Comparison and Optimization of Methane Hydrate Production Process Using Different Methods in a Single Vertical Well
Abstract
:1. Introduction
2. Experiments
2.1. Apparatus
2.2. Well Configuration
2.3. Hydrate Formation Procedure
2.4. Decomposition Procedure
- (1)
- PD. After the depressurizing stage, the pressure was decreased to 5.0 MPa. During the production stage, the production pressure was fixed at approximately 5.0 MPa with the accuracy of ±0.05 MPa by adjusting the back pressure regulator. There was no electric heat supplied during the whole experiment.
- (2)
- PH. The resistance rod began to work at a constant electric heating power when the production pressure was adjusted at approximately 5.9 MPa, which indicated the start of the production stage. Since this pressure is a little higher than the equilibrium pressure of 5.7 MPa, there was no depressurizing driving force for hydrate dissociation in this case.
- (3)
- SDH. The experimental process of SDH was basically the same as that of PH. And the only difference was the production pressure of SDH (5.0 MPa), which was lower than the phase equilibrium pressure.
- (4)
- H&P. The production stage was composed of several cycles and there were three steps in each cycle, which were the heat injection, the soaking and the production step. The first step of this stage was the heat injection, in which the heating was lasted at a constant electric heating power for 5 min with the reactor closed. The soaking was the second step, in which the electric heating was stopped, and the reactor was closed for 3 min for heat diffusion. During this step, the pressure in the reactor increased gradually. And in the final step (the production step), the back pressure regulator was opened and the gas and water began to produce. Once the pressure in the reactor decreased to 5.0 MPa again, the reactor was closed and the electric heating started again, indicating the beginning of the next production cycle. Each cycle of the above three steps was called a H&P cycle. This was thus a kind of combination method of depressurization and thermal stimulation operated in a single vertical well.
3. Results and Discussion
3.1. Temperature Profiles
3.2. Gas Production
- (1)
- During the heat injection and the soaking step, the reactor was closed, and the gas was discharged quickly after the opening of the reactor in the production step. The VP curve of H&P thus increased in a ladder shape. It was observed that the step height of the VP curve with H&P continuously decreased, indicating that the gas production during each cycle gradually dropped with time. This was due to the continuous reduction of the total amount of the hydrate and the increase of the hydrate decomposition regions. The heat transfer rate and the heat utilization efficiency from the vertical well to the hydrate zone were significantly reduced, resulting in a gradual decline in gas production in the later cycles of huff and puff process.
- (2)
- In each cycle of huff and puff process, during the heat injection and the soaking step, the production well was not open for gas production, and the pressure in the reactor continued rising, leading to the decrease of the pressure driving force. Electric heating at this time mainly provided the driving force for hydrate decomposition. When gas was produced from the vertical well, the pressure rapidly dropped and the pressure driving force increased. Under the dual driving forces, the decomposition rate of hydrate was faster than that before the production step. The mH curve thus continuously reduced in a serrated shape. However, in the later period of the production stage, the serrated shape became less obvious. This was due to the heat loss and hydrate consumption in the reactor, making the difference between the slopes of the mH curve increasingly smaller during the closure of the reactor and the gas generation period.
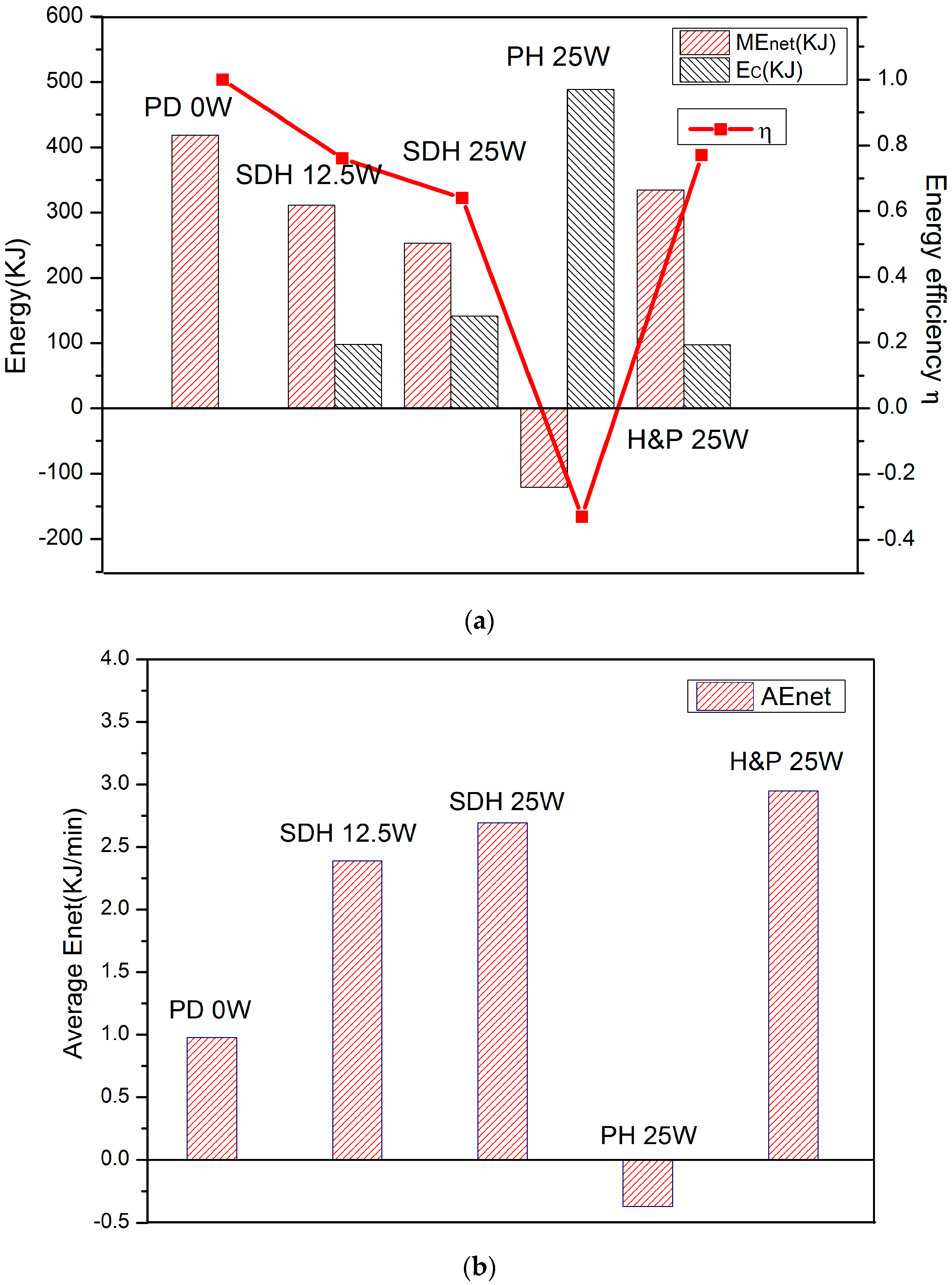
3.3. Energy Consumption and Gain
3.4. Energy Efficiency
4. Conclusions
- (1)
- The heat from electric heating can be effectively transferred to the hydrate deposit. The hydrate distribution in the reactor is considered to be relatively uniform. The temperatures of PD, SDH and PH method increase at different growth rates until the heat transfer between the internal and the external of the reactor reaches in a balance. However, the temperature-changing trend in the H&P process is a cyclical rise that fluctuates with time. Besides, the temperature change in the reactor is affected not only by the production method but also by the produced water in the production process.
- (2)
- The VP and mH curves in the same run of the experiment intersected at one point, showing the total amount of undecomposed hydrate was just equal to that of the dissociated in the reactor. Besides, the hydrate decomposition is the only source of gas production.
- (3)
- In the PD method, the decomposition front moves from the boundary to the central vertical well by absorbing heat from water bath. In contrast to PD, additional heating driving force is provided in the PH, SDH and H&P method, with which the hydrate mainly decomposes from the center well to the boundary of the reactor. In addition, the hydrate cannot be completely exploited by PH, indicating that the heat offered by it shows a limited influence range.
- (4)
- The VP in the H&P process continues increasing in the step shape, and the quality of the undecomposed hydrate shows a gradual serrated decrease. The qualities of gas production and undecomposed hydrate are gradually changed with time in PD, SDH and H&P. From the aspect of gas production time, gas production rate, and hydrate decomposition rate, the SDH method is considered relatively superior.
- (5)
- With different electric heating powers in SDH, the production effect is the same before the thermal driving force provided by the lower heating power can meet the rapid decomposition of hydrates. After this duration, the group with higher heating power shows a better decomposition effect. It is a mean for commercial exploitation to change heating power for the control of the decomposition rate and the production time. However, it should be realized that the increase of the electric heating power has limited impact on the net energy gain.
- (6)
- When consuming the same total energy, H&P with a bigger heating power provides a larger thermal driving force for decomposition than in SDH. In addition, the rise of the pressure in the reactor during the soaking step increases the driving force for decomposition, and the exploitation duration is shorter than that of SDH. On the other hand, at the same power, continuous heating with SDH consumes a large amount of energy to maintain a continuous temperature gradient in the reactor; while, during the production step of H&P, the heating process is stopped, and the hydrate absorbs the heat stored in quartz sand in the heated area and continues decomposing, resulting in less heat loss with H&P. After the analysis of the energy aspect and exploitation duration, the H&P method is believed to show the best exploitation effect among the four methods.
- (7)
- The use of different exploitation methods will have a significant impact on the hydrate production effect. The AEnet of PD is such small to have less commercial value. Besides, the PH method has no commercial extraction value as the energy efficiency and AEnet are the lowest in four methods, and the exploitation effect is not ideal. However, an ideal exploitation effect is shown in the H&P method, and it has the most commercial exploitation value in the four methods. Moreover, the production effect can be optimized by choosing a reasonable electric heating power.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.S., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Koh, C.A. Towards a fundamental understanding of natural gas hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Loveday, J.S.; Nelmes, R.J.; Guthrie, M.; Klug, D.D.; Tse, J.S. New gas hydrate structures. Dokl. Phys. Chem. 2001, 381, 303–305. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Sun, Z.; Xin, Y.; Sun, Q.; Ma, R.; Zhang, J.; Lv, S.; Cai, M.; Wang, H. Numerical simulation of the depressurization process of a natural gas hydrate reservoir: An attempt at optimization of field operational factors with multiple wells in a real 3D geological model. Energies 2016, 9, 714. [Google Scholar] [CrossRef]
- Li, B.; Li, X.S.; Li, G.; Feng, J.C.; Wang, Y. Depressurization induced gas production from hydrate deposits with low gas saturation in a pilot-scale hydrate simulator. Appl. Energy 2014, 129, 274–286. [Google Scholar] [CrossRef]
- Schicks, J.M.; Spangenberg, E.; Giese, R.; Luzihelbing, M.; Priegnitz, M.; Beeskowstrauch, B. A Counter-Current Heat-Exchange Reactor for the Thermal Stimulation of Hydrate-Bearing Sediments. Energies 2013, 6, 3002–3016. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yuan, Q.; Su, K.H.; Yang, X.; Wu, B.C. Experimental Simulation of the Exploitation of Natural Gas Hydrate. Energies 2012, 5, 466–493. [Google Scholar] [CrossRef] [Green Version]
- Qorbani, K.; Kvamme, B.; Kuznetsova, T. Utilizing Non-Equilibrium Thermodynamics and Reactive Transport to Model CH4 Production from the Nankai Trough Gas Hydrate Reservoir. Energies 2017, 10, 1064. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Song, Y.; Lim, X.-L.; Lam, W.-H. Opportunities and challenges of gas hydrate policies with consideration of environmental impacts. Renew. Sustain. Energy Rev. 2017, 70, 875–885. [Google Scholar] [CrossRef]
- Yin, Z.; Chong, Z.R.; Tan, H.K.; Linga, P. Review of gas hydrate dissociation kinetic models for energy recovery. J. Nat. Gas Sci. Eng. 2016, 35, 1362–1387. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Zhao, J.; Liu, W.; Yang, M.; Li, Y.; Liu, Y.; Li, Q. The status of natural gas hydrate research in China: A review. Renew. Sustain. Energy Rev. 2014, 31, 778–791. [Google Scholar] [CrossRef]
- Yamamoto, K. Overview and introduction: Pressure core-sampling and analyses in the 2012–2013 MH21 offshore test of gas production from methane hydrates in the eastern Nankai Trough. Mar. Pet. Geol. 2015, 66, 296–309. [Google Scholar] [CrossRef]
- Yamamoto, K.; Terao, Y.; Fujii, T.; Ikawa, T.; Seki, M.; Matsuzawa, M.; Kanno, T. Operational overview of the first offshore production test of methane hydrates in the Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2014. [Google Scholar]
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K.; Nagao, J. Key Findings of the World’s First Offshore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production. Energy Fuels 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kanno, T.; Wang, X.X.; Tamaki, M.; Fujii, T.; Chee, S.S.; Wang, X.W.; Pimenov, V.; Shako, V. Thermal responses of a gas hydrate-bearing sediment to a depressurization operation. RSC Adv. 2017, 7, 5554–5577. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Collett, T.S. Gas hydrate saturations estimated from fractured reservoir at Site NGHP-01-10, Krishna-Godavari Basin, India. J. Geophys. Res. Solid Earth 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Santamarina, J.C. Hydrate bearing clayey sediments: Formation and gas production concepts. Mar. Pet. Geol. 2016, 77, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, J.; Yoneda, J.; Tenma, N. Multiobjective optimization of the particle aspect ratio for gravel pack in a methane-hydrate reservoir using pore scale simulation. J. Nat. Gas Sci. Eng. 2016, 35, 920–927. [Google Scholar] [CrossRef]
- Qorbani, K.; Kvamme, B.; Kuznetsova, T. Using a Reactive Transport Simulator to Simulate CH4 Production from Bear Island Basin in the Barents Sea Utilizing the Depressurization Method. Energies 2017, 10, 187. [Google Scholar] [CrossRef]
- Ruan, X.; Li, X.S.; Xu, C.G. Numerical Investigation of the Production Behavior of Methane Hydrates under Depressurization Conditions Combined with Well-Wall Heating. Energies 2017, 10, 161. [Google Scholar] [CrossRef]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.S. Evaluation of Gas Production Potential from Marine Gas Hydrate Deposits in Shenhu Area of South China Sea. Energy Fuels 2010, 24, 6018–6033. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, G. Analytic modeling and large-scale experimental study of mass and heat transfer during hydrate dissociation in sediment with different dissociation methods. Energy 2015, 90, 1931–1948. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Zhang, L. Sensitivity analysis of gas production from Class I hydrate reservoir by depressurization. Energy 2012, 39, 281–285. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z.; Song, Y.; Liu, W.; Zhang, Y.; Wang, D. Analyzing the process of gas production for natural gas hydrate using depressurization. Appl. Energy 2015, 142, 125–134. [Google Scholar] [CrossRef]
- Li, X.-S.; Yang, B.; Zhang, Y.; Li, G.; Duan, L.-P.; Wang, Y.; Chen, Z.-Y.; Huang, N.-S.; Wu, H.-J. Experimental investigation into gas production from methane hydrate in sediment by depressurization in a novel pilot-scale hydrate simulator. Appl. Energy 2012, 93, 722–732. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.-S.; Zhang, Y.; Li, G. Large scale experimental evaluation to methane hydrate dissociation below quadruple point in sandy sediment. Appl. Energy 2016, 162, 372–381. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Dong, H.; Zhao, Y.; Liu, Y.; Zhang, Y.; Song, Y. Magnetic resonance imaging for in-situ observation of the effect of depressurizing range and rate on methane hydrate dissociation. Chem. Eng. Sci. 2016, 144, 135–143. [Google Scholar] [CrossRef]
- Yang, M.; Fu, Z.; Zhao, Y.; Jiang, L.; Zhao, J.; Song, Y. Effect of depressurization pressure on methane recovery from hydrate–gas–water bearing sediments. Fuel 2016, 166, 419–426. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, M.; Zheng, J.N.; Chen, B. Production characteristics of two class water-excess methane hydrate deposits during depressurization. Fuel 2018, 232, 99–107. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Y.; Li, X.S. Experimental study on characteristics of methane hydrate formation and dissociation in porous medium with different particle sizes using depressurization. Fuel 2018, 230, 37–44. [Google Scholar] [CrossRef]
- Kawamura, T.; Ohtake, M.; Sakamoto, Y.; Yamamoto, Y.; Haneda, H.; Komai, T.; Higuchi, S. Experimental study on steam injection method using methane hydrate core samples. In Proceedings of the Seventh ISOPE Ocean Mining Symposium, Lisbon, Portugal, 1–6 July 2007. [Google Scholar]
- Li, B.; Liang, Y.P.; Li, X.S.; Zhou, L. A pilot-scale study of gas production from hydrate deposits with two-spot horizontal well system. Appl. Energy 2016, 176, 12–21. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, W.; Song, Y. Analysis of heat transfer effects on gas production from methane hydrate by thermal stimulation. Int. J. Heat Mass Transf. 2015, 87, 145–150. [Google Scholar] [CrossRef]
- Chong, Z.R.; Pujar, G.A.; Yang, M.; Linga, P. Methane hydrate formation in excess water simulating marine locations and the impact of thermal stimulation on energy recovery. Appl. Energy 2016, 177, 409–421. [Google Scholar] [CrossRef]
- Li, G.; Li, X.-S.; Tang, L.-G.; Zhang, Y. Experimental Investigation of Production Behavior of Methane Hydrate under Ethylene Glycol Injection in Unconsolidated Sediment. Energy Fuels 2007, 21, 180–186. [Google Scholar] [CrossRef]
- Boswell, R.; Schoderbek, D.; Collett, T.S.; Ohtsuki, S.; White, M.D.; Anderson, B.J. The iġnik sikumi field experiment, alaska north slope: Design, operations, and implications for CO2-CH4 exchange in gas hydrate reservoirs. Energy Fuels 2016, 31, 140–153. [Google Scholar] [CrossRef]
- Darnell, K.N.; Flemings, P.B.; DiCarlo, D. Subsurface injection of combustion power plant effluent as a solid-phase carbon dioxide storage strategy: Injections of flue gas for CO2 storage. Geophys. Res. Lett. 2017, 44, 5521–5530. [Google Scholar] [CrossRef]
- Kvamme, B. Thermodynamic Limitations of the CO2/N2 Mixture Injected into CH4 Hydrate in the Ignik Sikumi Field Trial. J. Chem. Eng. Data 2016, 61, 1280–1295. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Liu, Y.; Zhao, J. Analysis of heat transfer influences on gas production from methane hydrates using a combined method. Int. J. Heat Mass Transf. 2016, 92, 766–773. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Yang, D.; Jiang, X. Determination of dissociation front and operational optimization for hydrate development by combining depressurization and hot brine stimulation. J. Nat. Gas Sci. Eng. 2017, 50, 215–230. [Google Scholar] [CrossRef]
- Giraldo, C.; Klump, J.; Clarke, M.; Schicks, J.M. Sensitivity Analysis of Parameters Governing the Recovery of Methane from Natural Gas Hydrate Reservoirs. Energies 2014, 7, 2148–2176. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, S.; Sung, W. An experimental study on the productivity of dissociated gas from gas hydrate by depressurization scheme. Energy Convers. Manag. 2010, 51, 2510–2515. [Google Scholar] [CrossRef]
- Li, X.-S.; Zhang, Y.; Li, G.; Chen, Z.-Y.; Wu, H.-J. Experimental Investigation into the Production Behavior of Methane Hydrate in Porous Sediment by Depressurization with a Novel Three-Dimensional Cubic Hydrate Simulator. Energy Fuels 2011, 25, 4497–4505. [Google Scholar] [CrossRef]
- Li, B.; Liang, Y.-P.; Li, X.-S.; Wu, H.-J. Numerical Analysis of Methane Hydrate Decomposition Experiments by Depressurization around Freezing Point in Porous Media. Fuel 2015, 159, 925–934. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, R.; Huang, C.; Feng, Z.; Fan, S. Experimental investigation of production behavior of gas hydrate under thermal stimulation in unconsolidated sediment. Energy Fuels 2005, 19, 2402–2407. [Google Scholar] [CrossRef]
- Falser, S.; Uchida, S.; Palmer, A.C.; Soga, K.; Tan, T.S. Increased Gas Production from Hydrates by Combining Depressurization with Heating of the Wellbore. Energy Fuels 2012, 26, 6259–6267. [Google Scholar] [CrossRef]
- Li, X.S.; Yang, B.; Duan, L.P.; Li, G.; Huang, N.S.; Zhang, Y. Experimental study on gas production from methane hydrate in porous media by SAGD method. Appl. Energy 2013, 112, 1233–1240. [Google Scholar] [CrossRef]
- Li, G.; Li, X.-S.; Yang, B.; Duan, L.-P.; Huang, N.-S.; Zhang, Y.; Tang, L.G. The use of dual horizontal wells in gas production from hydrate accumulations. Appl. Energy 2013, 112, 1303–1310. [Google Scholar] [CrossRef]
- Yang, X.; Sun, C.Y.; Yuan, Q.; Ma, P.C.; Chen, G.J. Experimental Study on Gas Production from Methane Hydrate-Bearing Sand by Hot-Water Cyclic Injection. Energy Fuels 2010, 24, 5912–5920. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Y.; Duan, L.P.; Li, G.; Zhang, Y.; Huang, N.S.; Chen, D.F. Experimental investigation into methane hydrate production during three-dimensional thermal huff and puff. Appl. Energy 2012, 94, 48–57. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.; Lv, Q.; Yang, M.; Ling, Z.; Zhao, J. Assessment of gas production from natural gas hydrate using depressurization, thermal stimulation and combined methods. RSC Adv. 2016, 6, 47357–47367. [Google Scholar] [CrossRef]
- Loh, M.; Too, J.L.; Falser, S.; Linga, P.; Khoo, B.C.; Palmer, A. Gas Production from Methane Hydrates in a Dual Wellbore System. Energy Fuels 2015, 29, 35–42. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S.; Li, G.; Zhang, Y.; Chen, Z.Y. Effect of horizontal and vertical well patterns on methane hydrate dissociation behaviors in pilot-scale hydrate simulator. Appl. Energy 2015, 145, 69–79. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.S.; Li, G.; Zhang, Y.; Li, B.; Chen, Z.Y. Experimental investigation into methane hydrate production during three-dimensional thermal stimulation with five-spot well system. Appl. Energy 2013, 110, 90–97. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.S.; Li, G.; Zhang, Y.; Li, B.; Feng, J.C. A three-dimensional study on methane hydrate decomposition with different methods using five-spot well. Appl. Energy 2013, 112, 83–92. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.S.; Li, G.; Huang, N.S.; Feng, J.C. Experimental study on the hydrate dissociation in porous media by five-spot thermal huff and puff method. Fuel 2014, 117, 688–696. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, T.; Song, Y.; Liu, D.; Liu, W.; Liu, Y.; Yang, M.; Ruan, X.; Li, Y. Numerical simulation of gas production from hydrate deposits using a single vertical well by depressurization in the Qilian Mountain permafrost, Qinghai-Tibet Plateau, China. Energy 2013, 52, 308–319. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, G. Evaluation of Gas Production from Marine Hydrate Deposits at the GMGS2-Site 8, Pearl River Mouth Basin, South China Sea. Energies 2016, 9, 222. [Google Scholar] [CrossRef]
- Li, B.; Li, X.-S.; Li, G. Kinetic Studies of Methane Hydrate Formation in Porous Media Based on Experiments in a Pilot-Scale Hydrate Simulator and a New Model. Chem. Eng. Sci. 2014, 105, 220–230. [Google Scholar] [CrossRef]
- Feng, J.C.; Wang, Y.; Li, X.S. Entropy generation analysis of hydrate dissociation by depressurization with horizontal well in different scales of hydrate reservoirs. Energy 2017, 125, 62–71. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.D.; Liang, Y.P.; Liu, H. The use of electrical heating for the enhancement of gas recovery from methane hydrate in porous media. Appl. Energy 2018, 227, 694–702. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, S.; Zhao, W.; Li, B.; Wan, Q.; Li, G. Effects of vertical center well and side well on hydrate exploitation by depressurization and combination method with wellbore heating. J. Nat. Gas Sci. Eng. 2018, 55, 154–164. [Google Scholar] [CrossRef]
- Callarotti, R.C. Energy Return on Energy Invested (EROI) for the Electrical Heating of Methane Hydrate Reservoirs. Sustainability 2011, 3, 2105–2114. [Google Scholar] [CrossRef] [Green Version]








| Run | Category | Power Q (W) | PW (MPa) | t (min) | SH1 | SG1 | SA1 | VP (L) | T5 (°C) | CP (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PD | 0 | 5.00 | 427.53 | 0.302 | 0.378 | 0.320 | 31.976 | 7.01 | 10.25 |
| 2 | SDH | 12.5 | 5.00 | 130.28 | 0.298 | 0.393 | 0.308 | 31.223 | 18.78 | 20.81 |
| 3 | SDH | 25 | 5.00 | 93.92 | 0.295 | 0.382 | 0.323 | 30.068 | 28.61 | 39.74 |
| 4 | PH | 25 | 5.90 | 325.93 | 0.298 | 0.415 | 0.287 | 28.110 | 27.06 | 1.73 |
| 5 | H&P | 25 | 5.00 | 113.43 | 0.314 | 0.428 | 0.258 | 32.984 | 16.76 | 34.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-P.; Liu, S.; Wan, Q.-C.; Li, B.; Liu, H.; Han, X. Comparison and Optimization of Methane Hydrate Production Process Using Different Methods in a Single Vertical Well. Energies 2019, 12, 124. https://doi.org/10.3390/en12010124
Liang Y-P, Liu S, Wan Q-C, Li B, Liu H, Han X. Comparison and Optimization of Methane Hydrate Production Process Using Different Methods in a Single Vertical Well. Energies. 2019; 12(1):124. https://doi.org/10.3390/en12010124
Chicago/Turabian StyleLiang, Yun-Pei, Shu Liu, Qing-Cui Wan, Bo Li, Hang Liu, and Xiao Han. 2019. "Comparison and Optimization of Methane Hydrate Production Process Using Different Methods in a Single Vertical Well" Energies 12, no. 1: 124. https://doi.org/10.3390/en12010124




