Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy
Abstract
1. Amiodarone Pharmacology and Toxicity
2. Clinically Defining Amiodarone-Associated Optic Neuropathy
3. Refining Diagnostic Criteria for AAON
4. Persistent Diagnostic Challenges for AAON
5. Investigating AAON Incidence
6. Early Mechanistic Investigations of AAON
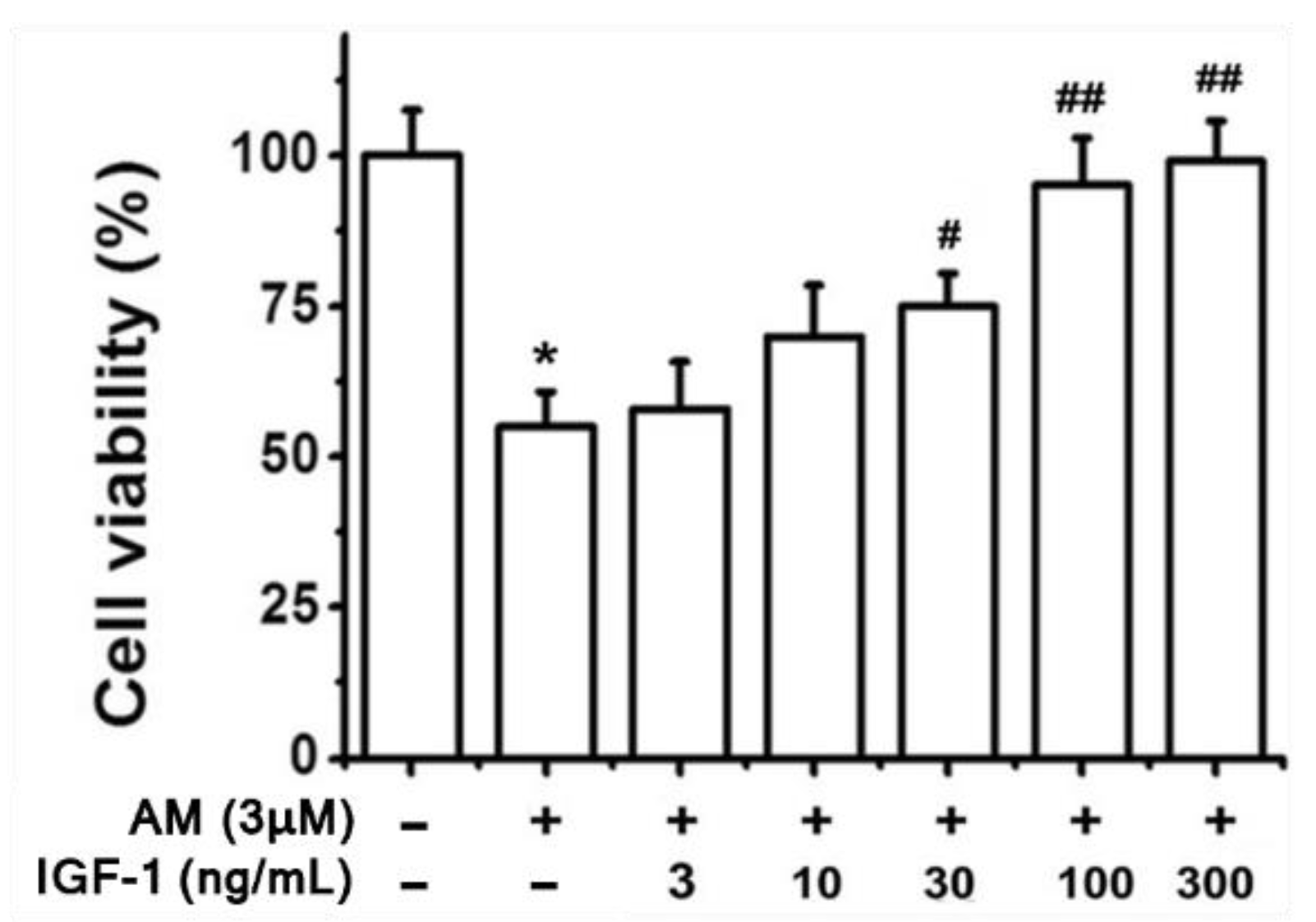
7. Retinal Pigment Epithelial Cell Models of Amiodarone Toxicity
8. Retinal Ganglion Cell Models of Amiodarone Toxicity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, M.A.; Murphy, J.F. Amiodarone and optic neuropathy: The heart of the matter. J. Neuroophthal. 2005, 25, 232–236. [Google Scholar] [CrossRef]
- McKenna, W.J.; Oakley, C.M.; Krikler, D.M.; Goodwin, J.F. Improved survival with amiodarone in patients with hypertrophic cardiomyopathy and ventricular tachycardia. Br. Heart J. 1985, 53, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Holt, D.W.; Storey, G.C.A.; Morley, A.R.; Callaghan, J.; Campbell, R.W. Amiodarone and its desethyl metabolite: Tissue distribution and morphologic changes during long-term therapy. Circulation 1985, 72, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.; Trohman, R.G. Prescribing amiodarone: An evidence-based review of clinical indications. JAMA 2007, 298, 1312–1322. [Google Scholar] [CrossRef]
- Singh, B.N. Amiodarone: A multifaceted antiarrhythmic drug. Curr. Cardiol. Rep. 2006, 8, 349–355. [Google Scholar] [CrossRef]
- Harris, L.; Hind, C.R.; McKenna, W.J.; Savage, C.; Krikler, S.J.; Storey, G.C.; Holt, D.W. Renal elimination of amiodarone and its desethyl metabolite. Postgrad. Med. J. 1983, 59, 440–442. [Google Scholar] [CrossRef]
- Raeder, E.A.; Podrid, P.J.; Lown, B. Side effects and complications of amiodarone therapy. Am. Heart J. 1985, 109, 975–983. [Google Scholar] [CrossRef]
- Johnson, L.N.; Krohel, G.B.; Thomas, E.R. The clinical spectrum of amiodarone-associated optic neuropathy. J. Natl. Med. Assoc. 2004, 96, 1477–1491. [Google Scholar]
- Charness, M.E.; Morady, F.; Scheinman, M.M. Frequent neurologic toxicity associated with amiodarone therapy. Neurology 1984, 34, 669–671. [Google Scholar] [CrossRef]
- Zhong, B.; Wang, Y.; Zhang, G.; Wang, Z. Environmental iodine content, female sex and age are associated with new-onset amiodarone-induced hypothyroidism: A systematic review and meta-analysis of adverse reactions of amiodarone on the thyroid. Cardiology 2016, 134, 366–371. [Google Scholar] [CrossRef]
- Ingram, D.V.; Jaggarao, N.S.; Chamberlain, D.A. Ocular changes resulting from therapy with amiodarone. Br. J. Ophthalmol. 1982, 66, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, T.; Atilla, H.; Batioglu, F.; Günalp, I. Amiodarone-related optic neuropathy. Jpn. J. Ophthalmol. 2000, 44, 565–568. [Google Scholar] [CrossRef]
- Nagra, P.K.; Foroozan, R.; Savino, P.J.; Castillo, I.; Sergott, R.C. Amiodarone induced optic neuropathy. Br. J. Ophthalmol. 2003, 87, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Speicher, M.A.; Goldman, M.H.; Chrousos, G.A. Amiodarone optic neuropathy without disc edema. J. Neuroophthalmol. 2000, 20, 171–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, A.G.; Cheng, H.C. Amiodarone-Associated Optic Neuropathy: Clinical Review. Neuroophthalmol. 2016, 41, 55–58. [Google Scholar] [CrossRef]
- Rucker, J.C.; Biousse, V.; Newman, N.J. Ischemic optic neuropathies. Curr. Opin. Neurol. 2004, 17, 27–35. [Google Scholar] [CrossRef]
- Berry, S.; Lin, W.V.; Sadaka, A.; Lee, A.G. Nonarteritic anterior ischemic optic neuropathy: Cause, effect, and management. Eye Brain 2017, 9, 23–28. [Google Scholar] [CrossRef]
- Barros, A.E.; Amram, A.L.; Derham, A.M.; Smith, S.V.; Lee, A.G. Management of ischemic optic neuropathies. Expert Rev. Ophthalmol. 2017, 12, 99–109. [Google Scholar] [CrossRef]
- Macaluso, D.C.; Shults, W.T.; Fraunfelder, F.T. Features of amiodarone-induced optic neuropathy. Am. J. Ophthalmol. 1999, 127, 610–612. [Google Scholar] [CrossRef]
- Purvin, V.; Kawasaki, A.; Borruat, F.-X. Optic neuropathy in patients using amiodarone. Arch. Ophthalmol. 2006, 124, 696–701. [Google Scholar] [CrossRef]
- Fasler, K.; Traber, G.L.; Jaggi, G.P.; Landau, K. Amiodarone-associated Optic Neuropathy—A Clinical Criteria-based Diagnosis? Neuro-Ophthalmology 2017, 42, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mindel, J.S.; Anderson, J.; Hellkamp, A.; Johnson, G.; Poole, J.E.; Mark, D.B.; Lee, K.L.; Bardy, G.H.; SCD-HeFT Investigators. Absence of bilateral vision loss from amiodarone: A randomized trial. Am. Heart J. 2007, 153, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Feiner, L.A.; Younge, B.R.; Kazmier, F.J.; Stricker, B.H.; Fraunfelder, F.T. Optic neuropathy and amiodarone therapy. Mayo Clin. Proc. 1987, 62, 702–717. [Google Scholar] [CrossRef]
- Stelfox, H.T.; Ahmed, S.B.; Fiskio, J.; Bates, D.W. Monitoring amiodarone’s toxicities: Recommendations, evidence, and clinical practice. Clin. Pharmacol. Ther. 2004, 75, 110–122. [Google Scholar] [CrossRef]
- Cheng, H.C.; Yeh, H.J.; Huang, N.; Chou, Y.J.; Yen, M.Y.; Wang, A.G. Amiodarone-associated optic neuropathy: A nationwide study. Ophthalmology 2015, 122, 2553–2559. [Google Scholar] [CrossRef]
- Passman, R.S.; Bennett, C.L.; Purpura, J.M.; Kapur, R.; Johnson, L.N.; Raisch, D.W.; West, D.P.; Edwards, B.J.; Belknap, S.M.; Liebling, D.B.; et al. Amiodarone-associated optic neuropathy: A critical review. Am. J. Med. 2012, 125, 447–453. [Google Scholar] [CrossRef]
- Su, V.Y.; Hu, Y.W.; Chou, K.T.; Ou, S.M.; Lee, Y.C.; Lin, E.Y.H.; Chen, T.J.; Tzeng, C.H.; Liu, C.J. Amiodarone and the risk of cancer: A nationwide population-based study. Cancer 2013, 119, 1699–1705. [Google Scholar] [CrossRef]
- Vorperian, V.R.; Havighurst, T.C.; Miller, S.; January, C.T. Adverse effects of low dose amiodarone: A meta-analysis. J. Am. Coll. Cardiol. 1997, 30, 791–798. [Google Scholar] [CrossRef]
- Lafuente-Lafuente, C.; Alvarez, J.C.; Leenhardt, A.; Mouly, S.; Extramiana, F.; Caulin, C.; Funck-Brentano, C.; Bergmann, J.F. Amiodarone concentrations in plasma and fat tissue during chronic treatment and related toxicity. Br. J. Clin. Pharmacol. 2009, 67, 511–519. [Google Scholar] [CrossRef]
- Nazarian, S.M.; Jay, W.M. Bilateral optic neuropathy associated with amiodarone therapy. J. Clin. Neuroophthalmol. 1988, 8, 25–28. [Google Scholar]
- Mansour, A.M.; Puklin, J.E.; O’Grady, R. Optic nerve ultrastructure following amiodarone therapy. J. Clin. Neuroophthalmol. 1988, 8, 231–237. [Google Scholar] [PubMed]
- Lu, J.; Miyakawa, K.; Roth, R.A.; Ganey, P.E. Tumor necrosis factor-alpha potentiates the cytotoxicity of amiodarone in Hepa1c1c7 cells: Roles of caspase activation and oxidative stress. Toxicol. Sci. 2013, 131, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, A.C.; Ji, Y.; Comeau, J.L.; Hill, B.C.; Takahashi, T.; Brien, J.F.; Racz, W.J.; Massey, T.E. Direct mitochondrial dysfunction precedes reactive oxygen species production in amiodarone-induced toxicity in human peripheral lung epithelial HPL1A cells. Toxicol. Appl. Pharmacol. 2008, 227, 370–379. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Giudetti, A.M.; Gnoni, G.V.; Capitanio, N.; Tamborra, R.; Romano, A.D.; Quinto, M.; Blonda, M.; Vendemiale, G.; et al. Mitochondrial oxidative stress and respiratory chain dysfunction account for liver toxicity during amiodarone but not dronedarone administration. Free Radic. Biol. Med. 2011, 51, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, A.E.; Zigmond, M.J.; Smith, A.D. IGF-1 protects dopamine neurons against oxidative stress: Association with changes in phosphokinases. Exp. Brain Res. 2016, 234, 1863–1873. [Google Scholar] [CrossRef]
- Liao, R.; Yan, F.; Zeng, Z.; Wang, H.; Qiu, K.; Xu, J.; Zheng, W. IGF-1 activates PI3K/Akt signaling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury. Br. J. Pharm. 2018, 175, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Koriyama, Y.; Mawatari, K.; Higuchi, Y.; Kosaka, J.; Kato, S. Early downregulation of IGF-I decides the fate of rat retinal ganglion cells after optic nerve injury. Neurochem. Int. 2007, 50, 741–748. [Google Scholar] [CrossRef]
- Sanes, J.R.; Masland, R.H. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef]
- Liao, R.; Yan, F.; Zeng, Z.; Farhan, M.; Little, P.; Quirion, R.; Srivastava, L.K.; Zheng, W. Amiodarone-Induced Retinal Neuronal Cell Apoptosis Attenuated by IGF-1 via Counter Regulation of the PI3k/Akt/FoxO3a Pathway. Mol. Neurobiol. 2017, 54, 6931–6943. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, R.; Chacko, J. Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy. Biomolecules 2022, 12, 1298. https://doi.org/10.3390/biom12091298
Mitchell R, Chacko J. Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy. Biomolecules. 2022; 12(9):1298. https://doi.org/10.3390/biom12091298
Chicago/Turabian StyleMitchell, Reece, and Joseph Chacko. 2022. "Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy" Biomolecules 12, no. 9: 1298. https://doi.org/10.3390/biom12091298
APA StyleMitchell, R., & Chacko, J. (2022). Clinical and Mechanistic Review of Amiodarone-Associated Optic Neuropathy. Biomolecules, 12(9), 1298. https://doi.org/10.3390/biom12091298




