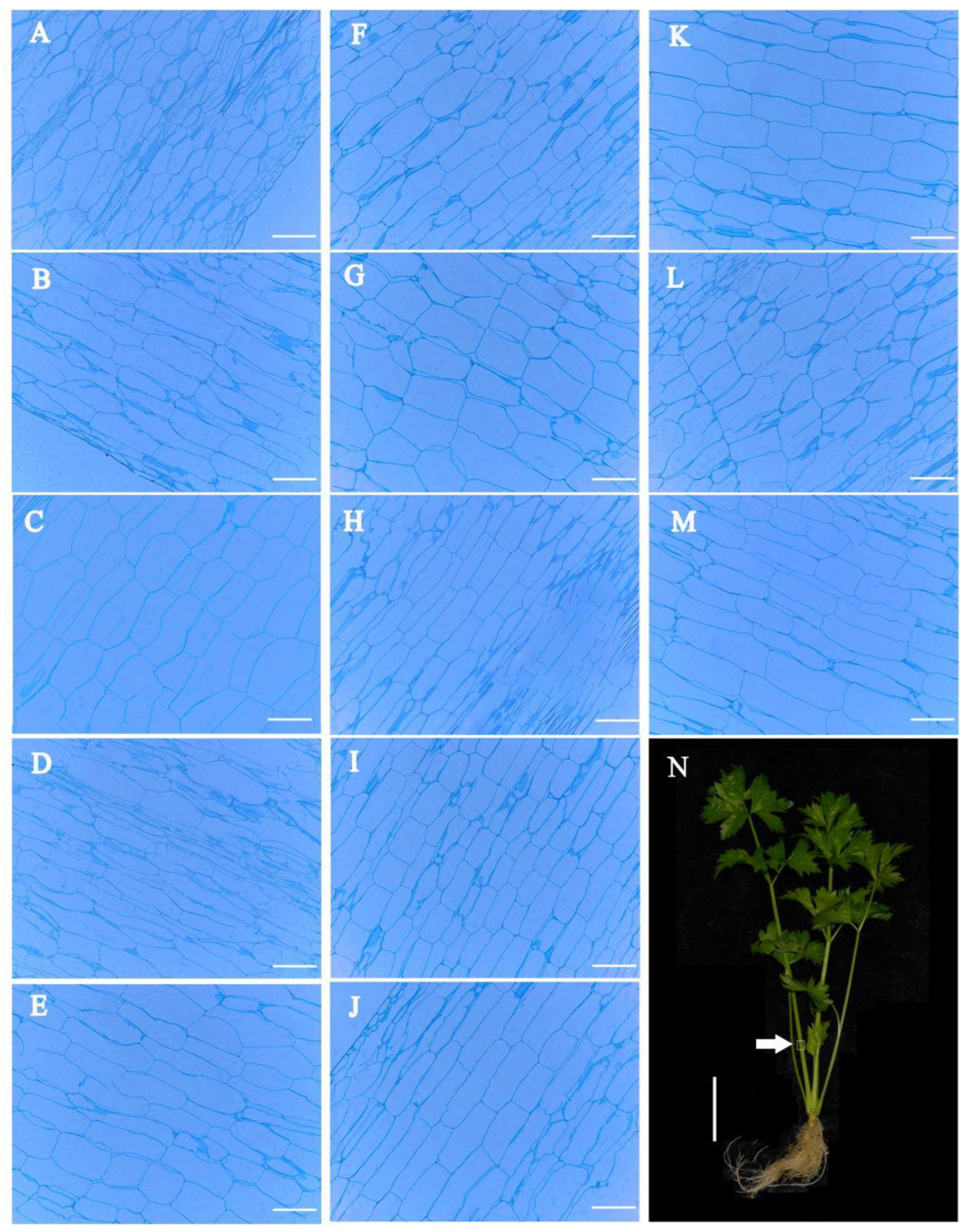
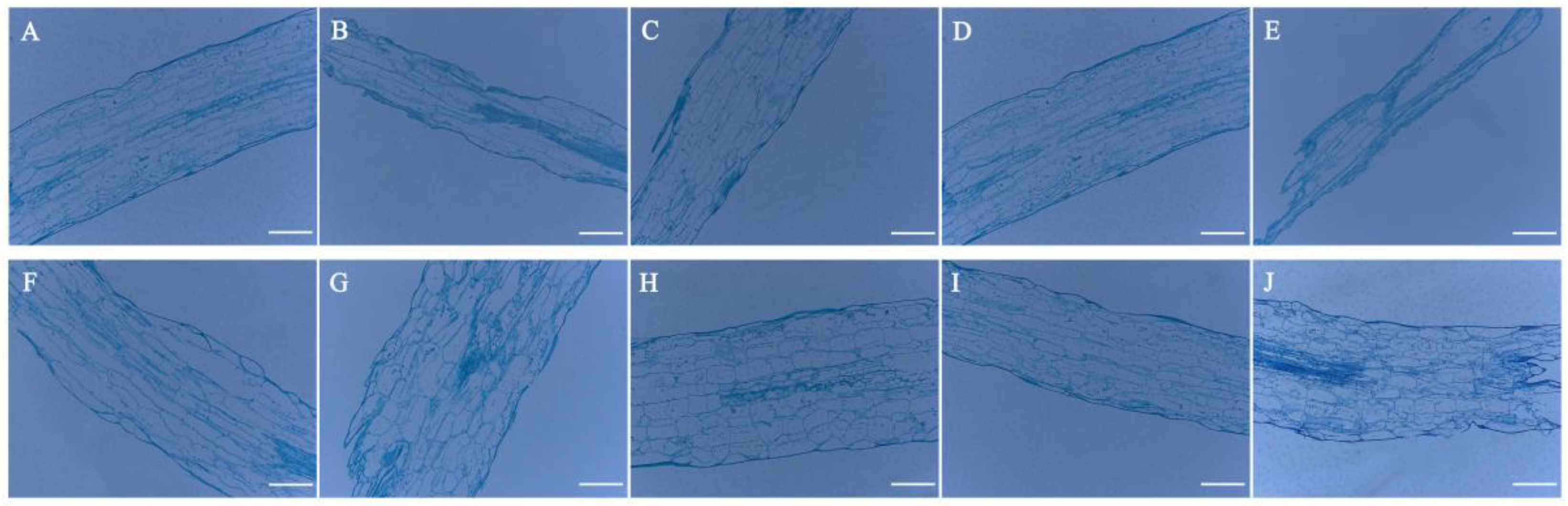
3.4. Anatomical Structure Analysis of Celery Petiole and Hypocotyl under 24-EBL, PCZ and DCB Treatments
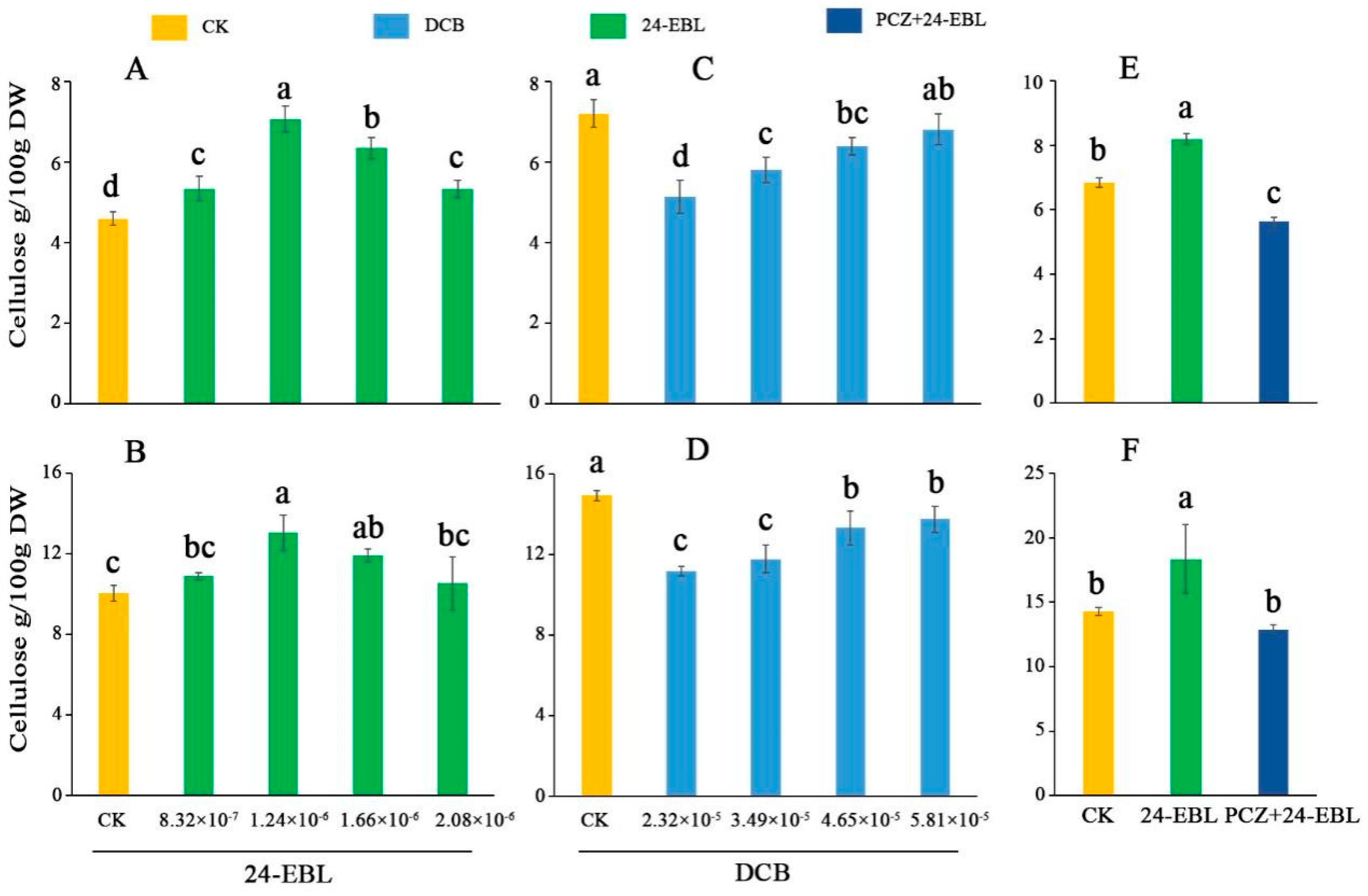
3.5. Cellulose Content in Celery Leaf Blades and Petioles under 24-EBL, PCZ and DCB Treatments
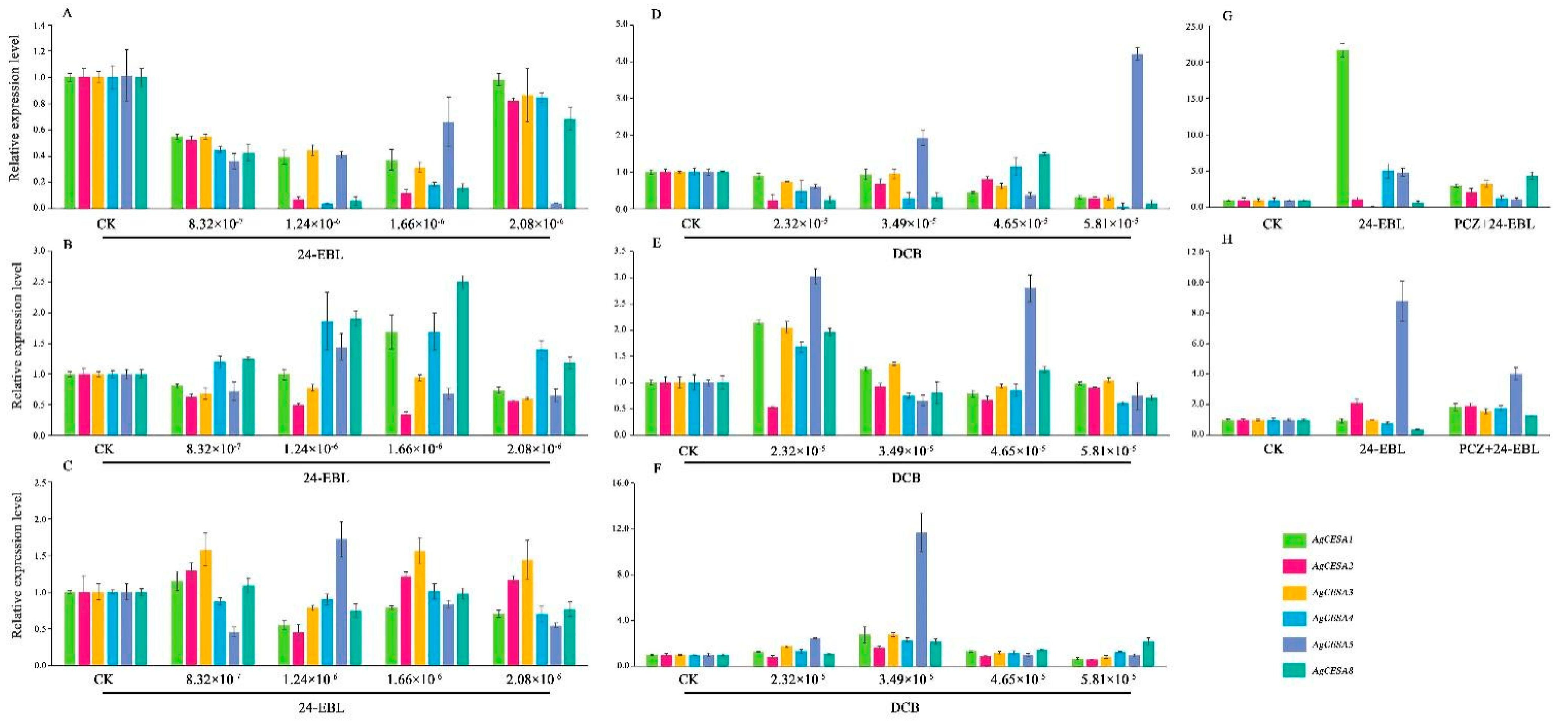
3.6. Expression Patterns of the Cellulose-Related Genes in Petioles, Leaf Blades and Hypocotyl of Celery under 24-EBL, PCZ and DCB Treatments
4. Discussion
4.1. Effects of BRs/PCZ/DCB on the Expression of Cellulose-Related Genes and Cellulose Accumulation in Celery
4.2. Effects of BRs/PCZ/DCB on Cell Elongation of Celery Petiole and Hypocotyl
4.3. Effects of BRs/PCZ/DCB on the Growth and Development of Celery
4.4. Application of BRs/PCZ/DCB in Celery Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.Y.; Hou, X.L.; Wang, F.; Tan, G.F.; Xu, Z.S.; Xiong, A.S. Advances in the research of celery, an important Apiaceae vegetable crop. Crit. Rev. Biotechnol. 2018, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.X.; Feng, K.; Li, T.; Duan, A.Q.; Liu, Y.H.; Liu, H.; Xiong, A.S. AgMYB12, a novel R2R3-MYB transcription factor, regulates apigenin biosynthesis by interacting with the AgFNS gene in celery. Plant Cell Rep. 2022, 41, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Fagard, M.; Desnos, T.; Desprez, T.; Goubet, F.; Refregier, G.; Mouille, G.; McCann, M.; Rayon, C.; Vernhettes, S.; Höfte, H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 2000, 12, 2409–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refrégier, G.; Pelletier, S.; Jaillard, D.; Höfte, H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiol. 2004, 135, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Pysh, L.D. Two alleles of the AtCesA3 gene in Arabidopsis thaliana display intragenic complementation. Am. J. Bot. 2015, 102, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Zhang, R.; Tao, Z.; Li, X.; Li, Y.; Huang, J.; Li, X.; Han, X.; Feng, S.; Zhang, G.; et al. Cellulose Synthase Mutants Distinctively Affect Cell Growth and Cell Wall Integrity for Plant Biomass Production in Arabidopsis. Plant Cell Physiol. 2018, 59, 1144–1157. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Tang, Y.; Peng, C.; Wu, L.; Feng, S.; Chen, P.; Wang, Y.; Du, X.; Peng, L. Cotton CSLD3 restores cell elongation and cell wall integrity mainly by enhancing primary cellulose production in the Arabidopsis cesa6 mutant. Plant Mol. Biol. 2019, 101, 389–401. [Google Scholar] [CrossRef]
- Asami, T.; Nakano, T.; Fujioka, S. Plant brassinosteroid hormones. Vitam. Horm. 2005, 72, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H. Designed Manipulation of the Brassinosteroid Signal to Enhance Crop Yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Yu, H.; Qin, S.; Li, Y.; Alam, A.; Xu, C.; Fan, D.; Zhang, Q.; Wang, Y.; Zhu, W.; et al. Brassinosteroid overproduction improves lignocellulose quantity and quality to maximize bioethanol yield under green-like biomass process in transgenic poplar. Biotechnol. Biofuels 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, F.; Khadr, A.; Wang, G.-L.; Li, T.; Wang, Y.-H.; Xu, Z.-S.; Xiong, A.-S. Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci. 2018, 277, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Wang, Y.H.; Xu, Z.S.; Xiong, A.S. DcBAS1, a Carrot Brassinosteroid Catabolism Gene, Modulates Cellulose Synthesis. J. Agric. Food Chem. 2019, 67, 13526–13533. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Corvalan, C.; Best, N.B.; Budka, J.S.; Zhu, J.Y.; Choe, S.; Schulz, B. Propiconazole is a specific and accessible brassinosteroid (BR) biosynthesis inhibitor for Arabidopsis and maize. PLoS ONE 2012, 7, e36625. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.R.; Barnes, W.S.; Bedinger, P. 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J. Plant Physiol. 2002, 159, 61–67. [Google Scholar] [CrossRef]
- Encina, A.; Sevillano, J.M.; Acebes, J.L.; Alvarez, J. Cell wall modifications of bean (Phaseolus vulgaris) cell suspensions during habituation and dehabituation to dichlobenil. Physiol. Plant. 2002, 114, 182–191. [Google Scholar] [CrossRef]
- Wells, B.; Mccann, M.C.; Shedletzky, E.; Delmer, D.; Roberts, K. Structural features of cell walls from tomato cells adapted to grow on the herbicide 2,6-dichlorobenzonitrile. J. Microsc. 2011, 173, 155–164. [Google Scholar] [CrossRef]
- Hao, H.; Chen, T.; Fan, L.; Li, R.; Wang, X.; Zabotina, O.A. 2,6-dichlorobenzonitrile causes multiple effects on pollen tube growth beyond altering cellulose synthesis in Pinus bungeana Zucc. PLoS ONE 2013, 8, e76660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBolt, S.; Gutierrez, R.; Ehrhardt, D.W.; Somerville, C. Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2,6-dichlorobenzonitrile treatment. Plant Physiol. 2007, 145, 334–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.X.; Jiang, Q.; Tao, J.P.; Feng, K.; Li, T.; Duan, A.Q.; Wang, H.; Xu, Z.S.; Liu, H.; Xiong, A.S. Integrative genome, transcriptome, microRNA, and degradome analysis of water dropwort (Oenanthe javanica) in response to water stress. Hortic. Res. 2021, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.L.; Li, M.Y.; Jiang, Q.; Xu, Z.S.; Liu, J.X.; Xiong, A.S. CeleryDB: A genomic database for celery. Database 2018, 2018, bay070. [Google Scholar] [CrossRef]
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xu, Z.S.; Wang, G.L.; Liu, J.X.; Wang, F.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Y.; Wang, F.; Jiang, Q.; Wang, G.L.; Tian, C.; Xiong, A.S. Validation and Comparison of Reference Genes for qPCR Normalization of Celery (Apium graveolens) at Different Development Stages. Front. Plant Sci. 2016, 7, 313. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L.). J. Evid. Based Complementary Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Liu, J.X.; Duan, A.Q.; Li, T.; Yang, Q.Q.; Xu, Z.S.; Xiong, A.S. AgMYB2 transcription factor is involved in the regulation of anthocyanin biosynthesis in purple celery (Apium graveolens L.). Planta 2018, 248, 1249–1261. [Google Scholar] [CrossRef]
- Wang, X.J.; Luo, Q.; Li, T.; Meng, P.H.; Pu, Y.T.; Liu, J.X.; Zhang, J.; Liu, H.; Tan, G.F.; Xiong, A.S. Origin, evolution, breeding, and omics of Apiaceae: A family of vegetables and medicinal plants. Hortic. Res. 2022, 9, uhac076. [Google Scholar] [CrossRef]
- Tan, G.F.; Ma, J.; Zhang, X.Y.; Xu, Z.S.; Xiong, A.S. AgFNS overexpression increase apigenin and decrease anthocyanins in petioles of transgenic celery. Plant Sci. 2017, 263, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, L.; He, L.; Zhu, L.; Xu, S.; Wan, Y.; Wang, H.; Wang, Y.; Zhu, W. Comparative Transcriptome Analysis of Celery Leaf Blades Identified an R2R3-MYB Transcription Factor that Regulates Apigenin Metabolism. J. Agric. Food Chem. 2019, 67, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, J.X.; Tao, J.P.; Xing, G.M.; Tan, G.F.; Li, S.; Duan, A.Q.; Ding, X.; Xu, Z.S.; Xiong, A.S. The gene encoding lycopene epsilon cyclase of celery enhanced lutein and β-carotene contents and confers increased salt tolerance in Arabidopsis. Plant Physiol. Biochem. 2020, 157, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jia, L.L.; Xing, G.M.; Tao, J.P.; Sun, S.; Tan, G.F.; Li, S.; Liu, J.X.; Duan, A.Q.; Wang, H.; et al. The Accumulation of Lutein and beta-Carotene and Transcript Profiling of Genes Related to Carotenoids Biosynthesis in Yellow Celery. Mol. Biotechnol. 2021, 63, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, K.; Hou, X.; Li, H.; Wang, G.; Xu, Z.; Xiong, A. Isolation, purification and characterization of an ascorbate peroxidase from celery and overexpression of the AgAPX1 gene enhanced ascorbate content and drought tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, A.Q.; Feng, K.; Wang, G.L.; Liu, J.X.; Xu, Z.S.; Xiong, A.S. Elevated gibberellin enhances lignin accumulation in celery (Apium graveolens L.) leaves. Protoplasma 2019, 256, 1507–1517. [Google Scholar] [CrossRef]
- Richmond, T.A.; Somerville, C.R. The cellulose synthase superfamily. Plant Physiol. 2000, 124, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Daras, G.; Rigas, S.; Penning, B.; Milioni, D.; McCann, M.C.; Carpita, N.C.; Fasseas, C.; Hatzopoulos, P. The thanatos mutation in Arabidopsis thaliana cellulose synthase 3 (AtCesA3) has a dominant-negative effect on cellulose synthesis and plant growth. New Phytol. 2009, 184, 114–126. [Google Scholar] [CrossRef]
- Goubet, F.; Barton, C.J.; Mortimer, J.C.; Yu, X.; Zhang, Z.; Miles, G.P.; Richens, J.; Liepman, A.H.; Seffen, K.; Dupree, P. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009, 60, 527–538. [Google Scholar] [CrossRef]
- Qiao, Z.; Lampugnani, E.R.; Yan, X.F.; Khan, G.A.; Saw, W.G.; Hannah, P.; Qian, F.; Calabria, J.; Miao, Y.; Grüber, G.; et al. Structure of Arabidopsis CESA3 catalytic domain with its substrate UDP-glucose provides insight into the mechanism of cellulose synthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2024015118. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Guo, Y.; Gan, X.; Yang, M.; Zeng, W.; Persson, S.; Li, J.; Xu, W. GhMYB7 promotes secondary wall cellulose deposition in cotton fibres by regulating GhCesA gene expression through three distinct cis-elements. New Phytol. 2021, 232, 1718–1737. [Google Scholar] [CrossRef] [PubMed]
- Polko, J.K.; Kieber, J.J. The regulation of cellulose biosynthesis in plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Delmer, D.P.; Read, S.M.; Cooper, G. Identification of a receptor protein in cotton fibers for the herbicide 2,6-Dichlorobenzonitrile. Plant Physiol. 1987, 84, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivakov, A.A.; Flis, A.; Apelt, F.; Fuenfgeld, M.; Scherer, U.; Stitt, M.; Kragler, F.; Vissenberg, K.; Persson, S.; Suslov, D. Cellulose synthesis and cell expansion are regulated by different mechanisms in growing Arabidopsis hypocotyls. Plant Cell 2017, 29, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Veerabomma, S.; Abdel-Mageed, H.A.; Fokar, M.; Asami, T.; Yoshida, S.; Allen, R.D. Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiol. 2005, 46, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, N.; Geng, Z.; Ji, M.; Wang, S.; Zhuang, Y.; Wang, D.; He, G.; Zhao, S.; Zhou, G.; et al. Integrated transcriptome and proteome analysis reveals brassinosteroid-mediated regulation of cambium initiation and patterning in woody stem. Hortic. Res. 2022, 9, uhab048. [Google Scholar] [CrossRef]
- Liu, J.; Feng, K.; Hou, X.; Li, H.; Wang, G.; Xu, Z.; Xiong, A. Transcriptome profiling reveals the association of multiple genes and pathways contributing to hormonal control in celery leaves. Acta Biochim. Biophys. Sin. 2019, 51, 524–534. [Google Scholar] [CrossRef]
- Müssig, C.; Shin, G.H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- González-García, M.P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Jin, Y.; Liu, W.; Li, F.; Fang, J.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. Dwarf and Low-Tillering, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 2009, 58, 803–816. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-H.; Sun, M.; Wang, H.; Liu, J.-X.; Tan, G.-F.; Yan, J.; Wang, Y.-H.; Yan, Z.-M.; Liu, H.; Tao, J.-P.; et al. 24-Epibrassinolide and 2,6-Dichlorobenzonitrile Promoted Celery Petioles and Hypocotyl Elongation by Altering Cellulose Accumulation and Cell Length. Agronomy 2022, 12, 1670. https://doi.org/10.3390/agronomy12071670
Liu Y-H, Sun M, Wang H, Liu J-X, Tan G-F, Yan J, Wang Y-H, Yan Z-M, Liu H, Tao J-P, et al. 24-Epibrassinolide and 2,6-Dichlorobenzonitrile Promoted Celery Petioles and Hypocotyl Elongation by Altering Cellulose Accumulation and Cell Length. Agronomy. 2022; 12(7):1670. https://doi.org/10.3390/agronomy12071670
Chicago/Turabian StyleLiu, Yan-Hua, Miao Sun, Hao Wang, Jie-Xia Liu, Guo-Fei Tan, Jun Yan, Yuan-Hua Wang, Zhi-Ming Yan, Hui Liu, Jian-Ping Tao, and et al. 2022. "24-Epibrassinolide and 2,6-Dichlorobenzonitrile Promoted Celery Petioles and Hypocotyl Elongation by Altering Cellulose Accumulation and Cell Length" Agronomy 12, no. 7: 1670. https://doi.org/10.3390/agronomy12071670








