Lef1 and Dlx3 May Facilitate the Maturation of Secondary Hair Follicles in the Skin of Gansu Alpine Merino
Abstract
:1. Introduction
2. Material and Methods
2.1. Collection of Animal Samples
2.2. RT-qPCR Analysis
2.3. Immunohistochemical Analysis
2.4. Immunofluorescence Analysis
2.5. Measurements and Statistical Analysis
3. Results
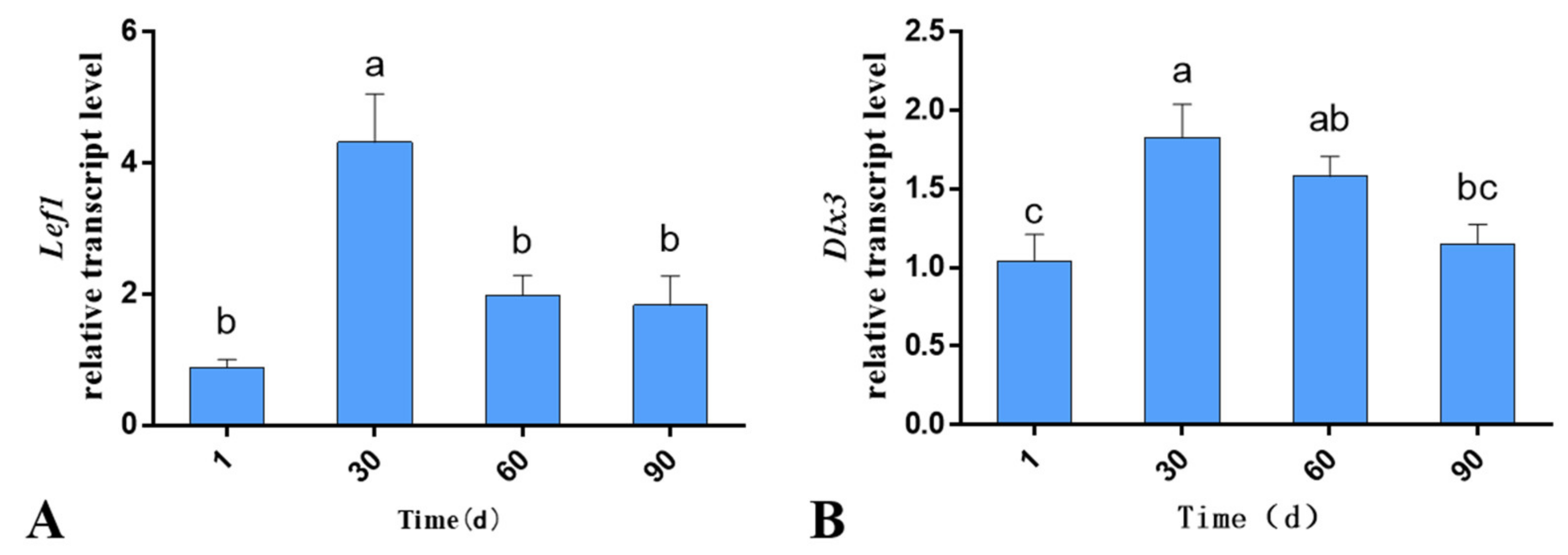
3.1. Expression of Lef1 and Dlx3 in the Skin Tissues of Gansu Alpine Merino at Various Ages
3.2. Histomorphological Analysis and Evaluation of Hair Follicles at Various Ages
3.3. The Average Optical Density of Lef1 and Dlx3 in the Skin Tissues of Gansu Alpine Merino at Various Ages
3.4. Distribution of Lef1 and Dlx3 in the Skin Tissues of Gansu Alpine Merino at Various Ages
4. Discussion
4.1. Study on the Morphological Development of Skin and Hair Follicles at Various Ages
4.2. mRNA and Protein Expression Studies of Lef1 and Dlx3 in Skin Tissues at Various Ages
4.3. Localization Study of Lef1 and Dlx3 in Skin Tissues at Various Ages
4.4. Prospects for Clinical Applications of Lef1 and Dlx3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulayman, A.; Tian, K.; Huang, X.; Tian, Y.; Xu, X.; Fu, X.; Zhao, B.; Wu, W.; Wang, D.; Yasin, A.; et al. Genome-wide identification and characterization of long non-coding RNAs expressed during sheep fetal and postnatal hair follicle development. Sci. Rep. 2019, 9, 8501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wu, H.; Lian, Z. Bioinformatics analysis of evolutionary characteristics and biochemical structure of FGF5 Gene in sheep. Gene 2019, 702, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sennett, R.; Rendl, M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.H.; Zhang, K.; Yang, K.; Ye, J.X.; Xing, Y.Z.; Guo, H.Y.; Deng, F.; Lian, X.H.; Yang, T. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J. Investig. Dermatol. 2013, 133, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, C.; Baarsma, H.A.; Wagner, D.E.; Hilgendorff, A.; Königshoff, M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: Role of WNT signaling. Mol. Cell. Pediatr. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asally, M.; Yoneda, Y. Beta-catenin can act as a nuclear import receptor for its partner transcription f actor, lymphocyte enhancer factor-1 (lef-1). Exp. Cell Res. 2005, 308, 357–363. [Google Scholar] [CrossRef]
- Driskell, R.R.; Goodheart, M.; Neff, T.; Liu, X.; Luo, M.; Moothart, C.; Sigmund, C.D.; Hosokawa, R.; Chai, Y.; Engelhardt, J.F. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev. Biol. 2007, 305, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhang, L.; Gong, K.; Lu, G.; Sheng, B.; Wang, A.; Zhao, N.; Zhang, X.; Gong, Y. LEF-1 activates the transcription of E2F1. Biochem. Biophys. Res. Commun. 2008, 365, 149–153. [Google Scholar] [CrossRef]
- Wang, S.H.; Nan, K.J.; Wang, Y.C. Endothelial cells promote the proliferation of lymphocytes partly through the Wnt pathway via LEF-1. Biochem. Biophys. Res. Commun. 2009, 388, 67–72. [Google Scholar] [CrossRef]
- Boras-Granic, K.; Chang, H.; Grosschedl, R.; Hamel, P.A. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev. Biol. 2006, 295, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, J.; Shi, C.; Huang, Y.; Wang, Y.; Yang, T.; Yang, J. Lef1 contributes to the differentiation of bulge stem cells by nuclear translocation and cross-talk with the Notch signaling pathway. Int. J. Med. Sci. 2013, 10, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.Q.; Javed, A.; Morasso, M.I.; Karlin, J.; Montecino, M.; van Wijnen, A.J.; Stein, G.S.; Stein, J.L.; Lian, J.B. Dlx3 transcriptional regulation of osteoblast differentiation: Temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol. Cell. Biol. 2004, 24, 9248–9261. [Google Scholar] [CrossRef] [Green Version]
- Depew, M.J.; Simpson, C.A.; Morasso, M.; Rubenstein, J.L. Reassessing the Dlx code: The genetic regulation of branchial arch skeletal pattern and development. J. Anat. 2005, 207, 501–561. [Google Scholar] [CrossRef] [PubMed]
- Morasso, M.I.; Markova, N.G.; Sargent, T.D. Regulation of epidermal differentiation by a Distal-less homeodomain gene. J. Cell Biol. 1996, 135, 1879–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beanan, M.J.; Sargent, T.D. Regulation and function of Dlx3 in vertebrate development. Dev. Dyn. Aug. 2000, 218, 545–553. [Google Scholar] [CrossRef]
- Radoja, N.; Guerrini, L.; Lo Iacono, N.; Merlo, G.R.; Costanzo, A.; Weinberg, W.C.; La Mantia, G.; Calabrò, V.; Morasso, M.I. Homeobox gene Dlx3 is regulated by p63 during ectoderm development: Relevance in the pathogenesis of ectodermal dysplasias. Development 2007, 134, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Price, J.A.; Bowden, D.W.; Wright, J.T.; Pettenati, M.J.; Hart, T.C. Identification of a mutation in DLX3 associated with tricho-dento-osseous (TDO) syndrome. Hum. Mol. Genet. 1998, 7, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.T.; Hong, S.P.; Simmons, D.; Daly, B.; Uebelhart, D.; Luder, H.U. DLX3 c.561_562delCT mutation causes attenuated phenotype of tricho-dento-osseous syndrome. Am. J. Med. Genet. A 2008, 146, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Eastman, Q.; Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 1999, 11, 233–240. [Google Scholar] [CrossRef]
- Mecklenburg, L.; Paus, R.; Halata, Z.; Bechtold, L.S.; Fleckman, P.; Sundberg, J.P. FOXN1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. J. Investig. Dermatol. 2004, 123, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000, 11, 273–282. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Aebischer, T.; Hülsken, J.; Birchmeier, W.; Klemm, U.; Scheidereit, C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development 2001, 128, 3843–3853. [Google Scholar] [CrossRef]
- Petersson, M.; Brylka, H.; Kraus, A.; John, S.; Rappl, G.; Schettina, P.; Niemann, C. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 2011, 30, 3004–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Zhang, Y.J.; Yue, W.B. Sheep Production; Agriculture Press: Beijing, China, 2016. [Google Scholar]
- Zhang, L.P.; Dong, H. Study-on the development ofskin and hair follicles of Gansu Alpine-fine-wool sheepand their hybrids. China Herbiv. Sci. 2019, 39, 67–69. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, H.Z. Study on the development ofskin and hair follicles of Liaoning cashmere goats atlamb stage. China Herbiv. Sci. 2020, 40, 70–73. [Google Scholar] [CrossRef]
- Wu, W.; Xu, R.F.; Gu, X.; Li, H.; Wu, C.X. Characterization of embryonic feather follicle development in the Chinese indigenous Jilin White goose. Asian-Australas. J. Anim. Sci. 2008, 21, 346–352. [Google Scholar] [CrossRef]
- Sennett, R.; Wang, Z.; Rezza, A.; Grisanti, L.; Roitershtein, N.; Sicchio, C.; Mok, K.W.; Heitman, N.J.; Clavel, C.; Ma’ayan, A.; et al. An Integrated Transcriptome Atlas of Embryonic Hair Follicle Progenitors, Their Niche, and the Developing Skin. Dev. Cell. 2015, 34, 577–591. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberger, B.M.; Mastrogiannaki, M.; Watt, F.M. Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 2016, 7, 10537. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, X.; Liu, Z.; Li, S.; Zheng, X.; Nie, Y.; Tao, Y.; Zhou, X.; Wu, W.; Yang, G.; et al. Exploration of key regulators driving primary feather follicle induction in goose skin. Gene 2020, 731, 144338. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Msuthwana, P.; Liu, J.; Liu, C.; Sello, C.T.; Song, Y.; Feng, Z.; Li, S.; Yang, W.; et al. The role of CTNNB1 and LEF1 in feather follicles development of Anser cygnoides and Anser anser. Genes Genom. 2020, 42, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Fan, Y.; Yan, X.; Li, W.; Yan, X.; Liu, H.; Zhang, L.; Su, Y.; Zhang, J.; Jiang, W.; et al. Identification of Genes Related to Hair Follicle Cycle Development in Inner Mongolia Cashmere Goat by WGCNA. Front. Vet. Sci. 2022, 9, 894380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, P.; Ye, N.; Zhou, X.; Zhang, Y.; Liang, C.; Guo, X.; Chu, M.; Pei, J.; Yan, P. Identification of the Key Genes Associated with the Yak Hair Follicle Cycle. Genes 2021, 13, 32. [Google Scholar] [CrossRef]
- Hwang, J.; Mehrani, T.; Millar, S.E.; Morasso, M.I. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development 2018, 135, 3149–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardaryev, A.N.; Ahmed, M.I.; Vlahov, N.V.; Fessing, M.Y.; Gill, J.H.; Sharov, A.A.; Botchkareva, N.V. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. FASEB J. 2010, 24, 3869–3881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.K.; Lee, H.Y.; Choi, J.H.; Kim, J.K.; Yoon, J.B.; Yoon, S.K. Hairless plays a role in formation of inner root sheath via regulation of Dlx3 gene. J. Biol. Chem. 2012, 287, 16681–16688. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.H.; Joo, H.J.; Kim, J.E.; Park, Y.M.; Kang, H. Effect of mycophenolic acid on proliferation of dermal papilla cells and induction of anagen hair follicles. Clin. Exp. Dermatol. 2015, 40, 894–902. [Google Scholar] [CrossRef]
- Morgan, B.A. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [Green Version]
- Botchkarev, V.A.; Kishimoto, J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003, 8, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Yan, W.; Guo, X.; Zhu, B.; Liu, D. Regulatory role of LEF-1 in the Proliferation of Arbas White Cashmere Goat Dermal Papilla Cells. Can. J. Anim. Sci. 2018, 98. [Google Scholar] [CrossRef]
- Lévy, J.; Capri, Y.; Rachid, M.; Dupont, C.; Vermeesch, J.R.; Devriendt, K.; Verloes, A.; Tabet, A.C.; Bailleul-Forestier, I. LEF1 haploinsufficiency causes ectodermal dysplasia. Clin. Genet. 2020, 97, 595–600. [Google Scholar] [CrossRef]
- Dufour, W.; Alawbathanim, S.; Jourdainm, A.S.; Asif, M.; Baujat, G.; Becker, C.; Hussain, M.S. Monoallelic and biallelic variants in LEF1 are associated with a new syndrome combining ectodermal dysplasia and limb malformations caused by altered WNT signaling. Genet. Med. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xiong, Z.M.; Xue, H.; Brown, M.A.; Gete, Y.G.; Yu, R.; Sun, L.; Cao, K. Impaired LEF1 Activation Accelerates iPSC-Derived Keratinocytes Differentiation in Hutchinson-Gilford Progeria Syndrome. Int. J. Mol. Sci. 2022, 23, 5499. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Phan, Q.M.; Fine, G.M.; Salz, L.; Herrera, G.G.; Wildman, B.; Driskell, I.M.; Driskell, R.R. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 2020, 9, e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Li, Z.; Chen, D.; Han, W.; Wu, Z.; Shang, F.; Hai, E.; Wei, Y.; Su, R.; et al. Transcriptome profiling reveals transcriptional and alternative splicing regulation in the early embryonic development of hair follicles in the cashmere goat. Sci. Rep. 2019, 9, 17735. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 89. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Su, R. Editorial: Sheep and Goat Gene Exploration. Front. Genet. 2022, 13, 80. [Google Scholar] [CrossRef]






| Gene | GenBank Accession No. | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Lef1 | XM_042251154.1 | F: ACCATGACAAGGCCAGAGAA | 228 | 60 |
| R: TGATGAGAGGGGTGAGAGGA | ||||
| Dlx3 | XM_004012779.5 | F: AACCGCCGTTCCAAATTCAA | 230 | 60 |
| R: TGTGCATGGTACCAGGAGTT | ||||
| β-actin | NM_001009784 | F: AGCCTTCCTTCCTGGGCATGGA | 113 | 60 |
| R: GGACAGCACCGTGTTGGCGTAGA |
| Names | Days | Corneum | Inner Root Sheath | Outer Root Sheath | Hair Medulla | Sebaceous Gland | Dermal Papilla |
|---|---|---|---|---|---|---|---|
| Lef1 | 1 | +++ | +++ | ++++ | ++++ | ++++ | ++++ |
| 30 | − | − | − | − | − | ++++ | |
| 60 | − | − | − | − | − | ++++ | |
| 90 | − | − | − | − | − | ++++ | |
| Dlx3 | 1 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 30 | +++ | ++++ | ++++ | ++ | +++ | ++++ | |
| 60 | ++++ | ++++ | ++++ | +++ | ++++ | ++++ | |
| 90 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; He, Z.; Xi, Q.; Zhao, F.; Hu, J.; Wang, J.; Liu, X.; Zhao, Z.; Li, M.; Luo, Y.; et al. Lef1 and Dlx3 May Facilitate the Maturation of Secondary Hair Follicles in the Skin of Gansu Alpine Merino. Genes 2022, 13, 1326. https://doi.org/10.3390/genes13081326
Sun H, He Z, Xi Q, Zhao F, Hu J, Wang J, Liu X, Zhao Z, Li M, Luo Y, et al. Lef1 and Dlx3 May Facilitate the Maturation of Secondary Hair Follicles in the Skin of Gansu Alpine Merino. Genes. 2022; 13(8):1326. https://doi.org/10.3390/genes13081326
Chicago/Turabian StyleSun, Hongxian, Zhaohua He, Qiming Xi, Fangfang Zhao, Jiang Hu, Jiqing Wang, Xiu Liu, Zhidong Zhao, Mingna Li, Yuzhu Luo, and et al. 2022. "Lef1 and Dlx3 May Facilitate the Maturation of Secondary Hair Follicles in the Skin of Gansu Alpine Merino" Genes 13, no. 8: 1326. https://doi.org/10.3390/genes13081326






