Performance of C2H4 Reductant in Activated-Carbon- Supported MnOx-based SCR Catalyst at Low Temperatures
Abstract
:1. Introduction
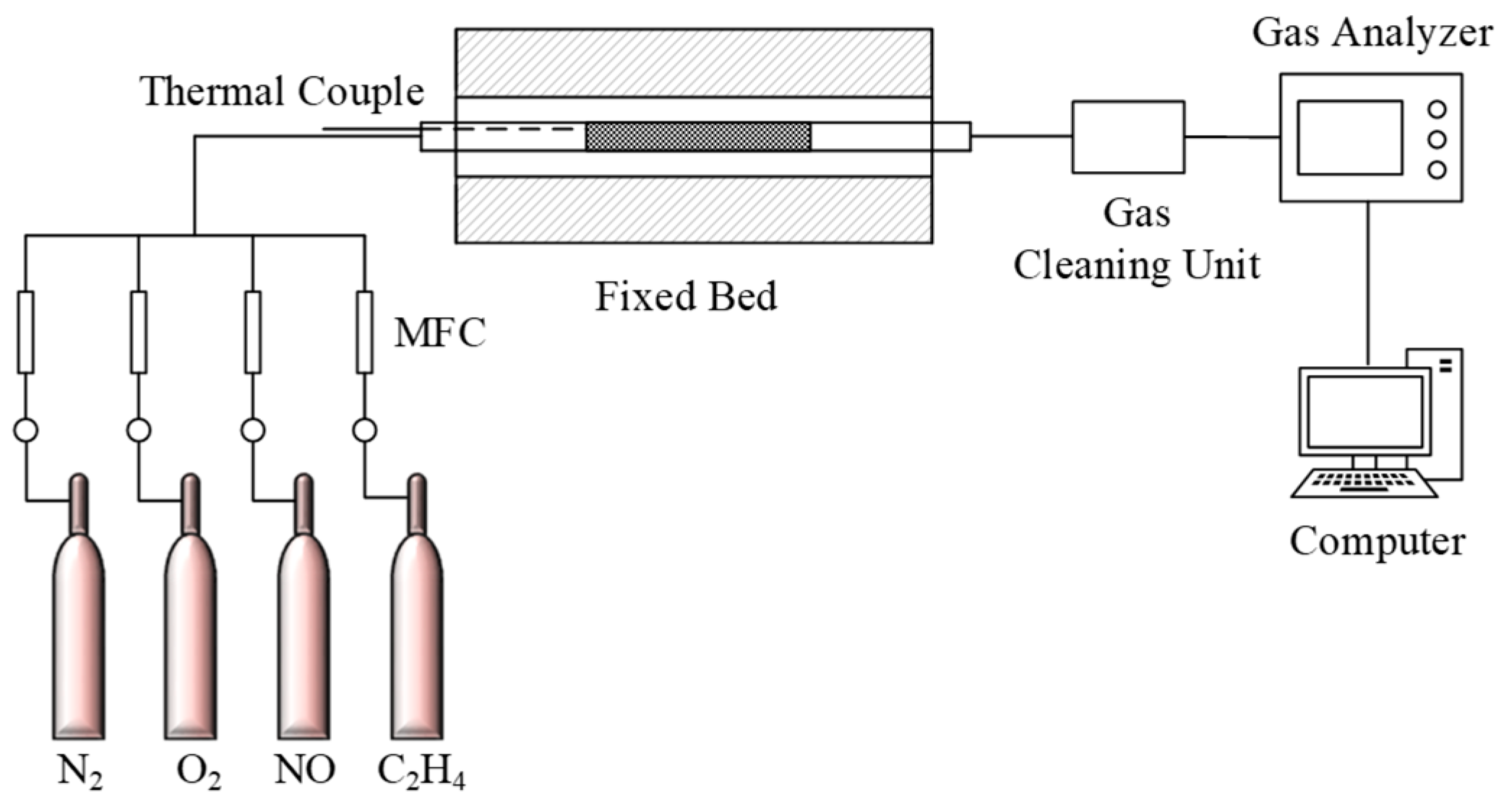
2. Experimental Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Activity Test
3. Results and Discussion
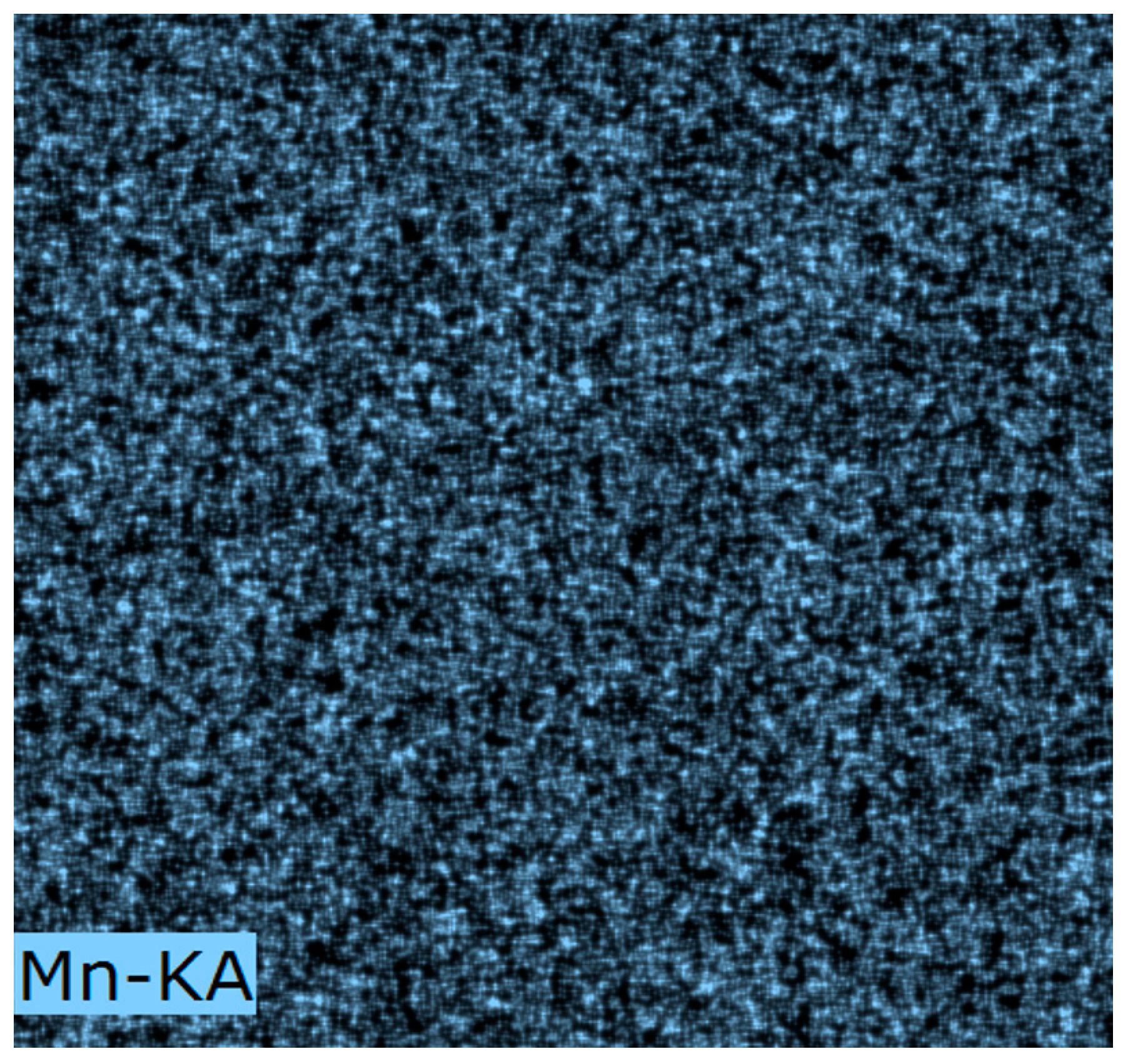
3.1. Dispersion of Active Component
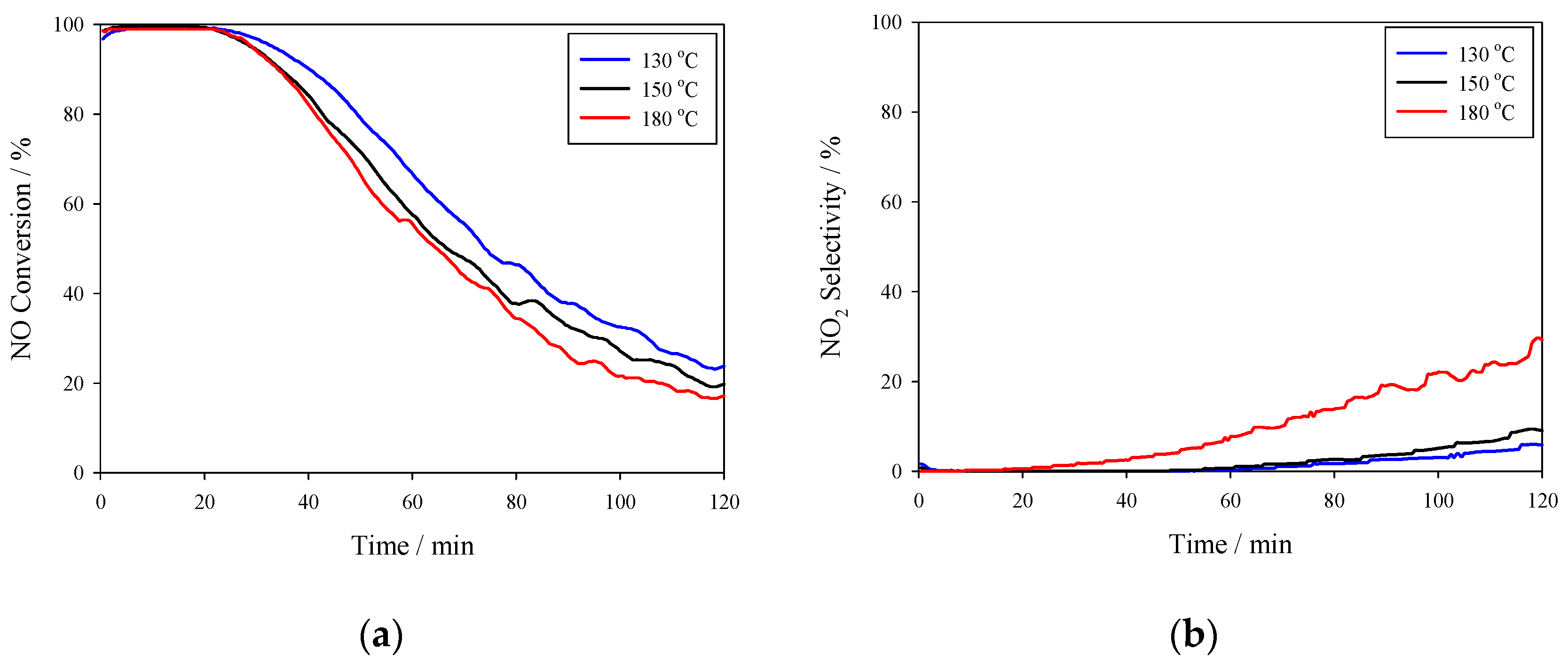
3.2. Catalytic Performance
3.3. Deactivation Mechanism
3.3.1. Surface Area and Porosity
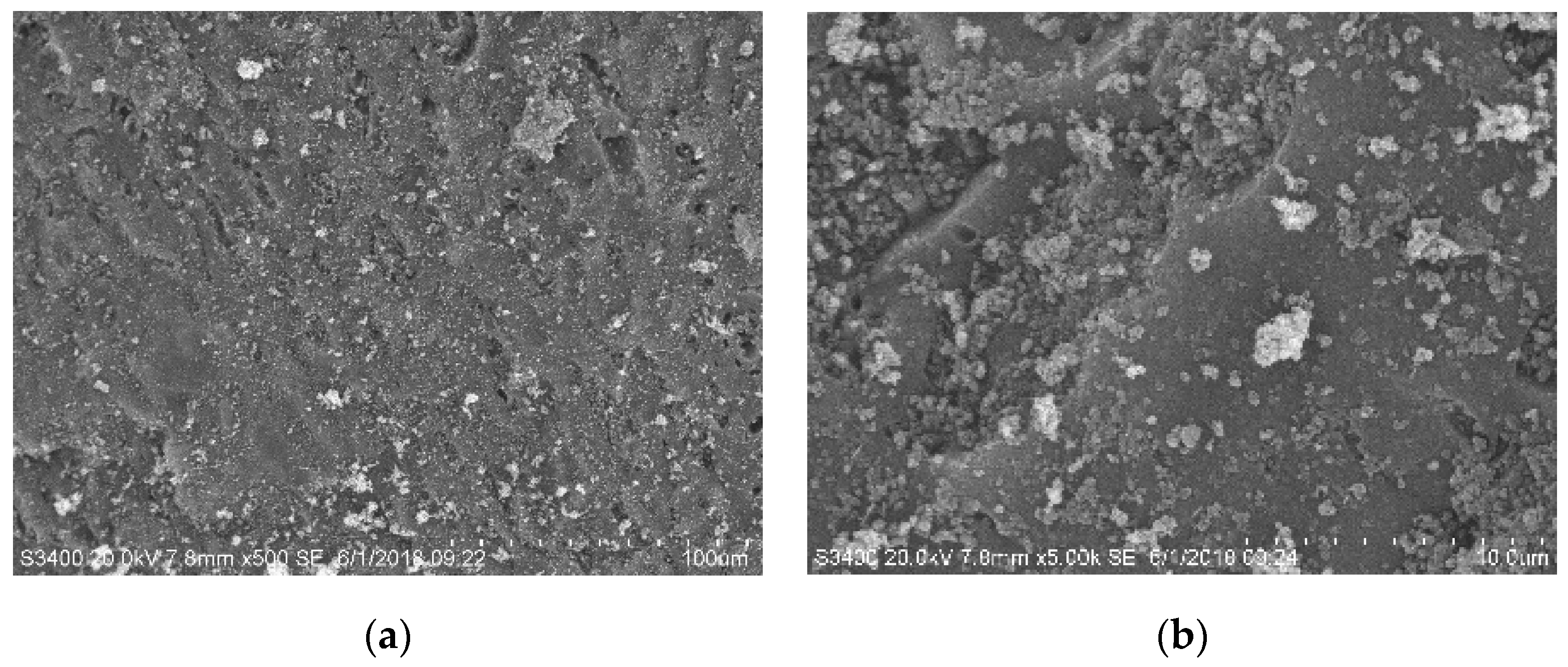
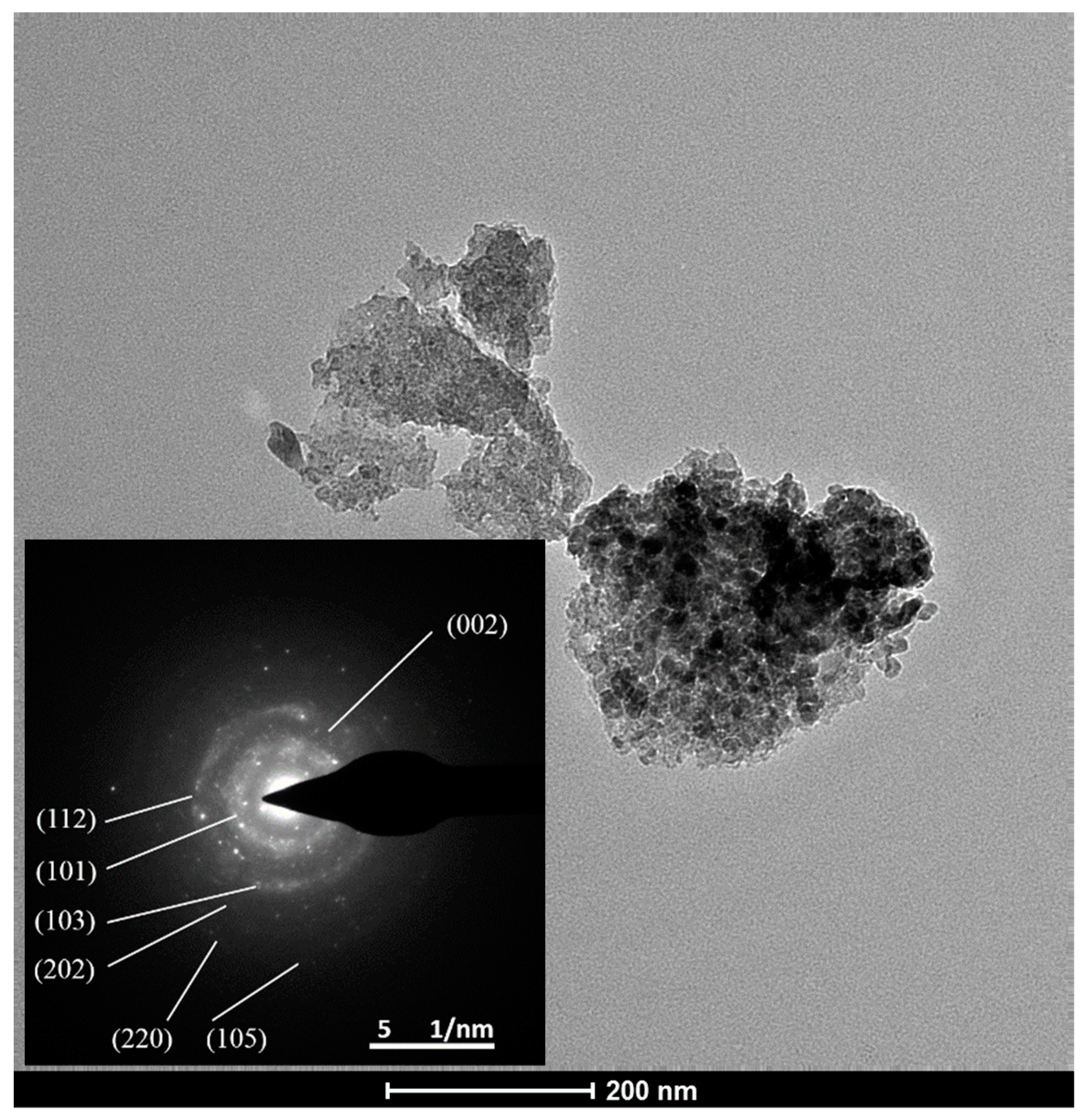
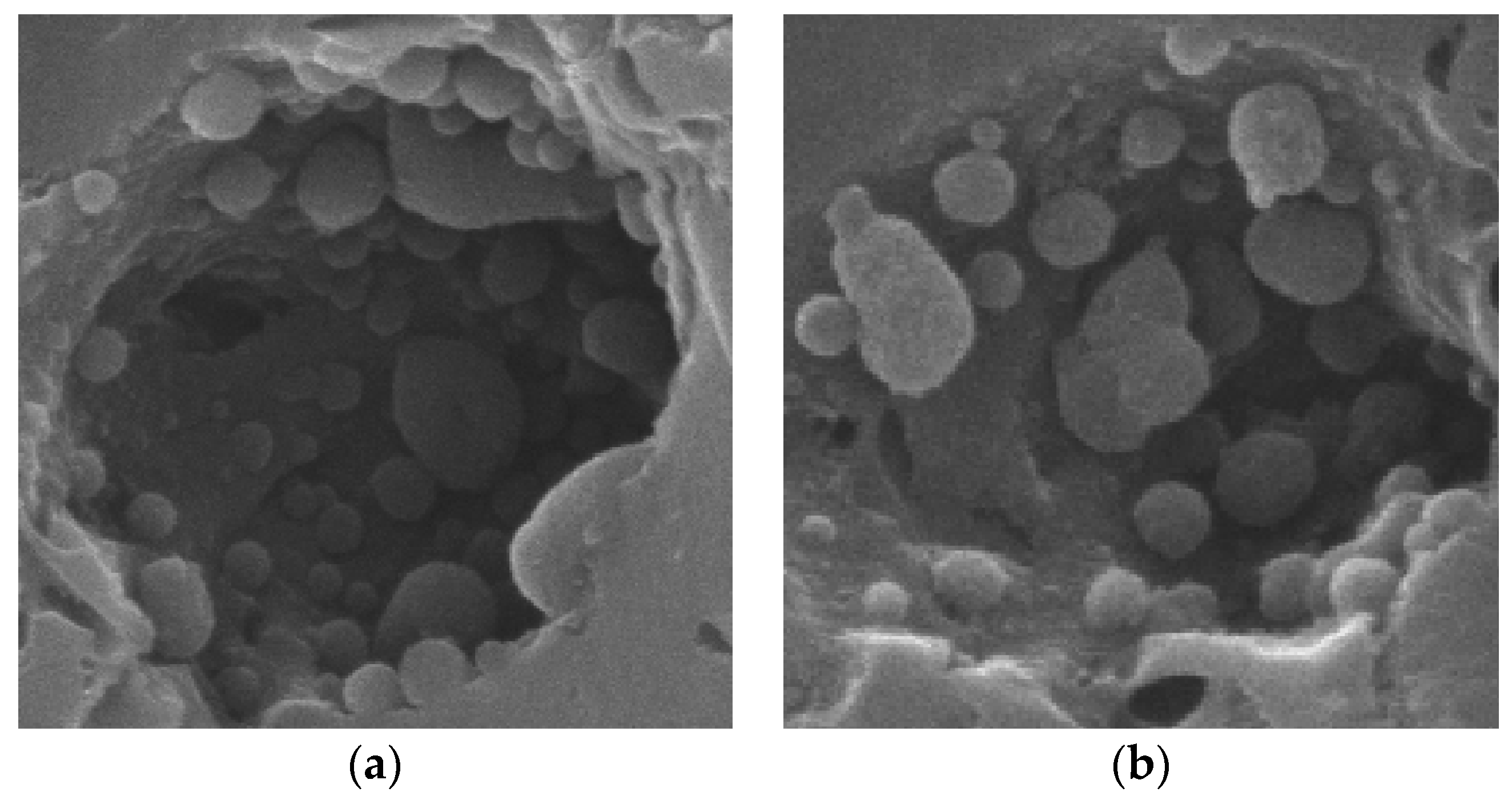
3.3.2. Morphology Evolution
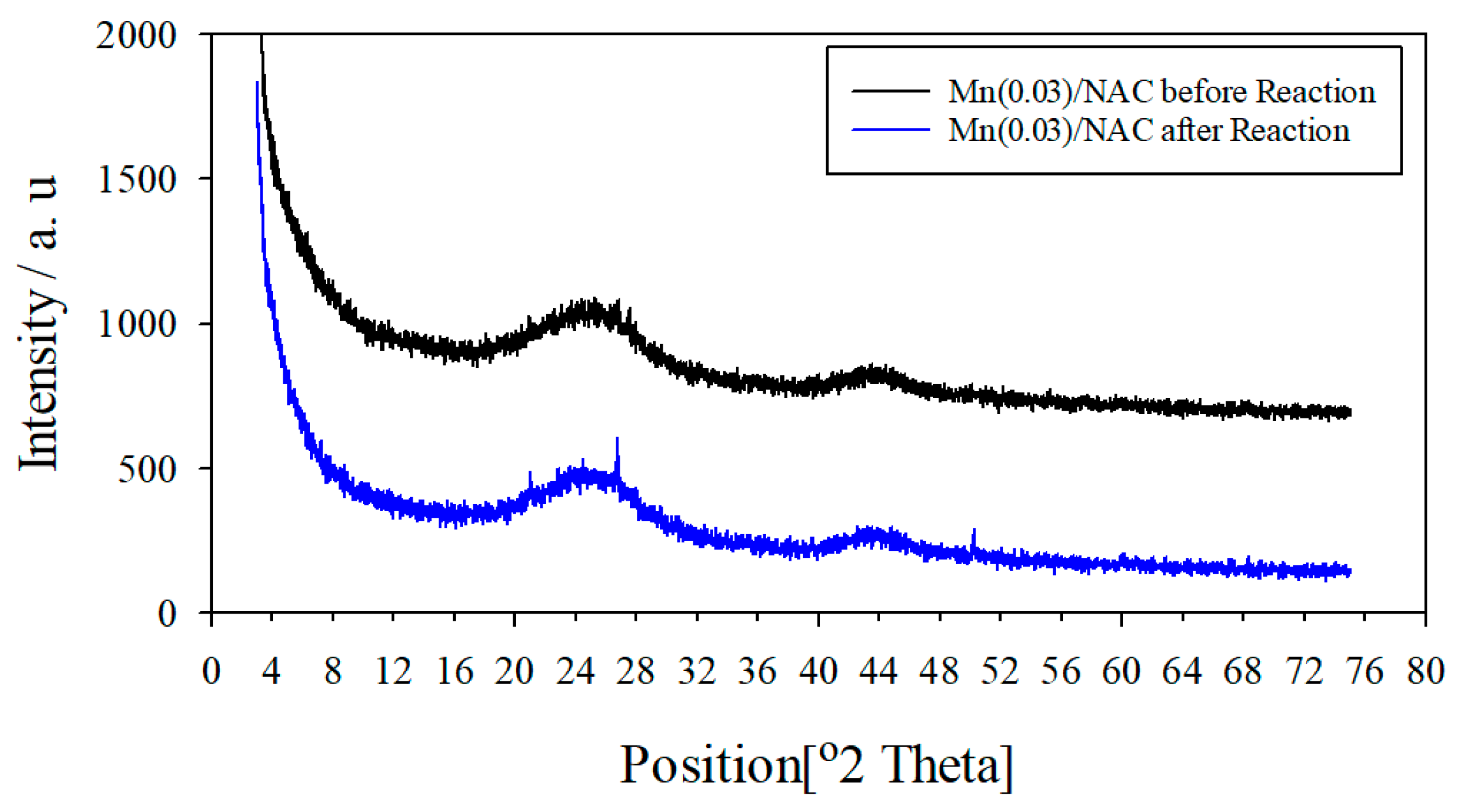
3.3.3. Crystalline Phase Change
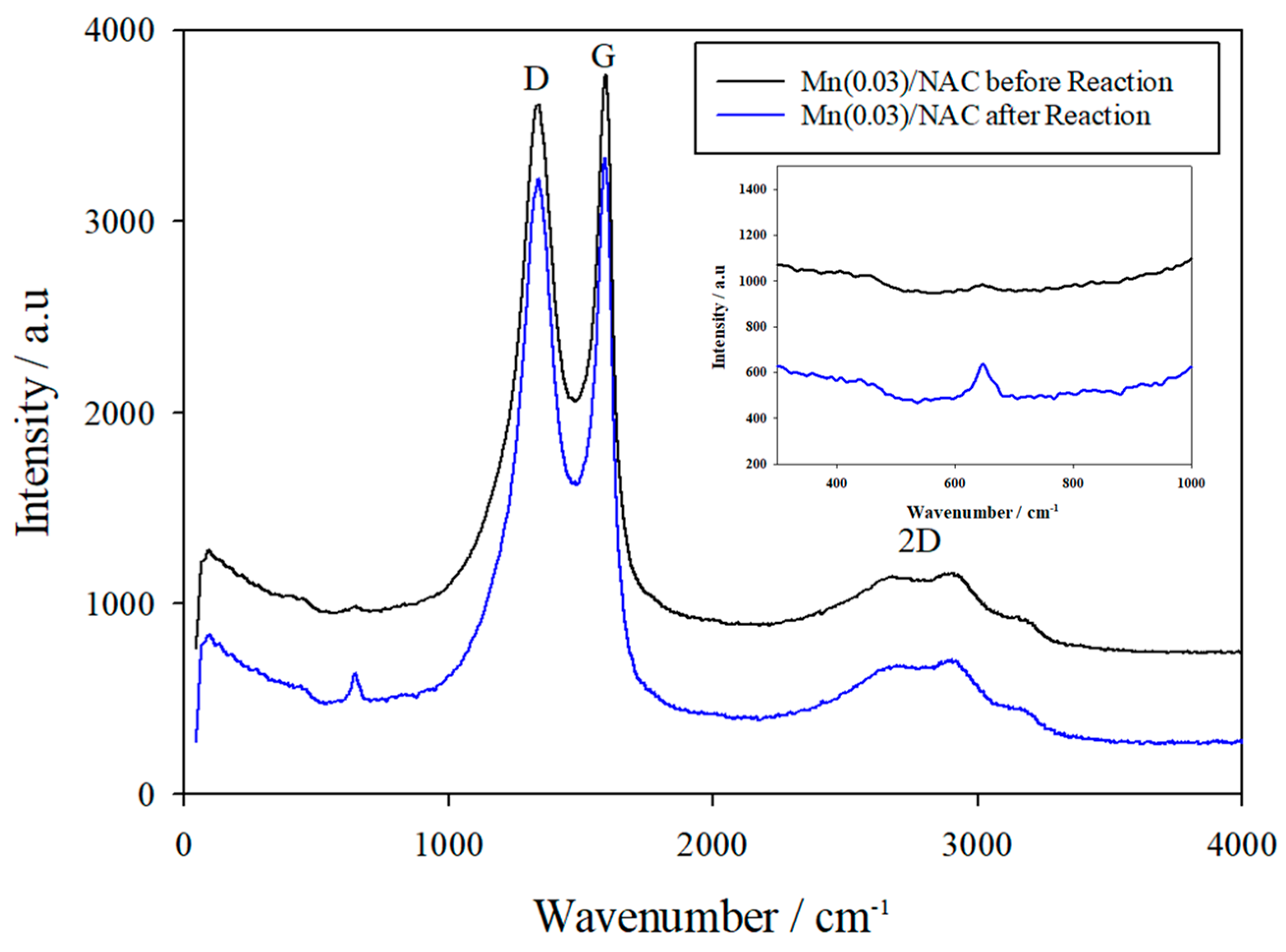
3.3.4. Raman Spectra
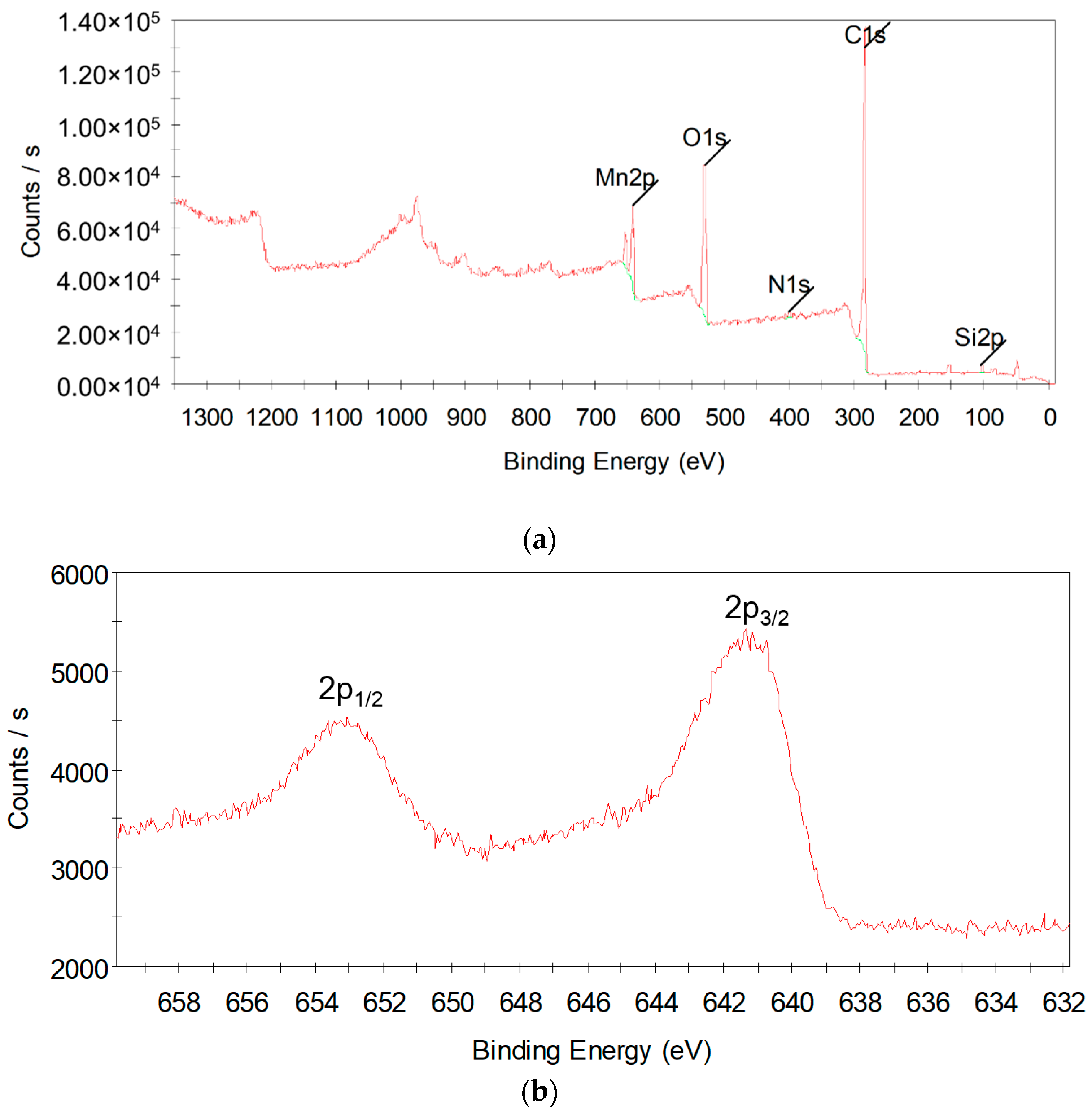
3.3.5. TEM-SAED and XPS
3.3.6. Formation of Carbon Spheres
4. Conclusions
- (1)
- The HNO3-treated activated carbon contained a high surface area and significant pore volume to act as a support for the impregnation of manganese oxide; changes in the surface areas and pore volumes as a consequence of catalytic testing were insignificant.
- (2)
- Three manganese concentrations were impregnated onto the NAC (3%, 5%, and 7%) to create Mn (0.0X)/NAC catalysts that initially exhibited nearly 100% NO conversion at temperatures between 130 and 180 °C for up to 30 min after beginning NO conversion testing. Independent of the temperature and the manganese concentration, the conversion activity decreased rapidly beyond 30 min of testing and attained only ~20–30% conversion after 2 h.
- (3)
- According to XRD, Raman, SEM-EDS, TEM-SAED, and XPS data, the as-prepared catalysts contained highly dispersed and highly disordered manganese species, whereas after testing, the catalysts contained crystalline Mn3O4 species.
- (4)
- The deactivation of the low-temperature HC-SCR catalyst is attributed to two causes: (a) although the manganese oxide on the NAC support was initially highly active for de-NOx conversion, it was reduced to Mn3O4 during reaction testing, a species having less activity than MnO2 and Mn2O3; (b) simultaneously, the crystallinity of the manganese oxides and their size were increased during reaction testing, and carbon black was formed that contained encapsulated manganese; the carbon black in the shape of spheres were deposited within pores and covered the active manganese sites on the NAC surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, P.A.; Jeong, Y.E.; Ha, H.P. Low temperature NH3-SCR activity enhancement of antimony promoted vanadia-ceria catalyst. Catal. Today 2017, 293–294, 61–72. [Google Scholar] [CrossRef]
- Pourkhalil, M.; Izadi, N.; Rashidi, A.; Mahboobeh, M. Synthesis of CeOx/γ-Al2O3 catalyst for the NH3-SCR of NOx. Mater. Res. Bull. 2018, 97, 1–5. [Google Scholar] [CrossRef]
- Nam, K.B.; Kwon, D.W.; Hong, S.C. DRIFT study on promotion effects of tungsten-modified Mn/Ce/Ti catalysts for the SCR reaction at low-temperature. Appl. Catal. A-Gen. 2017, 542, 55–62. [Google Scholar] [CrossRef]
- Ying, W.; Fan, H.; Wang, R. Transition metals (Co, Zr, Ti) modified iron-samarium oxide as efficient catalysts for selective catalytic reduction of NOx at low-temperature. Appl. Surf. Sci. 2018, 459, 63–73. [Google Scholar]
- Prieto-Centurion, D.; Eaton, T.R.; Roberts, C.A.; Fanson, P.T.; Notestein, J.M. Catalytic reduction of NO with H2 over redox-cycling Fe on CeO2. Appl. Catal. B-Environ. 2015, 168–169, 68–76. [Google Scholar]
- Chakraborty, S.; Nayak, S.C.; Deo, G. TiO2/SiO2 supported vanadia catalysts for the ODH of propane. Catal. Today 2015, 254, 62–71. [Google Scholar] [CrossRef]
- Muzio, L.; Bogseth, S.; Himes, R.; Chien, Y.C.; Dunn-Rankin, D. Ammonium bisulfate formation and reduced load SCR operation. Fuel 2017, 206, 180–189. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.M.; Guo, F.; Gao, S.Q.; Zhang, Z.G.; Matsuoka, K.; Xu, G.W. The pilot demonstration of a honeycomb catalyst for the DeNOx of low-temperature flue gas from an industrial coking plant. Fuel 2018, 219, 37–49. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Yang, J.; Ma, H.; Yamamoto, Y.; Yu, J.; Xu, G.; Zhang, Z.; Suzuki, Y. SCR catalyst coated on low-cost monolith support for flue gas denitration of industrial furnaces. Chem. Eng. J. 2013, 230, 513–521. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A simplified approach to modeling of dual-layer ammonia slip catalysts. Chem. Eng. Sci. 2012, 75, 75–83. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Wang, J.; Wang, J.; Zhai, Y. New insights into the promotional mechanism of ceria for activity and ammonium bisulfate resistance over V/WTi catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. A-Gen. 2018, 560, 153–164. [Google Scholar] [CrossRef]
- Chegrouche, S.; Kebir, A. Study of ammonium uranyl carbonate re-extraction-crystallization process by ammonium carbonate. Hydrometallurgy 1992, 28, 135–147. [Google Scholar] [CrossRef]
- Gummow, B. Vanadium: Environmental Pollution and Health Effects; Elsevier Press: Burlington, NJ, USA, 2011; pp. 628–636. [Google Scholar]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A-Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Männikkö, M.; Skoglundh, M.; Ingelsten, H.H. Selective catalytic reduction of NOx with methanol over supported silver catalysts. Appl. Catal. B-Environ. 2012, 119–120, 256–266. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B-Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Chen, Z.H.; Li, X.H.; Yang, Q.; Li, H.; Gao, X.; Jiang, Y.B.; Wang, F.R.; Wang, L.F. Removal of nox using novel fe-mn mixed-oxide catalysts at low temperature. Acta Phys.-Chim. Sin. 2009, 25, 601–605. [Google Scholar]
- Huang, B.; Huang, R.; Jin, D.; Ye, D. Low temperature SCR of NO with NH3 over carbon nanotubes supported vanadium oxides. Catal. Today 2007, 126, 279–283. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Liu, B. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: Reaction mechanism and catalyst deactivation. RSC Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef]
- Isabella, N.; Enrico, T. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer Press: New York, NY, USA, 2014. [Google Scholar]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B-Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Saqer, S.; Kondarides, D.; Verykios, X. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on g-Al2O3. Appl. Catal. B-Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of g-MnO2 as a VOC removal catalyst: Comparison with a noble metal catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Sultana, A.; Sasaki, M.; Hamada, H. Influence of support on the activity of Mn supported catalysts for SCR of NO with ammonia. Catal. Today 2012, 185, 284–289. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Chen, H.; Zhang, G.; Han, Y.; Chang, Y.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Chen, Z.; Wang, R. Bauxite-supported Transition Metal Oxides: Promising Low-temperature and SO2-tolerant Catalysts for Selective Catalytic Reduction of NOx. Sci. Rep. 2015, 5, 9766. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wei, Z.; Kollar, M.; Gao, F.; Wang, Y.; Szanyi, J.; Peden, C.H. NO oxidation on zeolite supported Cu catalysts: Formation and reactivity of surface nitrates. Catal. Today 2016, 267, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, X.; Zhao, Q.; Zhang, D.; Ndokoye, P. The selective catalytic reduction of NO with propene over Cu-supported Ti–Ce mixed oxide catalysts: Promotional effect of ceria. J. Mol. Catal. A-Chem. 2013, 378, 115–123. [Google Scholar] [CrossRef]
- Leistner, K.; Olsson, L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR. Appl. Catal. B-Environ. 2015, 165, 192–199. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania. Appl. Catal. B-Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Worch, D.; Suprun, W.; Gläser, R. Supported transition metal-oxide catalysts for HC-SCR DeNOx with propene. Catal. Today 2011, 176, 309–313. [Google Scholar] [CrossRef]
- Roy, S.; Baiker, A. NOx Storage−Reduction Catalysis: From Mechanism and Materials Properties to Storage−Reduction Performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar] [CrossRef] [PubMed]
- Santillan-Jimenez, E.; Miljković-Kocić, V.; Crocker, M.; Wilson, K. Carbon nanotube-supported metal catalysts for NOx reduction using hydrocarbon reductants. Part 1: Catalyst preparation, characterization and NOx reduction characteristics. Appl. Catal. B-Environ. 2011, 102, 1–8. [Google Scholar] [CrossRef]
- Adamowska-Teyssier, M.; Krztoń, A.; Da Costa, P.; Djéga-Mariadassou, G. SCR NOx mechanistic study with a mixture of hydrocarbons representative of the exhaust gas from coal combustion over Rh/Ce0.62Zr0.38O2 catalyst. Fuel 2015, 150, 21–28. [Google Scholar] [CrossRef]
- Gu, H.; Chun, K.M.; Song, S. The effects of hydrogen on the efficiency of NOx reduction via hydrocarbon-selective catalytic reduction (HC-SCR) at low temperature using various reductants. Int. J. Hydrogen Energy 2015, 40, 9602–9610. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A-Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Nicolae, S.; Neaţu, F.; Florea, M. Selective catalytic oxidation reaction of p-xylene on manganese–iron mixed oxide materials. CR Chim. 2018, 21, 354–361. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Koch, A. Effect of Ag, Pd and Co promoters on the Selective Catalytic Reduction (SCR) of NOx by ethanol over sulfated ceria-zirconia catalysts. Catal. Today 2012, 191, 65–74. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, H.G.; Kim, E.S.; Han, H.S.; Seo, G. Incorporation of zirconia onto silica for improved Pt/SiO2 catalysts for the selective reduction of NO by H2. Appl. Catal. A-Gen. 2012, 427–428, 155–164. [Google Scholar] [CrossRef]
- Liu, F.; Chen, L.; Neathery, J.; Saito, K.; Liu, K. Cerium oxide promoted iron-based oxygen carrier for chemical looping combustion. Ind. Eng. Chem. Res. 2014, 53, 16341–16348. [Google Scholar] [CrossRef]
- Komvokis, V.G.; Iliopoulou, E.F.; Vasalos, I.A.; Triantafyllidis, K.S.; Marshall, C.L. Development of optimized Cu–ZSM-5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives. Appl. Catal. A 2007, 325, 345–352. [Google Scholar] [CrossRef]
- Zhang, H. Fundamental Study on CH4-SCR of Nox; Zhejiang University: Hangzhou, China, 2005. [Google Scholar]
- Yan, J.Y.; Lei, G.D.; Sachtler, W.H.M.; Kung, H.H. Deactivation of Cu/ZSM-5 catalysts for lean NOx reduction: Characterization of changes of Cu state and zeolite support. J. Catal. 1996, 161, 43–54. [Google Scholar] [CrossRef]
- Xie, J.; Fang, D.; He, F.; Chen, J.; Fu, Z.; Chen, X. Performance and mechanism about MnOX species included in MnOX/TiO2 catalysts for SCR at low temperature. Catal. Commun. 2012, 28, 77–81. [Google Scholar] [CrossRef]
- Tamm, S.; Ingelsten, H.H.; Skoglundh, M.; Palmqvist, A.E. Mechanistic aspects of the selective catalytic reduction of NOx by dimethyl ether and methanol over γ-Al2O3. J. Catal. 2010, 276, 402–411. [Google Scholar] [CrossRef]
- Tseng, H.H.; Lu, C.Y.; Chang, F.Y.; Wey, M.Y.; Cheng, H.T. Catalytic removal of NO and PAHs over AC-supported catalysts from incineration flue gas: Bench-scale and pilot-plant tests. Chem. Eng. J. 2011, 169, 135–143. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Yi, H.; Li, J. Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catal. Today 2007, 126, 406–411. [Google Scholar] [CrossRef]
- Marbán, G.; Antuña, R.; Fuertes, A.B. Low-temperature SCR of NOx with NH3 over activated carbon fiber composite-supported metal oxides. Appl. Catal. B-Environ. 2003, 41, 323–338. [Google Scholar] [CrossRef]
- Boyano, A.; Galvez, M.E.; Moliner, R.; Lazaro, M.J. Carbon based catalytic briquettes for the reduction of NO: Catalyst scale-up. Catal. Today 2008, 137, 209–214. [Google Scholar] [CrossRef]
- Chu, Y.H.; Zhang, T.; Guo, X.J.; Liu, C.; Yin, H.Q.; Zhu, X.F.; Liu, Y.J. Low temperature selective catalytic reduction of NO by C3H6 over CeOx loaded on AC treated by HNO3. J. Rare Earth 2015, 33, 371–381. [Google Scholar] [CrossRef]
- Marbán, G.; Fuertes, A.B. Kinetics of the low-temperature selective catalytic reduction of NO with NH3 over activated carbon fiber composite-supported iron oxides. Catal. Lett. 2002, 84, 13–19. [Google Scholar] [CrossRef]
- Anthonysamy, S.B.I.; Afandi, S.B.; Khavarian, M.; Mohamed, A.R.B. A review of carbon-based and non-carbon-based catalyst supports for the selective catalytic reduction of nitric oxide. Beilstein J. Nanotechnol. 2018, 9, 740–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Yang, L.; Song, C. Chemical looping hydrogen production using activated carbon and carbon black as multi-function carriers. Int. J. Hydrogen Energy 2018, 43, 5501–5511. [Google Scholar] [CrossRef]
- Liu, F.; Chen, L.; Yang, L.; Fan, Z.; Nikolic, H.; Liu, K. Application of chemical looping process for continuous high purity hydrogen production by methane thermocatalytic decomposition. Int. J. Hydrogen Energy 2016, 41, 4592–4602. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC-Trend Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Kaczmarczyk, J.; Zasada, F.; Janas, J.; Indyka, P.; Piskorz, W.; Kotarba, A.; Zbigniew, S. Thermodynamic Stability, Redox Properties, and Reactivity of Mn3O4, Fe3O4, and Co3O4 Model Catalysts for N2O Decomposition: Resolving the Origins of Steady Turnover. ACS Catal. 2016, 6, 1235–1246. [Google Scholar] [CrossRef]
- Amer, A.; Reda, S.M.; Mousa, M.A.; Mohamed, M.M. Mn3O4/graphene nanocomposites: Outstanding performances as highly efficient photocatalysts and microwave absorbers. RSC Adv. 2017, 7, 826–839. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B-Environ. 2017, 7, 126–136. [Google Scholar] [CrossRef]
- Raj, A.M.E.; Victoria, S.G.; Jothy, V.B.; Ravidhas, C.; Wollschläger, J.; Suendorf, M.; Neumann, M.; Jayachandran, M.; Sanjeeviraja, C. XRD and XPS characterization of mixed valence Mn3O4 hausmannite thin films prepared by chemical spray pyrolysis technique. Appl. Surf. Sci. 2010, 256, 2920–2926. [Google Scholar]



| BET Surface Area (m2/g) | Average Pore Width (nm) | Pore Volume (cm³/g) | |
|---|---|---|---|
| Mn(0.03)/NAC Before Reaction | 668.54 | 2.46 | 0.41 |
| Mn(0.03)/NAC After Reaction | 632.62 | 2.37 | 0.375 |
| Element | wt.% | at.% | Error (3 Sigma) |
|---|---|---|---|
| Carbon | 89.97 | 92.98 | 35.26 |
| Oxygen | 8.65 | 6.71 | 7.75 |
| Manganese | 1.37 | 0.31 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Han, D.; Cheng, J.; Feng, Y.; Quan, W.; Yang, L.; Saito, K. Performance of C2H4 Reductant in Activated-Carbon- Supported MnOx-based SCR Catalyst at Low Temperatures. Energies 2019, 12, 123. https://doi.org/10.3390/en12010123
Liu G, Han D, Cheng J, Feng Y, Quan W, Yang L, Saito K. Performance of C2H4 Reductant in Activated-Carbon- Supported MnOx-based SCR Catalyst at Low Temperatures. Energies. 2019; 12(1):123. https://doi.org/10.3390/en12010123
Chicago/Turabian StyleLiu, Guangli, Dongtai Han, Jie Cheng, Yongshi Feng, Wenbin Quan, Li Yang, and Kozo Saito. 2019. "Performance of C2H4 Reductant in Activated-Carbon- Supported MnOx-based SCR Catalyst at Low Temperatures" Energies 12, no. 1: 123. https://doi.org/10.3390/en12010123





