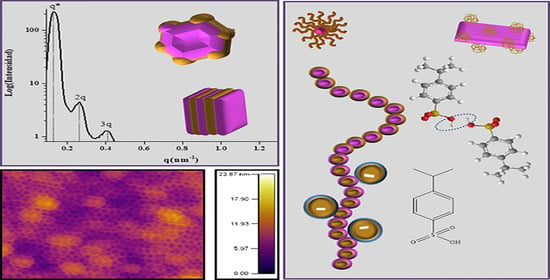
Sulfonated Block Copolymers: Synthesis, Chemical Modification, Self-Assembly Morphologies, and Recent Applications
Abstract
:1. Introduction
2. Phase Diagram of BCs
3. Techniques for Obtaining BCs
- (1)
- The initiation stage must be much faster than the propagation because it needs the chains to start and grow simultaneously.
- (2)
- The concentration of propagating radicals is very low to substantially decrease the termination events, whereas the chains are growing with very few side events like termination or chain transfer reactions.
- (3)
- A rapid exchange between dormant and active species is also required, favoring most of the growing chains that remain in the “dormant” state and only a small fraction of active radicals as end groups in the chain.
4. Amphiphilic Character of BCs through the Sulfonation
5. Morphological and Structural Changes Induced by the Sulfonation
5.1. Phase Behavior of Sulfonated Block-Copolymers
5.2. Nanoscale Morphologies Self-Assembly of Sulfonated Block-Copolymers
6. Applications of ABCs
6.1. Applications in Electronics
6.2. Applications in Lithography
6.3. Applications in Photovoltaics
6.4. Applications in Fuel Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, S.; Cohen, Y. Phase Separation of Polymer Blends in Thin Films. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1255–1267. [Google Scholar] [CrossRef]
- Yu, W. Rheology and Phase Separation of Polymer Blends with Weak Dynamic Asymmetry. Polymer 2011, 52, 2693–2700. [Google Scholar]
- Henderson, I.C. Two-Step Phase Separation in Polymer Blends. Macromolecules 2004, 37, 1952–1959. [Google Scholar]
- Fredrickson, G.H.; Eugene, H. Fluctuation Effects in the Theory of Microphase Separation in Block Copolymers. J. Chem. Phys. 1987, 87, 697–705. [Google Scholar] [CrossRef]
- Liu, C.C.; Franke, E.; Mignot, Y.; Xie, R.; Yeung, C.W.; Zhang, J.; Corliss, D. Directed Self-Assembly of Block Copolymers for 7 Nanometre FinFET Technology and Beyond. Nat. Electron. 2018, 1, 562–569. [Google Scholar]
- Kim, H.C.; Park, S.M.; Hinsberg, W.D. Block Copolymer Based Nanostructures: Materials, Processes, and Applications to Electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [PubMed]
- Meier-Haack, J.; Taeger, A.; Vogel, C.; Schlenstedt, K.; Lenk, W.; Lehmann, D. Membranes from Sulfonated Block Copolymers for Use in Fuel Cells. Sep. Purif. Technol. 2005, 41, 207–220. [Google Scholar]
- Elabd, Y.A.; Hickner, M.A. Block Copolymers for Fuel Cells. Macomolecules 2011, 44, 1–11. [Google Scholar]
- Orilall, M.C.; Wiesner, U. Block Copolymer Based Composition and Morphology Control in Nanostructured Hybrid Materials for Energy Conversion and Storage: Solar Cells, Batteries, and Fuel Cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [PubMed]
- Miyahara, T.; Hayano, T.; Matsuno, S.; Watanabe, M.; Miyatake, K. Sulfonated Polybenzophenone/Poly (Arylene Ether) Block Copolymer Membranes for Fuel Cell Applications. ACS Appl. Mater. Interfaces 2012, 4, 2881–2884. [Google Scholar] [PubMed]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic Block Copolymers for Drug Delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [PubMed]
- Diterlizzi, M.; Ferretti, A.M.; Scavia, G.; Sorrentino, R.; Luzzati, S.; Boccia, A.C.; Destri, S. Amphiphilic PTB7-Based Rod-Coil Block Copolymer for Water-Processable Nanoparticles as an Active Layer for Sustainable Organic Photovoltaic: A Case Study. Polymer 2022, 14, 1588. [Google Scholar]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.A. Advanced Drug Delivery Devices via Self-Assembly of Amphiphilic Block Copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-Engineering Block Copolymer Aggregates for Drug Delivery. Colloids Surf. B Biointerfaces 1999, 19, 3–27. [Google Scholar]
- Shi, A.-C.; Noolandi, J. Binary Mixtures of Diblock Copolymers. Phase Diagrams with a New Twist. Macromolecules 1995, 28, 3103. [Google Scholar] [CrossRef]
- Shi, A.-C.; Noolandi, J. Effects of Short Diblocks at Interfaces of Strongly Segregated Long Diblocks. Macromolecules 1994, 27, 2936–2944. [Google Scholar]
- Matsen, M.W.; Bates, F.S. Unifying Weak- and Strong-Segregation Block Copolymer Theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Matsen, M.W. Block Copolymer/Homopolymer Blends. Macromolecules 1995, 28, 5765–5773. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Paria, S.; Mondal, S.; Lee, G.B.; Shin, B.; Kim, S.; Nah, C. Amphiphilic Block Co-Polymer and Silica Reinforced Epoxy Composite with Excellent Toughness and Delamination Resistance for Durable Electronic Packaging Application. Polymer 2022, 245, 124679. [Google Scholar]
- Selianitis, D.; Forys, A.; Trzebicka, B.; Alemayehu, A.; Tyrpekl, V.; Pispas, S. Amphiphilic P (OEGMA-Co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly. Nanomanufacturing 2022, 2, 53–68. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, C.C.; Cui, W.L.; Qu, J.; Zhang, H.; Liu, K.; Wang, J.Y. A Novel and Modified Fluorescent Amphiphilic Block Copolymer Simultaneously Targeting to Lysosomes and Lipid Droplets for Cell Imaging with Large Stokes Shift. Eur. Polym. J. 2022, 166, 111030. [Google Scholar]
- Chang, C.H.; Chang, C.H.; Yang, Y.W.; Chen, H.Y.; Yang, S.J.; Yao, W.C.; Chao, C. Quaternized Amphiphilic Block Copolymers as Antimicrobial Agents. Polymers 2022, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Kučera, F.; Jančář, J. Homogeneous and Heterogeneous Sulfonation of Polymers: A Review. Polym. Eng. Sci. 1998, 38, 783–792. [Google Scholar] [CrossRef]
- Sang, S.; Wang, S.; Yang, C.; Geng, Z.; Zhang, X. Sponge-Inspired Sulfonated Polyetheretherketone Loaded with Polydopamine-Protected Osthole Nanoparticles and Berberine Enhances Osteogenic Activity and Prevents Implant-Related Infections. Chem. Eng. J. 2022, 437, 135255. [Google Scholar]
- Huang, Z.; Lv, B.; Zhou, L.; Qin, X.; Shao, Z. Ultra-Thin h-BN Doped High Sulfonation Sulfonated Poly (Ether-Ether-Ketone) of PTFE-Reinforced Proton Exchange Membrane. J. Memb. Sci. 2022, 644, 120099. [Google Scholar]
- Min, J.; Jung, H.Y.; Jeong, S.; Lee, B.; Son, C.Y.; Parka, M.J. Enhancing Ion Transport in Charged Block Copolymers by Stabilizing Low Symmetry Morphology: Electrostatic Control of Interfaces. Proc. Natl. Acad. Sci. USA 2021, 118, 1–8. [Google Scholar] [CrossRef]
- Sivashinsky, N.; Tanny, G.B. The State of Water in Swollen Ionomers Containing Sulfonic Acid Salts. J. Appl. Polym. Sci. 1981, 26, 2625–2637. [Google Scholar] [CrossRef]
- Shim, S.Y.; Weiss, R.A. Sulfonated Poly(Ethylene-Ran-Styrene) Ionomers. Polym. Int. 2005, 54, 1220–1223. [Google Scholar] [CrossRef]
- Langner, R.; Zundel, G. FT-IR Investigation of Polarizable, Strong Hydrogen Bonds in Sulfonic Acid-Sulfoxide, -Phosphine Oxide, and -Arsine Oxide Complexes in the Middle- and Far-Infrared Region. J. Phys. Chem. 1995, 99, 12214–12219. [Google Scholar] [CrossRef]
- Gu, S.; Skovgard, J.; Yan, Y.S. Engineering the van Der Waals Interaction in Cross-Linking-Free Hydroxide Exchange Membranes for Low Swelling and High Conductivity. ChemSusChem 2012, 5, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.S.; Thurman, E.M.; Pedersen, M.J. Application of Mixed-Mode, Solid-Phase Extraction in Environmental and Clinical Chemistry. Combining Hydrogen-Bonding, Cation-Exchange and Van Der Waals Interactions. J. Chromatogr. A 1993, 629, 11–21. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.; Leyva-Porras, C.; Olayo-Valles, R.; Revilla-Vázquez, J.; Schubert, U.S.; Guerrero-Sanchez, C.; Bonilla-Cruz, J. Self-Assembly Investigations of Sulfonated Poly(Methyl Methacrylate-Block-Styrene) Diblock Copolymer Thin Films. Adv. Polym. Technol. 2019, 2019, 4375838. [Google Scholar] [CrossRef] [Green Version]
- Hamley, I.W. Melt Phase Behaviour of Block Copolymers. In The Physics of Bock Copolymers; Oxford University Press: Oxford, UK, 1998; pp. 24–130. ISBN 978-0-19-850218-0. [Google Scholar]
- Cochran, E.W.; Garcia-Cervera, C.J.; Fredrickson, G.H. Stability of the Gyroid Phase in Diblock Copolymers at Strong Segregation. Macromolecules 2006, 39, 2449–2451. [Google Scholar] [CrossRef]
- Lynd, N.A.; Meuler, A.J.; Hillmyer, M.A. Polydispersity and Block Copolymer Self-Assembly. Prog. Polym. Sci. 2008, 33, 875–893. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization Initiated by Electron Transfer to Monomer. A New Method of Formation of Block Polymers. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Fink, Y.; Urbas, A.M.; Bawendi, M.G.; Joannopoulos, J.D.; Thomas, E.L. Block Copolymers as Photonic Bandgap Materials. J. Light. Technol. 1999, 17, 1963–1969. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.Y.; Ma, Y.Q. High Density Multiplication of Graphoepitaxy Directed Block Copolymer Assembly on Two-Dimensional Lattice Template. Soft Matter 2010, 6, 4460–4465. [Google Scholar] [CrossRef]
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, D.H. Block Copolymer Lithography: Periodic Arrays of ~1011 Holes in 1 Square Centimeter. Science 1997, 276, 1401–1404. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Schotter, J.; Kastle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-Density Nanowire Arrays Grown in Self-Assembled Diblock Copolymer Templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef] [Green Version]
- Yagci, Y.; Tasdelen, M.A. Mechanistic Transformations Involving Living and Controlled/Living Polymerization Methods. Prog. Polym. Sci. 2006, 31, 1133–1170. [Google Scholar] [CrossRef]
- Lutz, J.F.; Neugebauer, D.; Matyjaszewski, K. Stereoblock Copolymers and Tacticity Control in Controlled/Living Radical Polymerization. J. Am. Chem. Soc. 2003, 125, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/Living Radical Polymerization: Features, Developments, and Perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Zhang, H. Controlled/"living" Radical Precipitation Polymerization: A Versatile Polymerization Technique for Advanced Functional Polymers. Eur. Polym. J. 2013, 49, 579–600. [Google Scholar] [CrossRef] [Green Version]
- Goto, A.; Fukuda, T. Kinetics of Living Radical Polymerization. Prog. Polym. Sci. 2004, 29, 329–385. [Google Scholar] [CrossRef]
- Reynolds, D.D.; Kenyon, W.O. Preparation and Reactions of Sulfonic Esters. I. Preparation of Polyvinyl Sulfonates. J. Am. Chem. Soc. 1950, 72, 1584–1587. [Google Scholar] [CrossRef]
- Roth, H.H. Sulfonation of Poly(Vinyl Aromatics ). Ind. Eng. Chem. 1957, 49, 1820–1822. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Eisenberg, A. Dynamic Mechanial Properties of Sulfonated Cyclized Cis-1,4-polyisoprene. J. Appl. Polym. Sci. 1982, 27, 657–671. [Google Scholar] [CrossRef]
- Weiss, R.A.; Agarwal, P.K.; Lundberg, R.D. Control of Ionic Interactions in Sulfonated Polystyrene Ionomers by the Use of Alkyl-substituted Ammonium Counterions. J. Appl. Polym. Sci. 1984, 29, 2719–2734. [Google Scholar] [CrossRef]
- Storey, R.F.; George, S.E.; Nelson, M.E. Star-Branched Block Copolymer Ionomers. Synthesis, Characterization, and Properties. Macromolecules 1991, 24, 2920–2930. [Google Scholar] [CrossRef]
- Erdogan, T.; Unveren, E.E.; Inan, T.Y.; Birkan, B. Well-Defined Block Copolymer Ionomers and Their Blend Membranes for Proton Exchange Membrane Fuel Cell. J. Memb. Sci. 2009, 344, 172–181. [Google Scholar] [CrossRef]
- Tsang, E.M.W.; Shi, Z.; Holdcroft, S. Ionic Purity and Connectivity of Proton-Conducting Channels in Fluorous-Ionic Diblock Copolymers. Macromolecules 2011, 44, 8845–8857. [Google Scholar] [CrossRef]
- Huang, T.; Messman, J.M.; Hong, K.; Mays, J.W. Novel Amphiphilic Block Copolymers Derived from the Selective Fluorination and Sulfonation of Poly(Styrene-Block-1,3-Cyclohexadiene). J. Polym. Sci. Part A Polym. Chem. 2012, 50, 338–345. [Google Scholar] [CrossRef]
- Erdogan, T.; Bilir, C.; Erdal Unveren, E.; Demirel, A.L.; Tunca, U. Novel Multiarm Star Block Copolymer Ionomers as Proton Conductive Membranes. Polym. Chem. 2015, 6, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Colón, E.; Pérez-Pérez, M.; Suleiman, D. Synthesis and Characterization of Novel Phosphonated and Sulfonated Poly(Styrene–Isobutylene–Styrene) for Fuel Cell and Protective Clothing Applications. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1424–1435. [Google Scholar] [CrossRef]
- Noshay, A.; Robeson, L.M. Sulfonated Polysulfone. J. Appl. Polym. Sci. 1976, 20, 1885–1903. [Google Scholar] [CrossRef]
- Rahrig, D.; MacKnight, W.J.; Lenz, R.W. Sulfonation of a Polypentenamer and Preparation of Its Hydrogenated Derivatives. Macromolecules 1979, 12, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Valint, P.L.; Bock, J. Synthesis and Characterization of Hydrophobically Associating Block Polymers. Macromolecules 1988, 21, 175–179. [Google Scholar] [CrossRef]
- Gatsouli, K.D.; Pispas, S.; Kamitsos, E.I. Development and Optical Properties of Cadmium Sulfide and Cadmium Selenide Nanoparticles in Amphiphilic Block Copolymer Micellar-like Aggregates. J. Phys. Chem. C 2007, 111, 15201–15209. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, H.; Hong, K.; Simonson, J.M.; Mays, J.W. Architecturally and Chemically Modified Poly(1,3-Cyclohexadiene). Macromol. Chem. Phys. 2008, 209, 308–314. [Google Scholar] [CrossRef]
- Politakos, N.; Moutsios, I.; Manesi, G.M.; Artopoiadis, K.; Tsitoni, K.; Moschovas, D.; Piryazev, A.A.; Kotlyarskiy, D.S.; Kortaberria, G.; Ivanov, D.A.; et al. Molecular and Structure–Properties Comparison of an Anionically Synthesized Diblock Copolymer of the PS-b-PI Sequence and Its Hydrogenated or Sulfonated Derivatives. Polymers 2021, 13, 4167. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ying, S. Charge-mosaic Membrane from Gamma-irradiated Poly(Styrene-butadiene-4-vinylpyridine) Triblock Copolymer. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1075–1081. [Google Scholar] [CrossRef]
- Xu, K.; Li, K.; Khanchaitit, P.; Wang, Q. Synthesis and Characterization of Self-Assembled Sulfonated Poly (Styrene-b-Vinylidene Fluoride-b-Styrene) Triblock Copolymers for Proton Conductive Membranes. Chem. Mater. 2007, 19, 5937–5945. [Google Scholar] [CrossRef]
- Leemans, L.; Fayt, R.; Teyssié, P. Synthesis of New Amphiphilic Block Copolymers. Block Copolymer of Sulfonated Glycidyl Methacrylate and Alkyl Methacrylate. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 1255–1262. [Google Scholar] [CrossRef]
- Yang, J.C.; Mays, J.W. Synthesis and Characterization of Neutral/Ionic Block Copolymers of Various Architectures. Macromolecules 2002, 35, 3433–3438. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.B.; Hu, C.P.; Ying, S.K.; Wu, S.S.; Xu, X.D. Synthesis and Characterization of Fluorinated Block Copolymers Containing Carboxylic or Sulfonic Groups. React. Funct. Polym. 2003, 56, 189–197. [Google Scholar] [CrossRef]
- Khawas, K.; Daripa, S.; Kumari, P.; Das, S.; Dey, R.K.; Kuila, B.K. Highly Water-Soluble Rod–Coil Conjugated Block Copolymer for Efficient Humidity Sensor. Macromol. Chem. Phys. 2019, 220, 24–26. [Google Scholar] [CrossRef]
- Kalaska, B.; Kamiński, K.; Miklosz, J.; Nakai, K.; Yusa, S.I.; Pawlak, D.; Nowakowska, M.; Mogielnicki, A.; Szczubiałka, K. Anticoagulant Properties of Poly(Sodium 2-(Acrylamido)-2-Methylpropanesulfonate)-Based Di- and Triblock Polymers. Biomacromolecules 2018, 19, 3104–3118. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Bethani, A.; Bokias, G.; Kallitsis, J.K. Poly(Sodium Styrene Sulfonate)-b-Poly(Methyl Methacrylate) Diblock Copolymers through Direct Atom Transfer Radical Polymerization: Influence of Hydrophilic-Hydrophobic Balance on Self-Organization in Aqueous Solution. Eur. Polym. J. 2011, 47, 752–761. [Google Scholar] [CrossRef]
- Sheng, L.; Higashihara, T.; Maeda, R.; Hayakawa, T.; Ueda, M. Block Copolystyrene Derivatives Having Flexible Alkylsulfonated Side Chains and Hydrophobic Alkoxy Chains as a Proton Exchange Membrane for Fuel Cell Application. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2216–2224. [Google Scholar] [CrossRef]
- Won, J.; Park, H.H.; Kim, Y.J.; Choi, S.W.; Ha, H.Y.; Oh, I.H.; Kim, H.S.; Kang, Y.S.; Ihn, K.J. Fixation of Nanosized Proton Transport Channels in Membranes. Macromolecules 2003, 36, 3228–3234. [Google Scholar] [CrossRef]
- Deng, S.; Hassan, M.K.; Nalawade, A.; Perry, K.A.; More, K.L.; Mauritz, K.A.; McDonnell, M.T.; Keffer, D.J.; Mays, J.W. High Temperature Proton Exchange Membranes with Enhanced Proton Conductivities at Low Humidity and High Temperature Based on Polymer Blends and Block Copolymers of Poly(1,3-Cyclohexadiene) and Poly(Ethylene Glycol). Polymer 2015, 77, 208–217. [Google Scholar] [CrossRef]
- Yang, J.; Nonidez, W.K.; Mays, J.W. MALDI/TOF/MS as a Method for Characterizing Micelle-Forming Polymers: A MALDI/TOF/MS Study of Amphiphilic Diblock Copolymers Based on Sulfonated Polystyrene. Int. J. Polym. Anal. Charact. 2001, 6, 547–563. [Google Scholar] [CrossRef]
- Gromadzki, D.; Černoch, P.; Janata, M.; Kůdela, V.; Nallet, F.; Diat, O.; Štěpánek, P. Morphological Studies and Ionic Transport Properties of Partially Sulfonated Diblock Copolymers. Eur. Polym. J. 2006, 42, 2486–2496. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, E.; Joo, T.; Park, M.J. Morphology and Conductivity in Ionic Liquid Incorporated Sulfonated Block Copolymers. Macromolecules 2011, 44, 5289–5298. [Google Scholar] [CrossRef]
- Peddini, S.K.; Pham, H.N.; Spinu, L.; Weston, J.L.; Nikles, D.E.; Mauritz, K.A. Morphology and Magnetic Properties of Sulfonated Poly[Styrene-(Ethylene/Butylene)-Styrene]/Iron Oxide Composites. Eur. Polym. J. 2015, 69, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.C.; Lim, H.; Su, W.F.; Chao, C.Y. Novel Sulfonated Block Copolymer Containing Pendant Alkylsulfonic Acids: Syntheses, Unique Morphologies, and Applications in Proton Exchange Membrane. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2325–2338. [Google Scholar] [CrossRef]
- Storey, R.F.; Baugh, D.W. Poly(Styrene-b-Isobutylene-b-Styrene) Block Copolymers and Ionomers Therefrom: Morphology as Determined by Small-Angle X-Ray Scattering and Transmission Electron Microscopy. Polymer 2000, 41, 3205–3211. [Google Scholar] [CrossRef]
- Isaacs Sodeye, A.I.; Huang, T.; Gido, S.P.; Mays, J.W. Polymer Electrolyte Membranes from Fluorinated Polyisoprene-Block- Sulfonated Polystyrene: Membrane Structure and Transport Properties. Polymer 2011, 52, 1963–1970. [Google Scholar] [CrossRef]
- Matsuoka, H.; Matsutani, M.; Mouri, E.; Matsumoto, K. Polymer Micelle Formation without Gibbs Monolayer Formation: Synthesis and Characteristic Behavior of an Amphiphilic Diblock Copolymer Having Strong Acid Groups. Macromolecules 2003, 36, 5321–5330. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, L.; Wang, H.; Jiang, M. Preparation and Association Behavior of Diblock Copolymer Ionomers Based on Poly(Styrene-b-Ethylene-Co-Propylene). Eur. Polym. J. 2000, 36, 61–68. [Google Scholar] [CrossRef]
- Hernández, N.; Benson, C.; Cochran, E.W. Thermodynamics of Symmetric Diblock Copolymers Containing Poly(Styrene-Ran-Styrenesulfonic Acid). Macromolecules 2013, 46, 179–187. [Google Scholar] [CrossRef]
- Wang, X.; Hong, K.; Baskaran, D.; Goswami, M.; Sumpter, B.; Mays, J. Asymmetrical Self-Assembly from Fluorinated and Sulfonated Block Copolymers in Aqueous Media. Soft Matter 2011, 7, 7960–7964. [Google Scholar] [CrossRef]
- Mountz, D.A.; Storey, R.F.; Mauritz, K.A. Fourier Transform Infrared/Attenuated Total Reflectance Analysis of Water Diffusion in Poly[Styrene-b-Isobutylene-b-Styrene] Block Copolymer Membranes. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 764–776. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, S.; Kong, Y.; Yan, W.; Chen, H.; Liu, L.; Chang, W.; Li, J. Well-Defined Core-Shell Nanostructural Block Copolymer Supported Recyclable Bronsted Acidic Ionic Liquid Catalyst for the Synthesis of Biodiesel. Eur. Polym. J. 2020, 140, 109922. [Google Scholar] [CrossRef]
- Kajita, T.; Noro, A.; Seki, T.; Matsushita, Y.; Nakamura, N. Acidity Effects of Medium Fluids on Anhydrous Proton Conductivity of Acid-Swollen Block Polymer Electrolyte Membranes. RSC Adv. 2021, 11, 19012–19020. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Geng, W.; Gu, J.; Zhou, Y.; Zhang, J.; Zhang, Q. Synthesis of Poly (Methyl Methacrylate)-b-Polystyrene with High Molecular Weight by DPE Seeded Emulsion Polymerization and Its Application in Proton Exchange Membrane. J. Colloid Interface Sci. 2013, 406, 154–164. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, Y.W.; Yeh, C.F.; Wang, L. Synthesis of Conductive Core-Shell Nanoparticles Based on Amphiphilic Starburst Poly(n-Butyl Acrylate)-b-Poly(Styrenesulfonate). Macromolecules 2008, 41, 5632–5640. [Google Scholar] [CrossRef]
- Nisticò, R. Block Copolymers for Designing Nanostructured Porous Coatings. Beilstein J. Nanotechnol. 2018, 9, 2332–2344. [Google Scholar] [CrossRef] [PubMed]
- Stefik, M.; Guldin, S.; Vignolini, S.; Wiesner, U.; Steiner, U. Block Copolymer Self-Assembly for Nanophotonics. Chem. Soc. Rev. 2015, 44, 5076–5091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.C.; Darling, S.B. Block Copolymer Nanostructures for Technology. Polymers 2010, 2, 470–489. [Google Scholar] [CrossRef] [Green Version]
- Sing, C.E.; Zwanikken, J.W.; Olvera De La Cruz, M. Electrostatic Control of Block Copolymer Morphology. Nat. Mater. 2014, 13, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.M.; Jack, K.S.; Thurecht, K.J.; Blakey, I. Perturbation of the Experimental Phase Diagram of a Diblock Copolymer by Blending with an Ionic Liquid. Macromolecules 2016, 49, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Knychala, P.; Banaszak, M.; Park, M.J.; Balsara, N.P. Microphase Separation in Sulfonated Block Copolymers Studied by Monte Carlo Simulations. Macromolecules 2009, 42, 8925–8932. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.; Park, M.J. Enhanced Proton Transport in Nanostructured Polymer Electrolyte/Ionic Liquid Membranes under Water-Free Conditions. Nat. Commun. 2010, 1, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.J.; Balsara, N.P.; Jackson, A. Order-Disorder Transitions in Block Copolymer Electrolytes at Equilibrium with Humid Air. Macromolecules 2009, 42, 6808–6815. [Google Scholar] [CrossRef]
- Yu, X.; Nagarajan, M.R.; Li, C.; Gibson, P.E.; Cooper, S.L. Poly (Chloropropylmethyl-dimethylsiloxane)–Polyurethane Elastomers: Synthesis and Properties of Segmented Copolymers and Related Zwitterionomers. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 2681–2702. [Google Scholar]
- Wang, T.; Li, S.; Corrado, T.; Yang, L.; Schaefer, J.L.; Guo, R. Sulfonated Poly(Arylene Ether Sulfone) Multi-Block Copolymers with Selectively Cross-Linked Domains for Proton Exchange Membranes. ACS Appl. Polym. Mater. 2022, 4, 7476–7486. [Google Scholar] [CrossRef]
- Tachibana, S.; Hashimoto, K.; Mizuno, H.; Ueno, K.; Watanabe, M. Effects of Polyimide Sequence and Monomer Structures on CO2 Permeation and Mechanical Properties of Sulfonated Polyimide/Ionic Liquid Composite Membranes. Polymer 2022, 241, 124533. [Google Scholar]
- Yang, X.; Kim, J.H.; Kim, Y.J. Enhanced Proton Conductivity of Poly(Ether Sulfone) Multi-Block Copolymers Grafted with Densely Pendant Sulfoalkoxyl Side Chains for Proton Exchange Membranes. Polymer 2022, 242, 124604. [Google Scholar]
- Loveday, D.; Wilkes, G.L.; Deporter, C.D.; McGrath, J.E. Structure and Properties of Butadiene–Tert-Butyl Methacrylate and Butadiene/Styrene–Tert-Butyl Methacrylate Triblock Copolymer Ionomers. Macromolecules 1995, 28, 7822–7830. [Google Scholar] [CrossRef]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Bogart, T.D.; Lewis, R.L.; Beers, K.L.; Fasolka, M.J.; Hutchison, J.B.; Vogt, B.D.; Epps, T.H. Gradient Solvent Vapor Annealing of Block Copolymer Thin Films Using a Microfluidic Mixing Device. Nano Lett. 2011, 11, 1351–1357. [Google Scholar] [CrossRef]
- Nelson, G.; Drapes, C.S.; Grant, M.A.; Gnabasik, R.; Wong, J.; Baruth, A. High-Precision Solvent Vapor Annealing for Block Copolymer Thin Films. Micromachines 2018, 9, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshimo, T.; Maeda, R.; Odashima, R.; Takenaka, Y.; Kawana, D.; Ohmori, K.; Hayakawa, T. Perpendicularly Oriented Sub-10-Nm Block Copolymer Lamellae by Atmospheric Thermal Annealing for One Minute. Sci. Rep. 2016, 6, 4–11. [Google Scholar] [CrossRef]
- Campbell, I.P.; He, C.; Stoykovich, M.P. Topologically Distinct Lamellar Block Copolymer Morphologies Formed by Solvent and Thermal Annealing. ACS Macro Lett. 2013, 2, 918–923. [Google Scholar] [CrossRef]
- Shi, Z.; Holdcroft, S. Synthesis and Proton Conductivity of Partially Sulfonated Poly([Vinylidene Difluoride-Co-Hexafluoropropylene]-b-Styrene) Block Copolymers. Macromolecules 2005, 38, 4193–4201. [Google Scholar] [CrossRef] [Green Version]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Shokouhimehr, M.; Lee, Y.S. Polymer-Supported N-Heterocyclic Carbene-Palladium Complex for Heterogeneous Suzuki Cross-Coupling Reaction. J. Org. Chem. 2005, 70, 6714–6720. [Google Scholar] [CrossRef]
- Gu, Y.; Lodge, T.P. Synthesis and Gas Separation Performance of Triblock Copolymer Ion Gels with a Polymerized Ionic Liquid Mid-Block. Macromolecules 2011, 44, 1732–1736. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sun, W.; Plancher, H.; Radosz, M.; Shen, Y. Poly (Ionic Liquid) s: A New Material with Enhanced and Fast CO2 Absorption. Chem. Commun. 2005, 26, 3325–3327. [Google Scholar]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Young, W.S.; Kuan, W.F.; Epps, T.H. Block Copolymer Electrolytes for Rechargeable Lithium Batteries. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1–16. [Google Scholar] [CrossRef]
- Wijaya, F.; Woo, S.; Lee, H.; Nugraha, A.F.; Shin, D.; Bae, B. Sulfonated Poly(Phenylene-Co-Arylene Ether Sulfone) Multiblock Membranes for Application in High-Performance Fuel Cells. J. Memb. Sci. 2022, 645, 120203. [Google Scholar] [CrossRef]
- Rubatat, L.; Li, C.; Nyka, A.; Ruokolainen, J. Structure—Properties Relationship in Proton Conductive Sulfonated Polystyrene—Polymethyl Methacrylate Block Copolymers ( SPS—PMMA ). Macromolecules 2008, 41, 8130–8137. [Google Scholar] [CrossRef]
- Mineart, K.P.; Dickerson, J.D.; Love, D.M.; Lee, B.; Zuo, X.; Spontak, R.J. Hydrothermal Conditioning of Physical Hydrogels Prepared from a Midblock-Sulfonated Multiblock Copolymer. Macromol. Rapid Commun. 2017, 38, 1600666. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Franzka, S.; Quintieri, G.; Dai, X.; Wong, C.K.; Gröschel, A.H. Size-Controlled Formation of Polymer Janus Discs. Angew. Chemie-Int. Ed. 2021, 60, 21668–21672. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, A.A.; Chertovich, A.V.; Potemkin, I.I. Phase Behavior of Melts of Diblock-Copolymers with One Charged Block. Polymers 2019, 11, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.J.; Balsara, N.P. Phase Behavior of Symmetric Sulfonated Block Copolymers. Macromolecules 2008, 41, 3678–3687. [Google Scholar] [CrossRef]
- Liu, D.; Xie, Y.; Zhong, J.; Yang, F.; Pang, J.; Jiang, Z. High Methanol Resistance Semi-Crystalline Sulfonated Poly(Ether Ketone) Proton Exchange Membrane for Direct Methanol Fuel Cell. J. Memb. Sci. 2022, 650, 120413. [Google Scholar] [CrossRef]
- Lazzari, M.; Torneiro, M. A Global View on Block Copolymers. Polymers 2020, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, D.; Padovani, A.M.; Negrón, A.A.; Sloan, J.M.; Napadensky, E.; Crawford, D.M. Mechanical and Chemical Properties of Poly (Styrene-isobutylene-styrene) Block Copolymers: Effect of Sulfonation and Counter Ion Substitution. Ournal Appl. Polym. Sci. 2014, 131, 40344. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.H.; Liu, N.; Zou, H.; Wu, Z.Q. A Facile Synthetic Route to Multifunctional Poly(3-Hexylthiophene)- b-Poly(Phenyl Isocyanide) Copolymers: From Aggregation-Induced Emission to Controlled Helicity. Macromolecules 2018, 51, 7546–7555. [Google Scholar] [CrossRef]
- Appold, M.; Gallei, M. Bio-Inspired Structural Colors Based on Linear Ultrahigh Molecular Weight Block Copolymers. ACS Appl. Polym. Mater. 2019, 1, 239–250. [Google Scholar] [CrossRef]
- He, Y.J.; Tu, T.H.; Su, M.K.; Yang, C.W.; Kong, K.V.; Chan, Y.T. Facile Construction of Metallo-Supramolecular Poly(3-Hexylthiophene)-Block-Poly(Ethylene Oxide) Diblock Copolymers via Complementary Coordination and Their Self-Assembled Nanostructures. J. Am. Chem. Soc. 2017, 139, 4218–4224. [Google Scholar] [CrossRef]
- Chen, K.; Liang, D.; Tian, J.; Shi, L.; Zhao, H. In-Situ Polymerization at the Interfaces of Micelles: A “Grafting from” Method to Prepare Micelles with Mixed Coronal Chains. J. Phys. Chem. B 2008, 112, 12612–12617. [Google Scholar] [CrossRef]
- Hofman, A.H.; Fokkink, R.; Kamperman, M. A Mild and Quantitative Route towards Well-Defined Strong Anionic/Hydrophobic Diblock Copolymers: Synthesis and Aqueous Self-Assembly. Polym. Chem. 2019, 10, 6109–6115. [Google Scholar] [CrossRef] [Green Version]
- Cong, Z.; Yang, F.; Cao, L.; Wen, H.; Fu, T.; Ma, S.; Liu, C.; Quan, L.; Liao, Y. Multispectral Optoacoustic Tomography (MSOT) for Imaging the Particle Size-Dependent Intratumoral Distribution of Polymeric Micelles. Int. J. Nanomed. 2018, 13, 8549. [Google Scholar] [CrossRef] [Green Version]
- Braga, C.B.; Pilli, R.A.; Ornelas, C.; Weck, M. Near-Infrared Fluorescent Micelles from Poly(Norbornene) Brush Triblock Copolymers for Nanotheranostics. Biomacromolecules 2021, 22, 5290–5306. [Google Scholar] [CrossRef]
- Yu, Z.P.; Liu, N.; Yang, L.; Jiang, Z.Q.; Wu, Z.Q. One-Pot Synthesis, Stimuli Responsiveness, and White-Light Emissions of Sequence-Defined ABC Triblock Copolymers Containing Polythiophene, Polyallene, and Poly(Phenyl Isocyanide) Blocks. Macromolecules 2017, 50, 3204–3214. [Google Scholar] [CrossRef]
- Föster, S.; Antonietti, M. Amphiphilic Block Copolymers in Structure-Controlled Nanomaterials Hybrids. Adv. Mater. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Yao, S.; Bethani, A.; Ziane, N.; Brochon, C.; Fleury, G.; Hadziioannou, G.; Poulin, P.; Salmon, J.B.; Cloutet, E. Synthesis of a Conductive Copolymer and Phase Diagram of Its Suspension with Single-Walled Carbon Nanotubes by Microfluidic Technology. Macromolecules 2015, 48, 7473–7480. [Google Scholar] [CrossRef]
- Erothu, H.; Kolomanska, J.; Johnston, P.; Schumann, S.; Deribew, D.; Toolan, D.T.W.; Gregori, A.; Dagron-Lartigau, C.; Portale, G.; Bras, W.; et al. Synthesis, Thermal Processing, and Thin Film Morphology of Poly(3-Hexylthiophene)-Poly(Styrenesulfonate) Block Copolymers. Macromolecules 2015, 48, 2107–2117. [Google Scholar] [CrossRef]
- Brendel, J.C.; Liu, F.; Lang, A.S.; Russell, T.P.; Thelakkat, M. Macroscopic Vertical Alignment of Nanodomains in Thin Films of Semiconductor Amphiphilic Block Copolymers. ACS Nano 2013, 7, 6069–6078. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Rahimi, K.; Zhong, M.; Mourran, A.; Luebke, D.R.; Nulwala, H.B.; Möller, M.; Matyjaszewski, K. Cubosomes from Hierarchical Self-Assembly of Poly(Ionic Liquid) Block Copolymers. Nat. Commun. 2017, 8, 14057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zhang, J.; Qiao, Y.; Ganewatta, M.; Tang, C. Ruthenocene-Containing Homopolymers and Block Copolymers via ATRP and RAFT Polymerization. Macromolecules 2013, 46, 8816–8823. [Google Scholar] [CrossRef]
- Byard, S.J.; O’Brien, C.T.; Derry, M.J.; Williams, M.; Mykhaylyk, O.O.; Blanazs, A.; Armes, S.P. Unique Aqueous Self-Assembly Behavior of a Thermoresponsive Diblock Copolymer. Chem. Sci. 2020, 11, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, K.Y.; Kim, H.J.; Lee, S.H.; Cho, K.Y.; Kim, H.T.; Hwang, S.S. Morphology Control of Highly Sulfonated Block Copolymers by a Simple Thermal Process. Macromol. Chem. Phys. 2010, 211, 613–617. [Google Scholar] [CrossRef]
- Wang, X.; Goswami, M.; Kumar, R.; Sumpter, B.G.; Mays, J. Morphologies of Block Copolymers Composed of Charged and Neutral Blocks. Soft Matter 2012, 8, 3036–3052. [Google Scholar] [CrossRef]
- Chen, L.; Roarke, M. Endoscopic Ultrasound-Guided Fine Needle Aspiration of a Duodenal Submucosal Mass: Cytomorphological Clues and Radiological Correlation. Cytojournal 2020, 17, 10. [Google Scholar] [CrossRef]
- Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. A Facile, Effective Thermal Crosslinking to Balance Stability and Proton Conduction for Proton Exchange Membranes Based on Blend Sulfonated Poly(Ether Ether Ketone)/Sulfonated Poly(Arylene Ether Sulfone). Int. J. Hydrogen Energy 2021, 46, 15866–15877. [Google Scholar] [CrossRef]
- Yan, L.; Rank, C.; Mecking, S.; Winey, K.I. Gyroid and Other Ordered Morphologies in Single-Ion Conducting Polymers and Their Impact on Ion Conductivity. J. Am. Chem. Soc. 2020, 142, 857–866. [Google Scholar] [CrossRef] [PubMed]





| Polymerization Technique | BC System | Procedure for Obtaining of ABC | Applications | Reference |
|---|---|---|---|---|
| RAFT | Poly((sodium2-acrylamido-2-methylpropane-sulfonate)-b-ethylene glycol) | First, 2-(acrylamido)-2 methyl propanesulfonic (AMS) was polymerized by NMP. Subsequently extended with a macro RAFT agent of 2(methacryloyloxy)ethyl phosphorylcholine (MPC) for diblock copolymers and poly(ethyleneglycol)-based bifunctional chain transfer agent for the synthesis of tri-blocks PAMPS-b-PEG-b-PAMPS. | Medical science and drugs development. The diblock copolymer arquitectures based on PAMPS works more effectively for its anticoagulant activity. | Kalaska et al. 2018 [69] |
| ATRP | Poly(ethylene glycol monomethacrylate-b sodium 4-styrenesulfonate) | A membrane of chloromethylated of poly(ether imide) was used as a surface active initiators. The polymerization of PEG or PNaStS was carried out on the membrane and the block copolymerization was completed after reactivation of the preserved dormant chain ends with 2,2 –byridine, copper(I) chloride and copperchloride. | Membranes for ultra and microfiltration and protein adsorption. | Li et al. 2015 |
| ATRP | Poly(sodium styrene sulfonate-b-methyl methacrylate) | First, PSSNa macroinitiator was synthesized and then used used as initiator methyl-4-(bromomethyl)benzoate, copper(I) bromide (CuBr) as catalyzer, the ligands N,N,N′,N″,N‴ pentamethyldiethylenetriamine and 2,20-bipyridine, the surfactant hexadecyltrimethyl ammonium bromide and the solvents methanol and DMF. | Medical and food science for antimicrobial materials. | Oikonomou et al. 2011 [70] |
| Polymerization Technique | BC System | Obtention of ABC Character | Applications | Reference |
|---|---|---|---|---|
| Anionic polymerization | polystyrene-b poly(sulfonated isoprene co-isoprene) | Sulfur trioxide/1,4-dioxane complex was used as AS and added dropwise to a solution of the BC (T = 25 °C, trxn = 4 h) neutralization was with NaOH and methanol to stop the reaction. The sulfonated BC was recovered by dyalisis. | Not mentioned | Politakos et al. 2021 [62] |
| Anionic polymerization | Poly(4-(4sulfobutyloxy) styrene-b-(4-(n-butoxystyrene) | AS was a solution of methanesulfonic acid in DMSO (T = 80 °C, trxn = 36 h). | Proton exchange membranes | Sheng et al. 2013 [71] |
| Not synthesized | Crosslinked sulfonated poly(styrene-b-butadiene-b-styrene) (SBS) | AS was acetyl sulfate freshly prepared from acetic anhydride in DCE and sulfuric acid at T = 0 °C then added to a swollen film of crosslinked SBS (T = 50 °C, trxn = 30 min). | Proton exchange membranes | Won et al. 2003 [72] |
| Anionic polymerization | Sulfonated poly(1,3-cyclohexadiene-b-ethylene glycol) | 1,3- cyclohexadiene was polymerized and crosslinked previous to the copolymerization reaction with ethylene oxide. The BC was then sulfonated with a solution of chlorosulfonic acid in dichloroethane was used as AS (T = 25 °C, trxn = 1 h). | Proto exchange membranes | Deng et al. 2015 [73] |
| ATRP | Sulfonated poly(vinylidene difluoride-cohexafluoropropylene-b-styrene) | Trichloromethyl-terminated fluoropolymers of vinylidene difluoride and hexafluoropropylene were prepared by emulsion in chloroform as the chain transfer agent. The resulting CCl3- terminated were used as macroinitiators to extend the polystyrene block. The AS was acetyl sulfate. | Proton exchange membranes | Tsang, Shi and Holdcrof 2011 [53] |
| Anionic polymerization | poly(styrenesulfonate-b- tert-butylstyrene) | The BC were prepared by polymerization first of the tert-butyl styrene by using sec-butyllithium as initiator followed by the addition of styrene to the living chains, then a solution of sulfur trioxide in dichloroetane was employed as AS (T = 0 °C). | Not mentioned | Yang et al. 2001 [74] |
| Not synthesized | Sulfonated poly[(styrene-b- (ethylene-alt-propylene)] | Sulfonated with acetyl sulfate in various chlorinated solvents (CHCl3 or CH2Cl2 or 1,2-dichloroethane) prepared by reaction of sulfuric acid and acetic anhydride. (T = 50 °C, trxn = 3 h). | Proton exchange membranes | Gromadzki et al. 2006 [75] |
| Anionic polymerization | Poly(styrenesulfonate-b-methylbutylene) | First, the polymerization of styrene and isoprene was carried out and followed by selective hydrogenation of the polydiene. A solution of freshly prepared acetyl sulfate was used. (T = 40 °C, trxn = 1,4 and 25 h). | Proton exchange membranes | Kim, Kim and Park 2011 [76] |
| Sulfonated poly(styrene-b-ethylene/butylene-b-styrene) | The BC was dissolved in DCE and the AS (acetyl sulfate) was added to the solution. (T = 50–53 °C, trxn = 2 h). | Templates for magnetic nanocomposites | Peddini et al. 2015 [77] | |
| Anionic polymerization | Poly(styrene-b-sulfonated hydroxystyrene) | The sulfonic acid groups were grafted onto the PHS segment by reacting the BC with potassium hydride and 1,3-propanesultone in anhydrous THF (T = 60 °C, trxn = 24 h). | Proton exchange membranes | Lee et al. 2011 [78] |
| Cationic polymerization | Sulfonated poly(styrene-b-isobutylene-b-styrene) | BCs were lightly sulfonated with acetyl sulfate in refluxing methylene chloride. | Not mentioned | Storey et al. 2000 [79] |
| Anionic polymerization | Poly(isoprene-b-sulfonated styrene) | The BCs were fluorinated by reacting the double bonds of the PI with difluorocarbene and were then sulfonated. (sulfonation conditions not reported). | Polymer electrolyte membranes | Sodeye et al. 2011 [80] |
| Anionic polymerization | poly(diethylsilacyclobutane -b- methyl methacrylate) | Sulfonation of BCs employed 1,3-Propane Sultone as AS. The BC was dissolved in a sodium hydroxide aqueous solution and then an excess of 1,3- propane sultone was added and neutralized by the addition of sodium hydroxide. | Medical science as drug deliveries and biocompatibilizers. | Matsuoka et al. 2003 [81] |
| Not synthesized | Poly(styrene-b-ethylene-co-propylene) | Sulfonation was carried out with acetyl sulfate in 1,2-dichloroethane. It was prepared by by the reaction of concentrated sulfuric acid with 30 mol% excess of acetic anhydride. | Compatibilizers | Zhan et al. 2000 [82] |
| Anionic polymerization | Poly(styrene-b-isoprene) and Poly(styrene-b-dimethylsiloxane) | Blocks were hydrogenated to prevent degradation and to favoring the selective sulfonation of polystyrene. Acetyl sulfate prepared in acetic anhydride and sulfuric acid. (T = 40 °C and trx = 4 h). | Water purification and ion exchange membranes. | Hernández et al. 2013 [83] |
| Anionic polymerization | Poly(isoprene fluorinated -b- sulfonated styrene) | First, the PS/PI BC was synthesized followed by a fluorination procedure. The sulfonation was carried out with acetic anhydride and sulfuric acid (T = 25 °C, trxn = 24 h). | Molecular electronics, photovoltaic, and fuel-cell membranes | Goswami et al. 2010 [84] |
| Click chemistry and ATRP | Poly(3-hexylthiophene)-b-styrenesulfonic acid) | The obtention of the BC started with synthesis of bromobenzyl end-functionalized P3HT as ATRP macro initiator, followed by the polymerization of styrene and finally, the sulfonation of PS block carried out by solution of phosphorous pentoxide in concentrated sulphuric acid (T = 40 °C, trxn = 30 min). | Humidity sensors | Khawas et al. 2019 [68] |
| Anionic polymerization | Poly(styrene-b-isobutylene-b-styrene) | The BC was dissolved in dichloroethane and the sulfonation agent was (trxn = 2 h). | Membranes for water vapor-breathable films | Mountz et al. 2005 [85] |
| RAFT | Poly(methyl methacrylate-b-styrene sulfonate) | Methyl methacrylate macro RAFT agents were employed to reactive the copolymerization reaction with styrene monomer. Subsequently, the sulfonation of the PS segments was carried out by using freshly prepared acetyl sulfate (T = 40 °C, trxn= 5 h). | Proton exchange membranes | Piñón et al. 2019 [33] |
| RAFT | Poly(styrene-b-4 vinylpyridine) | Free pyridine unit were subjected to reacted with propane sultone to give catalyst precursors 3–6 which were followed by the acidification by trifluoromethanesulfonic acid (AS)to produce the corresponding acidic ionic liquid. | Catalysis for the synthesis of biodisel | Jiang et al. 2020 [86] |
| RAFT | Poly(styrene-b-4-vinylpyridine-b-styrene) | First, styrene was polymerized by a bifunctional RAFT agent and copolymerized and then extended with the styrene monomer. Then, acid-swollen membranes were prepared by dissolving the obtained BCs in pyridine. Finally, sulfonation procede through by a 98 wt% aqueous solution of sulfuric acid. | Electrolyte membranes | Kajita et al. 2021 [87] |
| RAFT | Poly (methyl methacrylate-b-styrenesulfonate) | The synthesis of the BC was carried out by seeded emulsion polymerization with 1,1-diphenylethylene (DPE) as a chain transfer agent. The sulfonation employed acetyl sulfate (T = 40 °C, trxn = 75 min and 130 min). | Proton exchange membranes | Wang et al. 2013 [88] |
| ATRP | Poly(styrenesulfonate-b-methyl methacrylate) | The BC were successfully converted to their ionomers by sulfonation using acetyl sulfate as sulfonating agent (T = 30 °C, trxn = 45 min). | Proton exchange membranes | Erdogan et al. 2009 [52] |
| ATRP | Poly(n-butyl acrylate-b-polystyrene sulfonate) | The outer PS shell of the star copolymer was converted into hydrophilic poly(p-styrenesulfonate) with acetyl sulfate (trxn = 24 h). Finally, the oxidative propagation of 3,4-ethylenedioxythiophene on the PSS chains was carried out by counterion-induced polymerization to produce a stable aqueous dispersion. | Electrically conductive core-shell nanoparticles | Chu et al. 2008 [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Balderrama, C.I.; Leyva-Porras, C.; Conejo-Dávila, A.S.; Zaragoza-Contreras, E.A. Sulfonated Block Copolymers: Synthesis, Chemical Modification, Self-Assembly Morphologies, and Recent Applications. Polymers 2022, 14, 5081. https://doi.org/10.3390/polym14235081
Piñón-Balderrama CI, Leyva-Porras C, Conejo-Dávila AS, Zaragoza-Contreras EA. Sulfonated Block Copolymers: Synthesis, Chemical Modification, Self-Assembly Morphologies, and Recent Applications. Polymers. 2022; 14(23):5081. https://doi.org/10.3390/polym14235081
Chicago/Turabian StylePiñón-Balderrama, Claudia I., César Leyva-Porras, Alain Salvador Conejo-Dávila, and Erasto Armando Zaragoza-Contreras. 2022. "Sulfonated Block Copolymers: Synthesis, Chemical Modification, Self-Assembly Morphologies, and Recent Applications" Polymers 14, no. 23: 5081. https://doi.org/10.3390/polym14235081
APA StylePiñón-Balderrama, C. I., Leyva-Porras, C., Conejo-Dávila, A. S., & Zaragoza-Contreras, E. A. (2022). Sulfonated Block Copolymers: Synthesis, Chemical Modification, Self-Assembly Morphologies, and Recent Applications. Polymers, 14(23), 5081. https://doi.org/10.3390/polym14235081