Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges
Abstract
:1. Introduction
2. Experimental Techniques
2.1. WCO Biodiesel Preparation and FAME Analysis Method
2.1.1. WCO Biodiesel Synthesis
2.1.2. FAMEs Analysis
2.2. High-Pressure Density Measurement
3. Results and Discussion
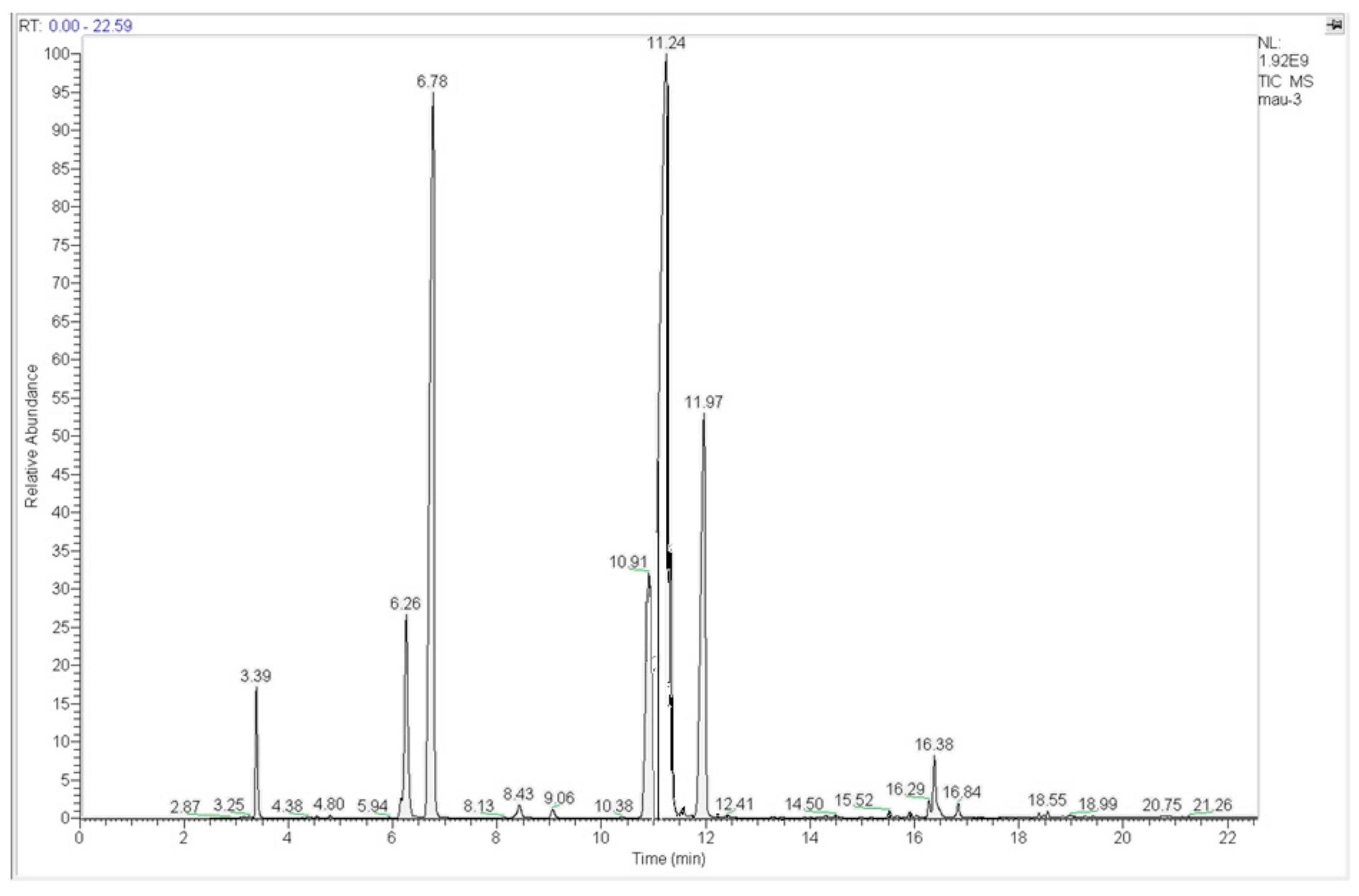
3.1. Fresh Biodiesel: Relative Compositions of Fatty Acid Contents
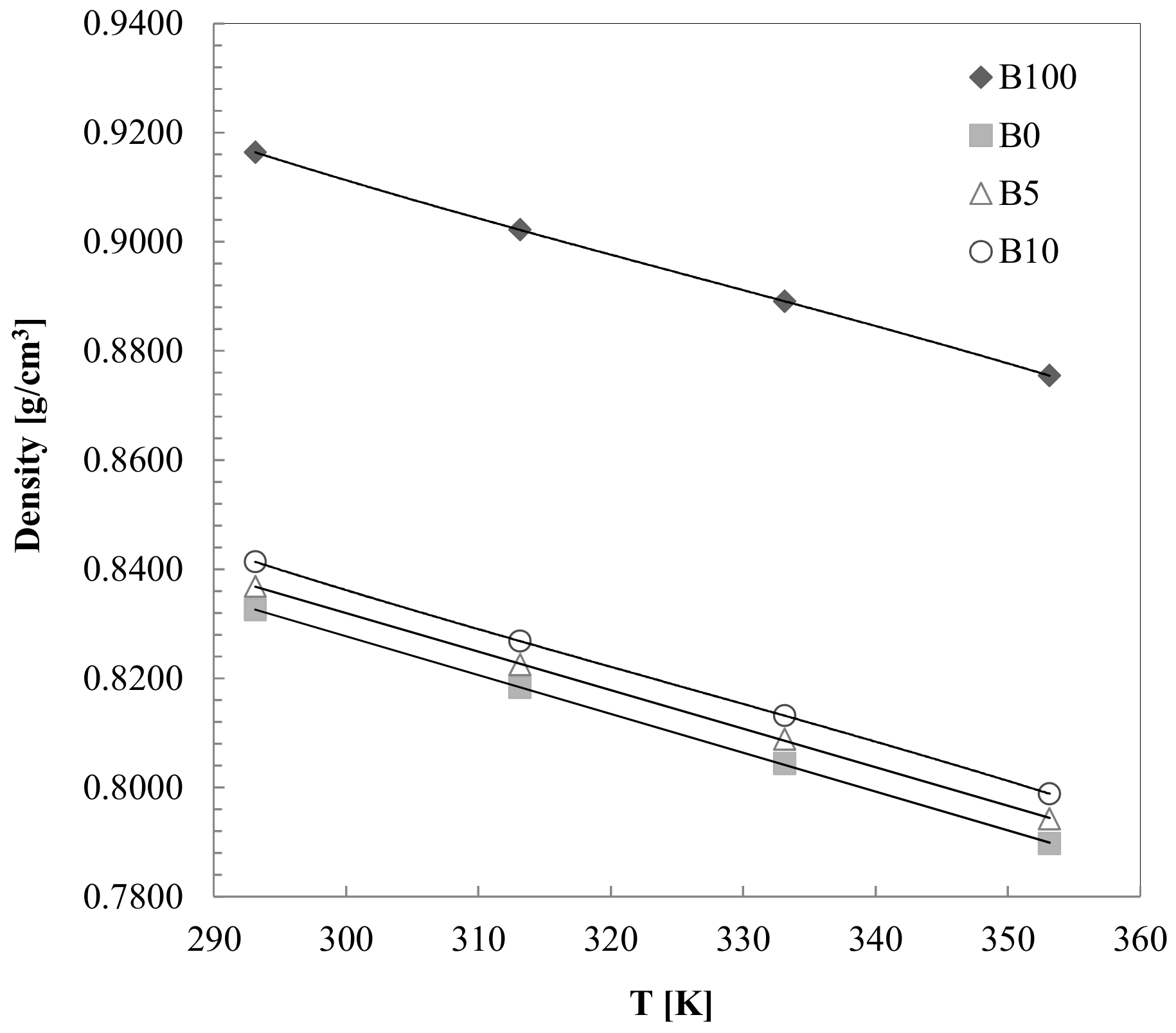
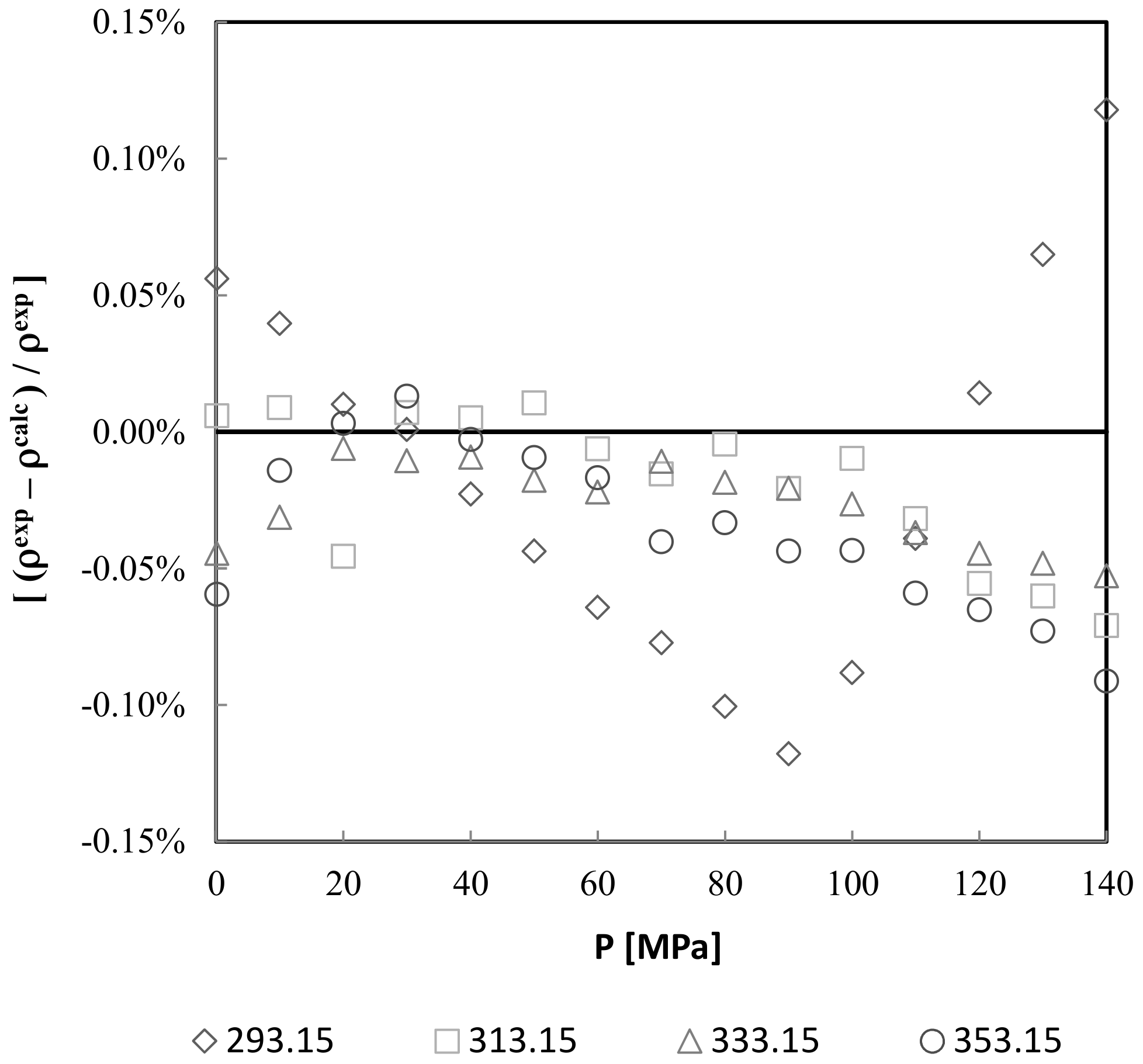
3.2. Density Measurements
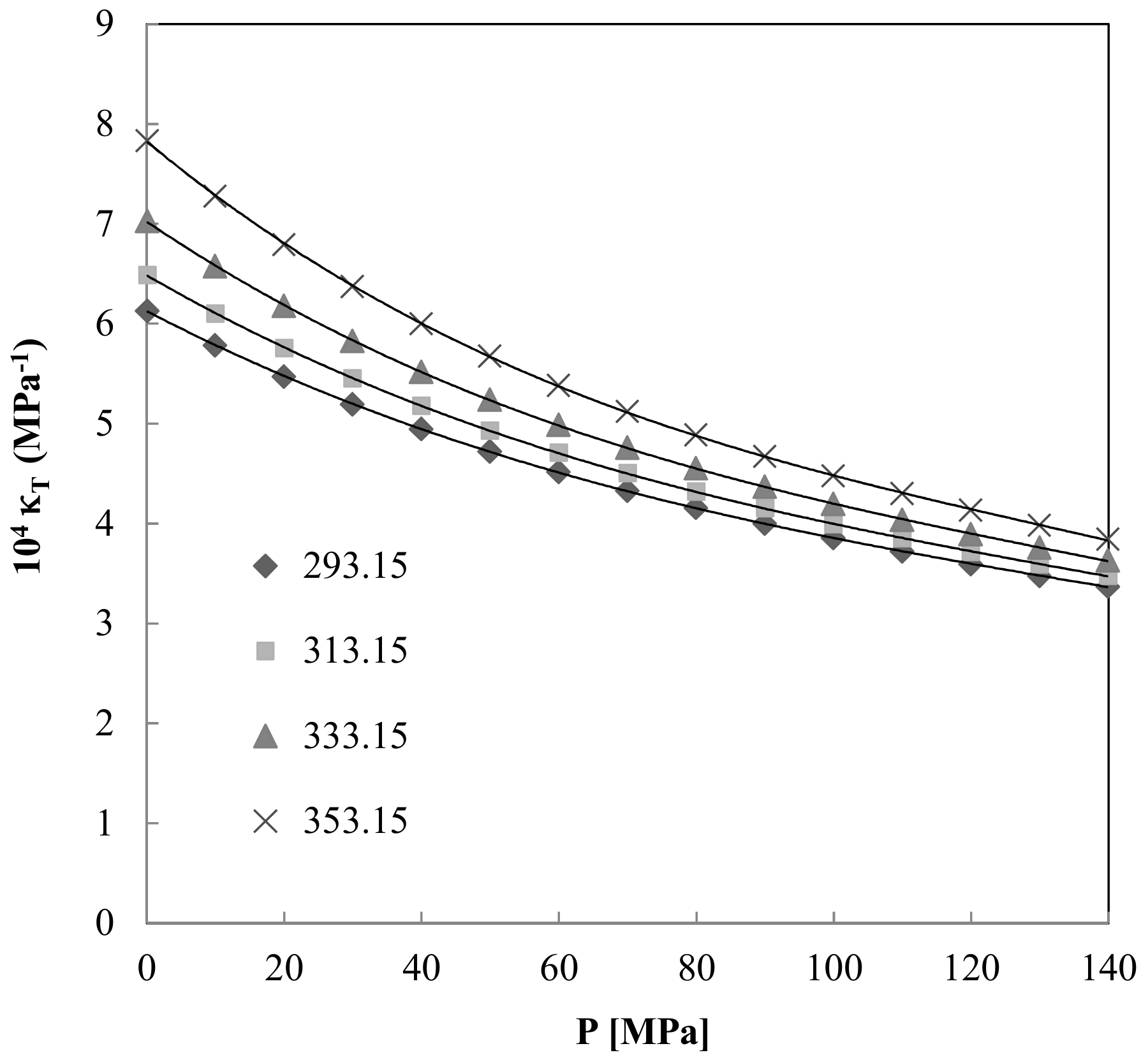
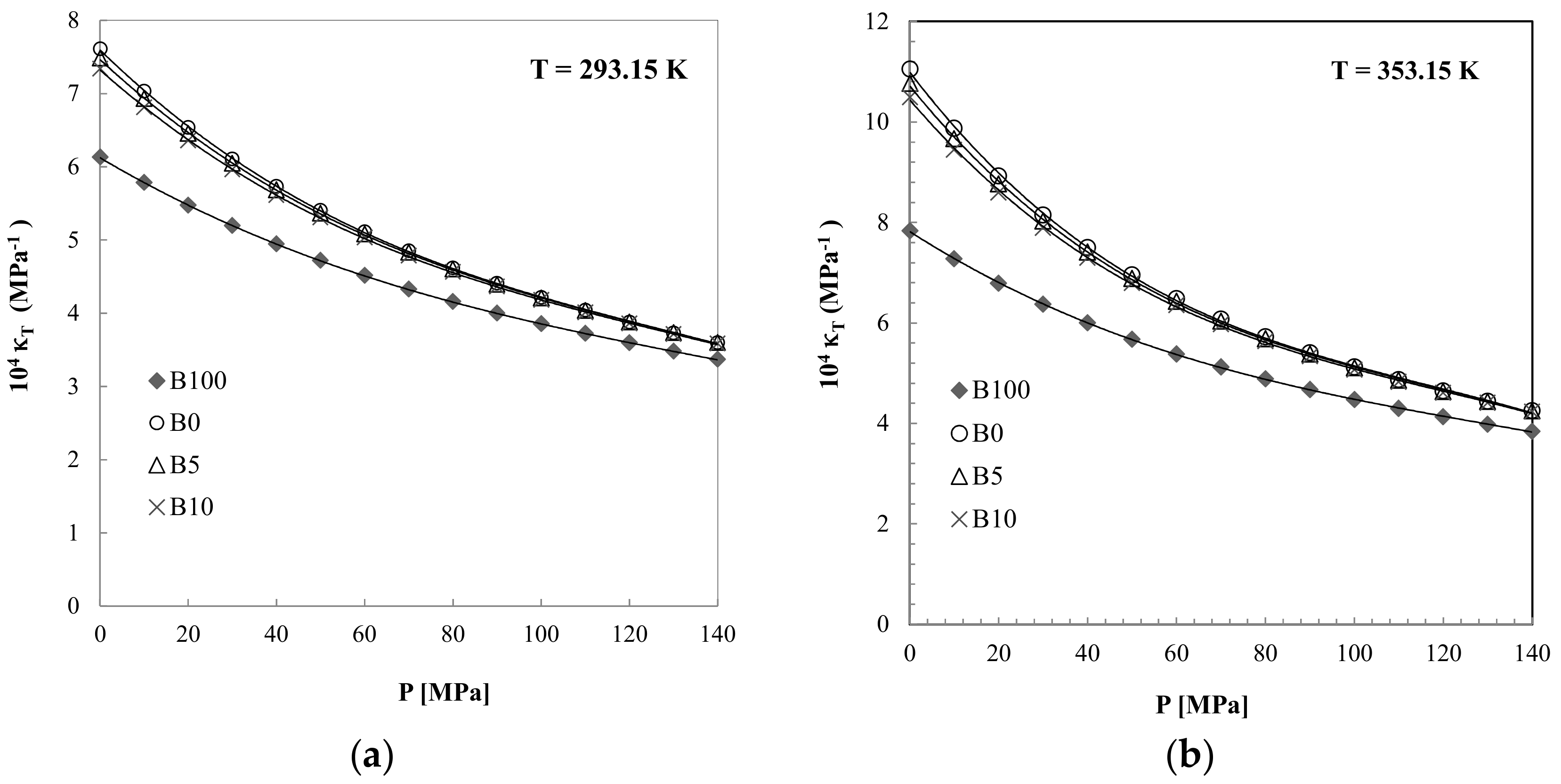
3.3. Isothermal Compressibility
4. Conclusions
- -
- The WCO biodiesel demonstrated high stability as well as suitable cold properties. This is due to its FAMEs profile.
- -
- The density value is somewhat higher that the allowed standards. At 293.15 (K) and for high pressures (120–140 MPa), the density seems to be affected by the high viscosity values of the biodiesel.
- -
- Nevertheless, these first results obtained are very promising in view of its transferability at the industrial scale.
- -
- This original set of experimental data measured for waste cooking oil biodiesel could be used to test predictive models developed for biodiesel thermodynamic properties.
Author Contributions
Conflicts of Interest
References
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and challenges for biodiesel fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khana, M.M.K.; Ashwath, N.; Azada, A.K.; Hazrata, M.A. Second Generation Biodiesel: Potential Alternative to Edible Oil-Derived Biodiesel. Energy Procedia 2014, 61, 1969–1972. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H.; Jayed, M.H.; Liaquat, A.M. Emission and performance characteristics of an indirect ignition diesel engine fuelled with waste cooking oil. Energy 2011, 36, 397–402. [Google Scholar] [CrossRef]
- Karmee, S.K.; Patria, R.D.; Ki Lin, C.S. Techno-Economic Evaluation of Biodiesel Production from Waste Cooking Oil -A Case Study of Hong Kong. Int. J. Mol. Sci. 2015, 16, 4362–4371. [Google Scholar] [CrossRef] [PubMed]
- Montefrio, M.J.F.; Obbard, J.P. The economics of biodiesel derived from waste cooking oil in the Philippines. Energy Sources Part B 2010, 5, 337–347. [Google Scholar] [CrossRef]
- Nas, B.; Berktay, A. Energy potential of biodiesel generated from waste cooking oil: An environmental approach. Energy Sources Part B 2007, 2, 63–71. [Google Scholar] [CrossRef]
- Sheinbaum-Pard, O.C.; Calderon-Irazoque, A. Ramırez-Suarez, M. Potential of biodiesel from waste cooking oil in Mexico. Biomass Bioenergy 2013, 56, 230–238. [Google Scholar] [CrossRef]
- Man, K.L.; Keat, T.L.; Abdul Rahman, M. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Ndiaye, E.H.I.; Bazile, J.P.; Nasri, D.; Boned, C.; Daridon, J.L. High pressure thermophysical characterization of fuel used for testing and calibrating diesel injection systems. Fuel 2012, 98, 288–294. [Google Scholar] [CrossRef]
- Fahd, M.E.A.; Lee, P.S.; Chou, S.K.; Wenming, Y.; Yap, C. Experimental study and empirical correlation development of fuel properties of waste cooking palm biodiesel and its diesel blends at elevated temperatures. Renew. Energy 2014, 68, 282–288. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, S.H.; Lee, C.S. Experimental investigation of the fuel properties of biodiesel and its blends at various temperatures. Energy Fuel 2008, 22, 652–656. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kılıç, M.; Özbay, N.; Pütün, A.E.; Pütün, E. Biodiesel production from waste frying oils: Optimization of reaction parameters and determination of fuel properties. Energy 2012, 44, 347–351. [Google Scholar] [CrossRef]
- Lagourette, B.; Boned, C.; Saint-Guirons, H.; Xans, P.; Zou, H. Densimeter calibration method versus temperature and pressure. Meas. Sci. Technol. 1992, 3, 699–703. [Google Scholar] [CrossRef]
- Miyake, Y.; Baylaucq, A.; Plantier, F.; Bessières, D.; Ushiki, H.; Boned, C. High-Pressure (up to 140MPa) density and derivative properties of some amines (Pentyl-, Hexyl- and Heptyl-amines) between (293.15 and 353.15) K. J. Chem. Thermodyn. 2008, 40, 836–845. [Google Scholar] [CrossRef]
- Pineiro, M.M.; Bessieres, D.; Gacio, J.M.; Saint-Guirons, H.; Legido, J.L. Determination of high-pressure liquid density for n-perfluorohexane and n-perfluorononane. Fluid Phase Equilib. 2004, 220, 127–136. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Sanli, H.; Canakci, M.; Alptekin, E. Predicting the higher heating values of waste frying oils as potential biodiesel feedstock. Fuel 2014, 115, 850–854. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” Biodiesel: Optimizing Fatty Ester Composition to Improve Fuel Properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Fu, J.; Hue, B.T.B.; Turn, S.Q. Oxidation stability of biodiesel derived from waste cat fish oil. Fuel 2017, 202, 455–463. [Google Scholar] [CrossRef]
- Segovia, J.J.; Fandiño, O.; López, E.R.; Lugo, L.; Martín, M.C.; Fernández, J. Automated densimetric system: Measurements and uncertainties for compressed fluids. J. Chem. Thermodyn. 2009, 41, 632–638. [Google Scholar] [CrossRef]
- Troncoso, J.; Bessieres, D.; Cerdeirina, C.A.; Carballo, E.; Romani, L. Automated measuring device of (p,rho,T) data-Application to the 1-hexanol plus n-hexane system. Fluid Phase Equilib. 2003, 208, 241–254. [Google Scholar] [CrossRef]
- Troncoso, J.; Bessieres, D.; Cerdeirina, C.A.; Carballo, E.; Romani, L. P rho Tx data for the dimethyl carbonate plus decane system. J. Chem. Eng. Data 2004, 49, 923–927. [Google Scholar] [CrossRef]




| Property | Units | Average Value |
|---|---|---|
| Saponification value | mg KOH/g | 192.64 |
| Acid value | mg KOH/g | 3.96 |
| Iodine number | g I2/100g | 87.76 |
| Retention Time (min) | FAME | wt % |
|---|---|---|
| 3.39 | Methyl myristate (C14:0) | 3.22 |
| 6.26 | Methyl palmitoleate (C16:1) | 7.16 |
| 6.78 | Methyl palmitate (C16:0) | 25.14 |
| 10.91 | Methyl linoleate (C18:2) | 9.76 |
| 11.24 | Methyl oleate (C18:1) | 37.59 |
| 11.97 | Methyl stearate (C18:0) | 13.18 |
| 16.29 | Methyl eicosadienoate (C20:2) | 0.53 |
| 16.38 | Methyl eicosenoate (C20:1) | 1.77 |
| 16.84 | Methyl eicosanoate (C20:0) | 0.62 |
| T (K) | ||||
|---|---|---|---|---|
| 293.15 | 313.15 | 333.15 | 353.15 | |
| P (MPa) | ρ (g·cm−3) | |||
| B100 | ||||
| 0.1 | 0.9164 | 0.9022 | 0.8891 | 0.8755 |
| 10 | 0.9216 | 0.9078 | 0.8952 | 0.8824 |
| 20 | 0.9266 | 0.9127 | 0.9012 | 0.8888 |
| 30 | 0.9314 | 0.9183 | 0.9065 | 0.8948 |
| 40 | 0.9359 | 0.9232 | 0.9117 | 0.9002 |
| 50 | 0.9403 | 0.9279 | 0.9165 | 0.9054 |
| 60 | 0.9444 | 0.9322 | 0.9212 | 0.9103 |
| 70 | 0.9485 | 0.9365 | 0.9258 | 0.9149 |
| 80 | 0.9523 | 0.9407 | 0.9300 | 0.9195 |
| 90 | 0.9560 | 0.9445 | 0.9342 | 0.9238 |
| 100 | 0.9601 | 0.9485 | 0.9381 | 0.9281 |
| 110 | 0.9642 | 0.9520 | 0.9419 | 0.9320 |
| 120 | 0.9682 | 0.9554 | 0.9456 | 0.9359 |
| 130 | 0.9721 | 0.9588 | 0.9491 | 0.9396 |
| 140 | 0.9760 | 0.9621 | 0.9526 | 0.9431 |
| B0 | ||||
| 0.1 | 0.8325 | 0.8183 | 0.8044 | 0.7897 |
| 10 | 0.8388 | 0.8250 | 0.8117 | 0.7982 |
| 20 | 0.8444 | 0.8312 | 0.8185 | 0.8057 |
| 30 | 0.8498 | 0.8371 | 0.8250 | 0.8127 |
| 40 | 0.8548 | 0.8425 | 0.8309 | 0.8191 |
| 50 | 0.8596 | 0.8475 | 0.8366 | 0.8250 |
| 60 | 0.8640 | 0.8525 | 0.8417 | 0.8304 |
| 70 | 0.8683 | 0.8570 | 0.8466 | 0.8356 |
| 80 | 0.8724 | 0.8614 | 0.8510 | 0.8404 |
| 90 | 0.8763 | 0.8657 | 0.8555 | 0.8452 |
| 100 | 0.8801 | 0.8698 | 0.8598 | 0.8497 |
| 110 | 0.8837 | 0.8737 | 0.8639 | 0.8539 |
| 120 | 0.8875 | 0.8773 | 0.8678 | 0.8580 |
| 130 | 0.8908 | 0.8807 | 0.8716 | 0.8621 |
| 140 | 0.8942 | 0.8842 | 0.8751 | 0.8658 |
| B5 | ||||
| 0.1 | 0.8368 | 0.8225 | 0.8089 | 0.7943 |
| 10 | 0.8427 | 0.8290 | 0.8161 | 0.8024 |
| 20 | 0.8484 | 0.8353 | 0.8228 | 0.8100 |
| 30 | 0.8538 | 0.8410 | 0.8290 | 0.8169 |
| 40 | 0.8587 | 0.8465 | 0.8349 | 0.8231 |
| 50 | 0.8634 | 0.8516 | 0.8405 | 0.8290 |
| 60 | 0.8679 | 0.8564 | 0.8457 | 0.8346 |
| 70 | 0.8722 | 0.8609 | 0.8505 | 0.8397 |
| 80 | 0.8764 | 0.8654 | 0.8550 | 0.8446 |
| 90 | 0.8804 | 0.8696 | 0.8595 | 0.8493 |
| 100 | 0.8844 | 0.8736 | 0.8638 | 0.8538 |
| 110 | 0.8878 | 0.8774 | 0.8679 | 0.8579 |
| 120 | 0.8911 | 0.8812 | 0.8719 | 0.8621 |
| 130 | 0.8947 | 0.8847 | 0.8756 | 0.8661 |
| 140 | 0.8980 | 0.8882 | 0.8791 | 0.8698 |
| B10 | ||||
| 0.1 | 0.8413 | 0.8268 | 0.8132 | 0.7989 |
| 10 | 0.8471 | 0.8333 | 0.8203 | 0.8069 |
| 20 | 0.8528 | 0.8395 | 0.8271 | 0.8141 |
| 30 | 0.8580 | 0.8452 | 0.8333 | 0.8211 |
| 40 | 0.8629 | 0.8506 | 0.8392 | 0.8272 |
| 50 | 0.8676 | 0.8557 | 0.8446 | 0.8330 |
| 60 | 0.8721 | 0.8605 | 0.8497 | 0.8386 |
| 70 | 0.8764 | 0.8650 | 0.8545 | 0.8436 |
| 80 | 0.8805 | 0.8695 | 0.8592 | 0.8486 |
| 90 | 0.8844 | 0.8736 | 0.8638 | 0.8533 |
| 100 | 0.8881 | 0.8776 | 0.8679 | 0.8576 |
| 110 | 0.8918 | 0.8814 | 0.8719 | 0.8620 |
| 120 | 0.8955 | 0.8852 | 0.8758 | 0.8661 |
| 130 | 0.8989 | 0.8888 | 0.8795 | 0.8699 |
| 140 | 0.9023 | 0.8922 | 0.8832 | 0.8737 |
| Coefficients | B100 | B0 | B5 | B10 |
|---|---|---|---|---|
| A0/g·cm−3 | 2.519378 | 1.662832 | 1.710722 | 1.837863 |
| A1/g·cm−3·K−1 | −0.013766 | −0.006533 | −0.006947 | −0.008047 |
| A2/g·cm−3·K−2 | 4.0498 × 10−5 | 1.8122 × 10−5 | 1.9405 × 10−5 | 2.2675 × 10−5 |
| A3/g·cm−3·K−3 | −4.1607 × 10−8 | −1.8749 × 10−8 | −2.0051 × 10−8 | −2.3271 × 10−8 |
| B0/MPa | −22.687549 | 438.128864 | 432.078831 | 480.962920 |
| B1/MPa·K−1 | 1.523006 | −1.576172 | −1.509124 | −1.786701 |
| B2/MPa·K−1 | −0.003194 | 0.001556 | 0.001440 | 0.001858 |
| C | 0.091540 | 0.083663 | 0.084928 | 0.085788 |
| σ/g·cm−3 | 0.000475 | 0.00011 | 0.000082 | 0.000084 |
| AAD/% | 0.03641% | 0.00936% | 0.00687% | 0.00716% |
| Dmax/% | 0.11784% | 0.02608% | 0.02830% | 0.02683% |
| Bias/% | −0.02453% | 0.00250% | 0.00054% | −0.00098% |
| T (K) | ||||
|---|---|---|---|---|
| 293.15 | 313.15 | 333.15 | 353.15 | |
| P (MPa) | 104 κT (MPa−1) | |||
| B100 | ||||
| 0.1 | 6.12791 | 6.48718 | 7.02636 | 7.83080 |
| 10 | 5.78100 | 6.09975 | 6.57421 | 7.27352 |
| 20 | 5.47017 | 5.75489 | 6.17567 | 6.78935 |
| 30 | 5.19265 | 5.44879 | 5.82495 | 6.36858 |
| 40 | 4.94329 | 5.17517 | 5.51383 | 5.99938 |
| 50 | 4.71796 | 4.92906 | 5.23586 | 5.67269 |
| 60 | 4.51330 | 4.70645 | 4.98596 | 5.38148 |
| 70 | 4.32655 | 4.50409 | 4.76002 | 5.12019 |
| 80 | 4.15544 | 4.31930 | 4.55470 | 4.88437 |
| 90 | 3.99803 | 4.14986 | 4.36727 | 4.67043 |
| 100 | 3.85274 | 3.99390 | 4.19546 | 4.47542 |
| 110 | 3.71819 | 3.84985 | 4.03736 | 4.29688 |
| 120 | 3.59322 | 3.71638 | 3.89137 | 4.13280 |
| 130 | 3.47683 | 3.59235 | 3.75614 | 3.98146 |
| 140 | 3.36815 | 3.47678 | 3.63050 | 3.84141 |
| B0 | ||||
| 0.1 | 7.61049 | 8.60011 | 9.74224 | 11.04942 |
| 10 | 7.03248 | 7.86956 | 8.81570 | 9.87327 |
| 20 | 6.53495 | 7.25255 | 8.04977 | 8.92402 |
| 30 | 6.10605 | 6.72913 | 7.41149 | 8.14823 |
| 40 | 5.73233 | 6.27928 | 6.87108 | 7.50188 |
| 50 | 5.40366 | 5.88833 | 6.40738 | 6.95472 |
| 60 | 5.11228 | 5.54530 | 6.00497 | 6.48529 |
| 70 | 4.85210 | 5.24179 | 5.65232 | 6.07796 |
| 80 | 4.61831 | 4.97125 | 5.34061 | 5.72102 |
| 90 | 4.40703 | 4.72854 | 5.06304 | 5.40555 |
| 100 | 4.21513 | 4.50950 | 4.81421 | 5.12465 |
| 110 | 4.04002 | 4.31081 | 4.58983 | 4.87285 |
| 120 | 3.87957 | 4.12970 | 4.38642 | 4.64580 |
| 130 | 3.73198 | 3.96393 | 4.20113 | 4.43997 |
| 140 | 3.59574 | 3.81159 | 4.03161 | 4.25249 |
| B5 | ||||
| 0.1 | 7.47859 | 8.42162 | 9.51242 | 10.76818 |
| 10 | 6.92808 | 7.73026 | 8.64008 | 9.66432 |
| 20 | 6.45196 | 7.14291 | 7.91387 | 8.76612 |
| 30 | 6.03980 | 6.64217 | 7.30517 | 8.02721 |
| 40 | 5.67939 | 6.20998 | 6.78728 | 7.40824 |
| 50 | 5.36144 | 5.83303 | 6.34108 | 6.88188 |
| 60 | 5.07879 | 5.50122 | 5.95247 | 6.42856 |
| 70 | 4.82579 | 5.20682 | 5.61085 | 6.03390 |
| 80 | 4.59795 | 4.94376 | 5.30809 | 5.68707 |
| 90 | 4.39165 | 4.70723 | 5.03783 | 5.37977 |
| 100 | 4.20394 | 4.49336 | 4.79504 | 5.10552 |
| 110 | 4.03237 | 4.29900 | 4.57568 | 4.85919 |
| 120 | 3.87494 | 4.12156 | 4.37648 | 4.63668 |
| 130 | 3.72993 | 3.95890 | 4.19474 | 4.43465 |
| 140 | 3.59592 | 3.80922 | 4.02823 | 4.25035 |
| B10 | ||||
| 0.1 | 7.33483 | 8.26828 | 9.32096 | 10.48672 |
| 10 | 6.80990 | 7.60755 | 8.49014 | 9.44723 |
| 20 | 6.35397 | 7.04369 | 7.79480 | 8.59544 |
| 30 | 5.95785 | 6.56113 | 7.20938 | 7.89066 |
| 40 | 5.61037 | 6.14327 | 6.70945 | 7.29748 |
| 50 | 5.30298 | 5.77778 | 6.27735 | 6.79103 |
| 60 | 5.02904 | 5.45527 | 5.89998 | 6.35339 |
| 70 | 4.78330 | 5.16849 | 5.56746 | 5.97125 |
| 80 | 4.56158 | 4.91175 | 5.27214 | 5.63457 |
| 90 | 4.36046 | 4.68051 | 5.00802 | 5.33559 |
| 100 | 4.17718 | 4.47109 | 4.77036 | 5.06823 |
| 110 | 4.00942 | 4.28050 | 4.55532 | 4.82768 |
| 120 | 3.85528 | 4.10629 | 4.35976 | 4.61003 |
| 130 | 3.71312 | 3.94641 | 4.18113 | 4.41213 |
| 140 | 3.58160 | 3.79912 | 4.01729 | 4.23136 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
NguyenThi, T.X.; Bazile, J.-P.; Bessières, D. Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges. Energies 2018, 11, 1212. https://doi.org/10.3390/en11051212
NguyenThi TX, Bazile J-P, Bessières D. Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges. Energies. 2018; 11(5):1212. https://doi.org/10.3390/en11051212
Chicago/Turabian StyleNguyenThi, Thanh Xuan, Jean-Patrick Bazile, and David Bessières. 2018. "Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends Over Extended Pressure and Temperature Ranges" Energies 11, no. 5: 1212. https://doi.org/10.3390/en11051212





