H2O2-Enhanced Shale Gas Recovery under Different Thermal Conditions
Abstract
:1. Introduction
2. Sample Preparation and Experiments
2.1. Fuel Properties
2.2. Experimental Set up and Pressure
2.3. Liquid Nitrogen Adsorption and Desorption Test
2.4. Uncertainty Analysis
3. Results and Discussions
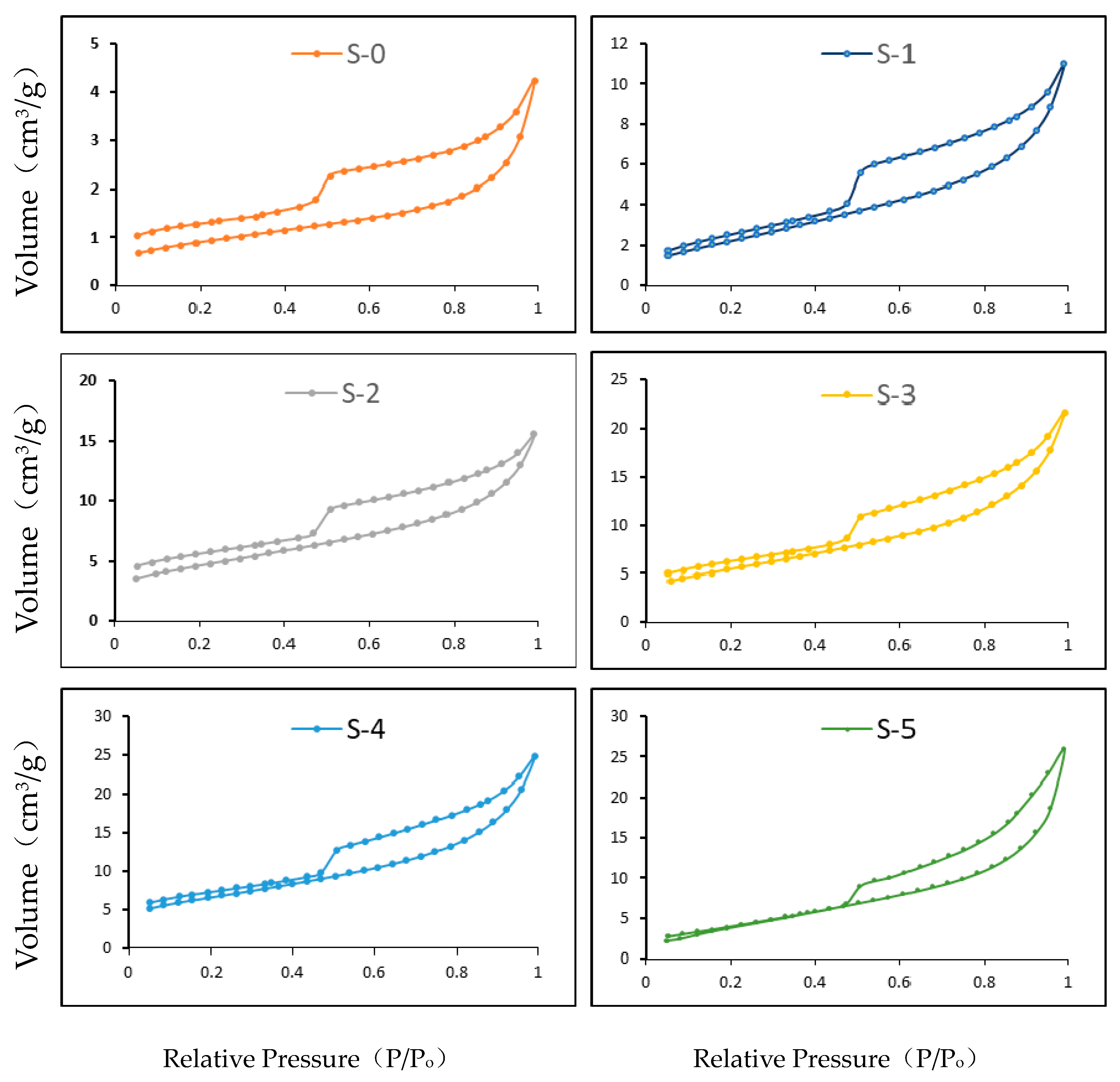
3.1. Liquid Nitrogen Adsorption and Desorption Test Result
3.1.1. Adsorption and Desorption Isotherms
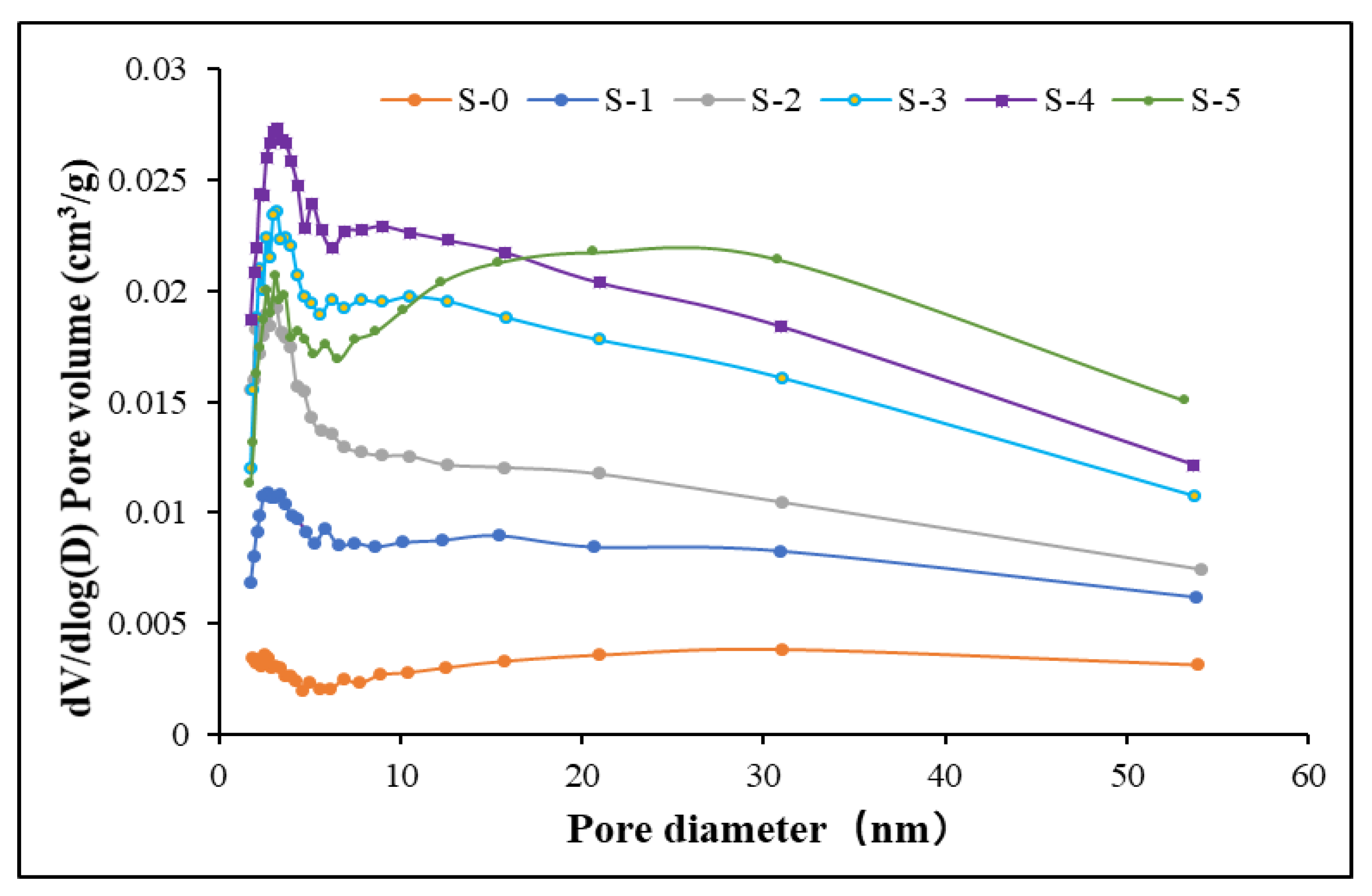
3.1.2. Diameter Distributions of Shale Samples
3.1.3. Mean Diameter of Shale Samples
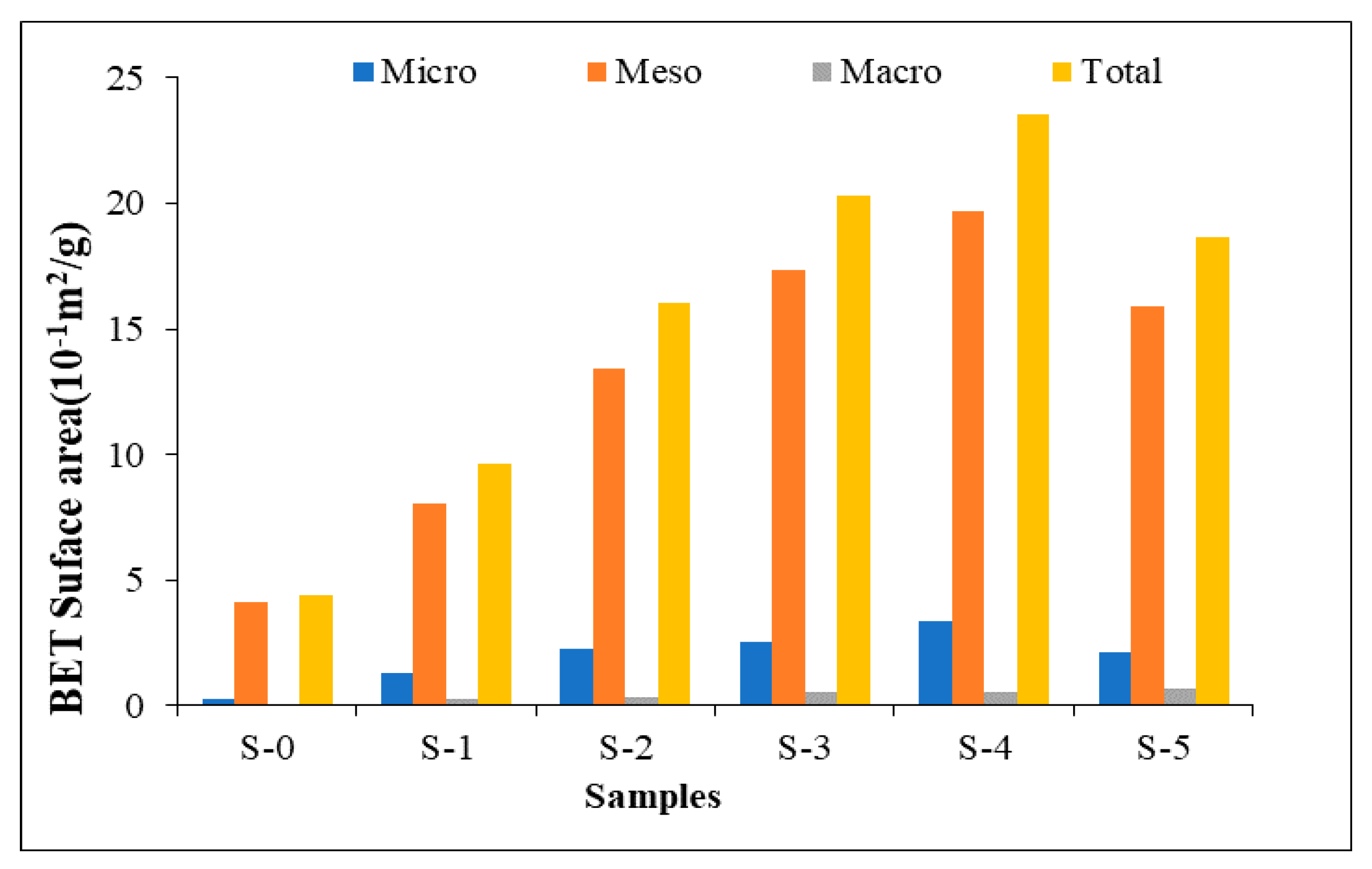
3.1.4. BET Surface Areas of the Samples
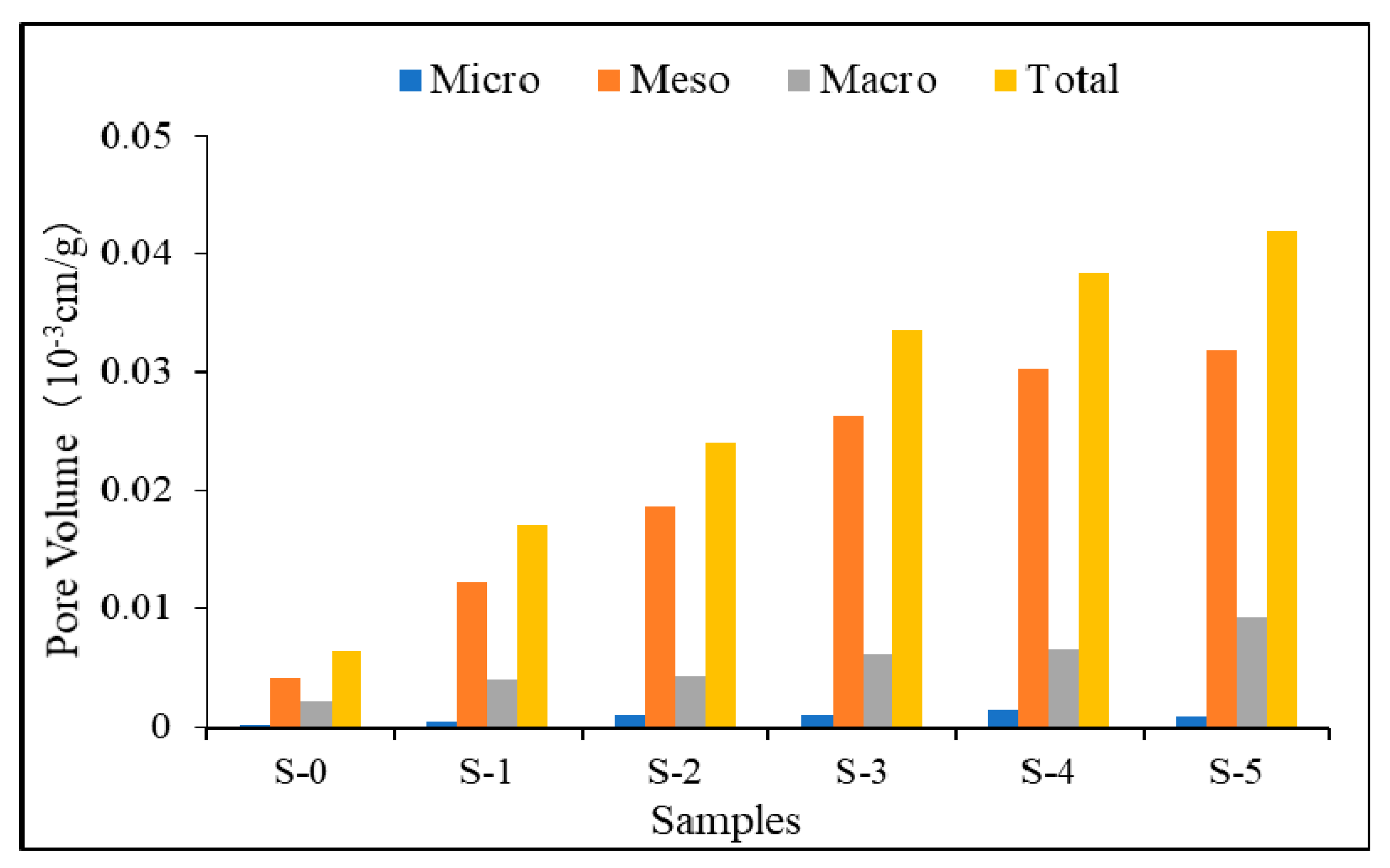
3.1.5. Pore Volume of the Samples
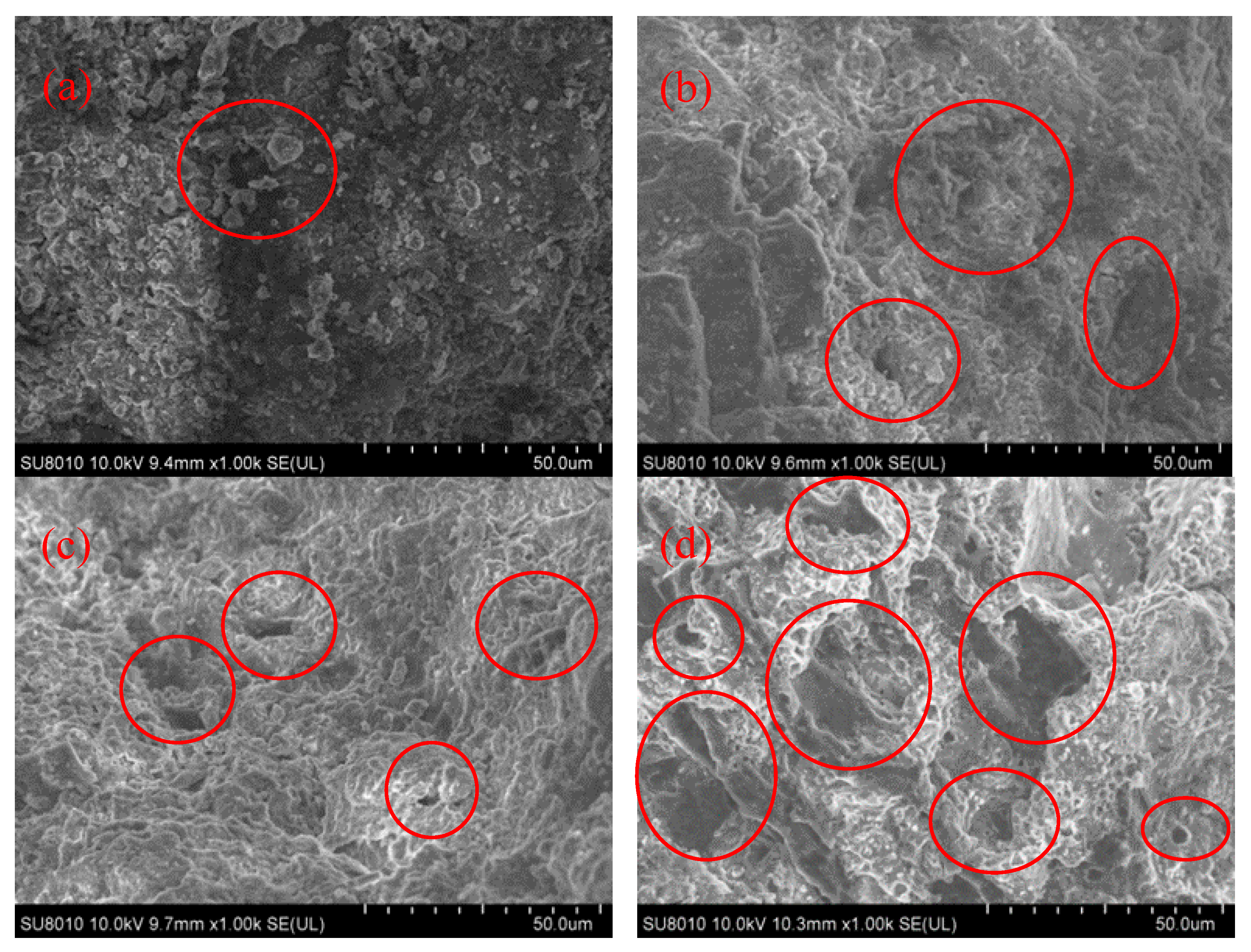
3.2. SEM Images of the Shale Samples
4. Conclusions
- 1)
- The pore diameter of the raw shale samples was in the range of 2 to 55 nm; most pore diameters ranged from 2 nm to 10 nm. The Knudsen number of the gas transportation was greater than 8 and free molecule diffusion was the main flow transportation mode inside the shale formation.
- 2)
- The mean diameter of the pores decreased first and increased again with the increase of reaction temperature and pressure. Compared to the sample under ambient condition, more micropores and mesopores were generated, which resulted in a decrease of mean pore diameter at 100 °C and 0.1 MPa. However, the diameter increased further with even higher reaction pressure and temperature.
- 3)
- Not only the total surface areas of samples but also the micropore and mesopore surface areas increased with temperature and pressure after 30 minutes of treatment. However, the total surface area decreased when the sample was heated at 180 °C and 1.58 MPa for 60 minutes. Higher temperature and pressure resulted in less small pores such as micropores and mesopores which have high surface area to volume ratio.
- 4)
- The total pore volume, mesopore volume, and macropore volume increased from the case S-1 to S5 due to the removal of organic matter. However, the volume of micropores increased from S-1 to S-4 first, and then decreased from S-4 to S-5.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| EIA | Energy Information Administration |
| FC | Fixed carbon |
| IUPAC | International Union of Pure and Applied Chemistry |
| Ro | Vitrinite-like macerals reflectance |
| SEM | Scanning electron microscopy |
| TOC | Total organic carbon |
| VM | Volatile matter |
References
- Cui, X.; Bustin, A.; Bustin, R. Measurements of gas permeability and diffusivity of tight reservoir rocks: Different approaches and their applications. Geofluids 2009, 9, 208–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Lei, Y. Combustion Characteristics of Tight Sandstone. Energy Fuels 2018, 32, 6293–6299. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Leung, C.; Gao, F. A Fully Coupled Numerical Model for Microwave Heating Enhanced Shale Gas Recovery. Energies 2018, 11, 1608. [Google Scholar] [CrossRef]
- Lee, T.; Bocquet, L.; Coasne, B. Activated desorption at heterogeneous interfaces and long-time kinetics of hydrocarbon recovery from nanoporous media. Nat. Commun. 2016, 7, 11890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Jiang, Z.; Xuanlong, S.; Zhang, C.; Zou, Q.; Li, Z.; Zhu, R. The effects of shale pore structure and mineral components on shale oil accumulation in the Zhanhua Sag, Jiyang Depression, Bohai Bay Basin, China. J. Petrol. Sci. Eng. 2018, 165, 365–374. [Google Scholar] [CrossRef]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Valenza, J.; Drenzek, N.; Marques, F.; Pagels, M.; Mastalerz, M. Geochemical controls on shale microstructure. Geology 2013, 41, 611–614. [Google Scholar] [CrossRef]
- Guo, H.; Jia, W.; Peng, P.a.; Lei, Y.; Luo, X.; Cheng, M.; Wang, X.; Zhang, L.; Jiang, C. The composition and its impact on the methane sorption of lacustrine shales from the Upper Triassic Yanchang Formation, Ordos Basin, China. Mar. Petrol. Geol. 2014, 57, 509–520. [Google Scholar] [CrossRef]
- Yang, J.; Hatcherian, J.; Hackley, P.C.; Pomerantz, A.E. Nanoscale geochemical and geomechanical characterization of organic matter in shale. Nat. Commun. 2017, 8, 2179. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Mastalerz, M.; Schimmelmann, A.; Chen, Y. Influence of Soxhlet-extractable bitumen and oil on porosity in thermally maturing organic-rich shales. Int. J. Coal Geol. 2014, 132, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Kuila, U.; Prasad, M.; Kazemi, H. Assessing Knudsen flow in gas-flow models of shale reservoirs. Can. Soc. Explor. Geophys. 2013, 38, 23–27. [Google Scholar]
- Adesida, A.G.; Akkutlu, I.; Resasco, D.E.; Rai, C.S. Characterization of Barnett Shale Kerogen Pore Size Distribution using DFT Analysis and Grand Canonical Monte Carlo Simulations. Soc. Petrol. Eng. 2011, 182, 401–417. [Google Scholar]
- Labani, M.; Rezaee, R. Petrophysical evaluation of gas shale reservoirs. In Fundamentals of Gas Shale Reverviors; Rezaee, R., Ed.; John Wiley& Sons Inc.: New Jersey, NJ, USA, 2015; pp. 117–138. [Google Scholar]
- Jin, L.; Mathur, R.; Rother, G.; Cole, D.; Bazilevskaya, E.; Williams, J.; Carone, A.; Brantley, S. Evolution of porosity and geochemistry in Marcellus Formation black shale during weathering. Chem. Geol. 2013, 356, 50–63. [Google Scholar] [CrossRef]
- Tamamura, S.; Ueno, A.; Aramaki, N.; Matsumoto, H.; Uchida, K.; Igarashi, T.; Kaneko, K. Effects of oxidative weathering on the composition of organic matter in coal and sedimentary rock. Org. Geochem. 2015, 81, 8–19. [Google Scholar] [CrossRef]
- Chen, W.; Lei, Y.; Ma, L.; Yang, L. Experimental Study of High Temperature Combustion for Enhanced Shale Gas Recovery. Energy Fuels 2017, 31, 10003–10010. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, Y.; Chen, W.; Yu, W. Enhance low temperature oxidization of shale gas recovery using hydrogen peroxide. J. Petrol. Sci. Eng. 2018, 164, 523–530. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Kaiser, K.; Jahn, R. Review: Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Sci. Soc. Am. J. 2005, 69, 120–135. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Abiven, S.; Schmidt, M.W.I.; Fuhrer, J. Sodium hypochlorite separates an older soil organic matter fraction than acid hydrolysis. Geoderma 2007, 139, 171–179. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Li, X.; Dong, X. Methane storage in nanoporous material at supercritical temperature over a wide range of pressures. Sci. Rep. 2016, 6, 33461. [Google Scholar] [CrossRef] [Green Version]
- Kuila, U.; McCarty, D.K.; Derkowski, A.; Fischer, T.B.; Topór, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Kang, Y.; You, L.; Yang, P.; Zhang, X.; Cheng, Q. Change in composition and pore structure of Longmaxi black shale during oxidative dissolution. Int. J. Coal Geol. 2017, 172, 95–111. [Google Scholar] [CrossRef]
- EIA. Technically Recoverable Shale Oil and Shale Gas Resources: An Asessment of 137 Shale Formations in 41 Countries outside the United States; EIA: Washington, DC, USA, June 2013.
- Chen, W.; Lei, Y.; Chen, Y.; Sun, J. Pyrolysis and Combustion Enhance Recovery of Gas for Two China Shale Rocks. Energy Fuels 2016, 30, 10298–10305. [Google Scholar] [CrossRef]
- Pan, S.; Zou, C.; Yang, Z.; Dong, D.; Wang, Y.; Wang, S.; Wu, S.; Huang, J.; Liu, Q.; Wang, D.; et al. Methods for shale gas play assessment: A comparison between Silurian Longmaxi shale and Mississippian Barnett shale. J. Earth Sci. 2015, 26, 285–294. [Google Scholar] [CrossRef]
- Kegel, T.M. Basic measurement uncertainty. In Proceedings of the 71st International School of Hydrocarbon Measurement, Oklahoma City, OK, USA, 9–11 April 1996; pp. 1–7. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas or solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Y.; Wei, C.; Huang, L.; Shi, Q.; Wu, C.; Zhang, X. Porosity changes in progressively pulverized anthracite subsamples: Implications for the study of closed pore distribution in coals. Fuel 2018, 225, 612–622. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, T.; Guo, J.; Zhang, Z.; Yang, Y. Characterization of the micropore systems in the high-rank coal reservoirs of the southern Sichuan Basin, China. AAPG Bull. 2015, 99, 2099–2119. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH4 adsorption capacity of coals. Int. J. Coal Geol. 2008, 73, 27–42. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of Coal and Shale: Insights from Gas Adsorption and SANS/USANS Techniques. Energy Fuels 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Chen, P.; Tang, X. The research on the adsorption of nitrogen in low temperature and micro-pore properties in coal. J. China Coal Soc. 2001, 5, 552–556. [Google Scholar]
- Deboer, J.H. The Structure and Properties of Porous Materials; Butterworths: London, UK, 1958; Volume 389. [Google Scholar]
- Kibodeaux, K.R. Evolution of porosity, permeability, and fluid saturations during thermal conversion of oil shale. Soc. Petrol. Eng. 2014. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Dong, M.; Wang, J.; Li, Y.; Gong, H.; Sang, Q. A model of dynamic adsorption–diffusion for modeling gas transport and storage in shale. Fuel 2016, 173, 115–128. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.; Zhou, Q. The influence of soluble organic matter on shale reservoir characterization. J. Nat. Gas Geosci 2016, 1, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale Gas Flow in Shale Gas Sediments. J. Can. Petrol. Technol. 2007, 46. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Zhang, R.; Zhao, H.; Krooss, B.M. Investigations on the methane sorption capacity of marine shales from Sichuan Basin, China. Int. J. Coal Geol. 2015, 146, 104–117. [Google Scholar] [CrossRef]
- Xiong, F.; Jiang, Z.; Chen, J.; Wang, X.; Huang, Z.; Liu, G.; Chen, F.; Li, Y.; Chen, L.; Zhang, L. The role of the residual bitumen in the gas storage capacity of mature lacustrine shale: A case study of the Triassic Yanchang shale, Ordos Basin, China. Mar. Petrol. Geol. 2016, 69, 205–215. [Google Scholar] [CrossRef]



| Moisture | 0.7 |
| Ash | 89.09 |
| VM | 8.11 |
| FC | 2.71 |
| Carbon | 6.24 |
| Oxygen | 3.09 |
| Hydrogen | 0.72 |
| Nitrogen | 0.16 |
| Sulfur | 0.63 |
| Thermal conductivity (kJ/m/s) | 1.56 |
| Samples | Exinite (%) | Vitrinite (%) | Inertinite (%) | Ro (%) | Organic Matter Type |
|---|---|---|---|---|---|
| Shale | 68 | 14 | 18 | 1.85 | II |
| Sample | Time (Minute) | Temperature (°C) | Pressure (MPa) |
|---|---|---|---|
| S-1 | 30 | 100 | 0.10 |
| S-2 | 30 | 120 | 0.48 |
| S-3 | 30 | 130 | 0.55 |
| S-4 | 30 | 140 | 1.48 |
| S-5 | 60 | 180 | 1.58 |
| Instrument | Accuracy | Resolution | Units | Total Instrument Uncertainty |
|---|---|---|---|---|
| Thermocouple | ±1% | 0.1 | °C | 1.0% |
| Pore Size Analyzer | ±2% | 1.0 | nm | 2.0% |
| BET | ±2% | 0.01 | m2/g | 2.0% |
| Pressure sensor | ±0.5% | 0.1 | kPa | 0.51% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Lei, J.; Wang, T.; Chen, W. H2O2-Enhanced Shale Gas Recovery under Different Thermal Conditions. Energies 2019, 12, 2127. https://doi.org/10.3390/en12112127
Yu W, Lei J, Wang T, Chen W. H2O2-Enhanced Shale Gas Recovery under Different Thermal Conditions. Energies. 2019; 12(11):2127. https://doi.org/10.3390/en12112127
Chicago/Turabian StyleYu, WeiGang, Jiang Lei, Tengxi Wang, and Wei Chen. 2019. "H2O2-Enhanced Shale Gas Recovery under Different Thermal Conditions" Energies 12, no. 11: 2127. https://doi.org/10.3390/en12112127
APA StyleYu, W., Lei, J., Wang, T., & Chen, W. (2019). H2O2-Enhanced Shale Gas Recovery under Different Thermal Conditions. Energies, 12(11), 2127. https://doi.org/10.3390/en12112127





