Emissivity Characteristics of Hydrocarbon Flame and Temperature Measurement by Color Image Processing
Abstract
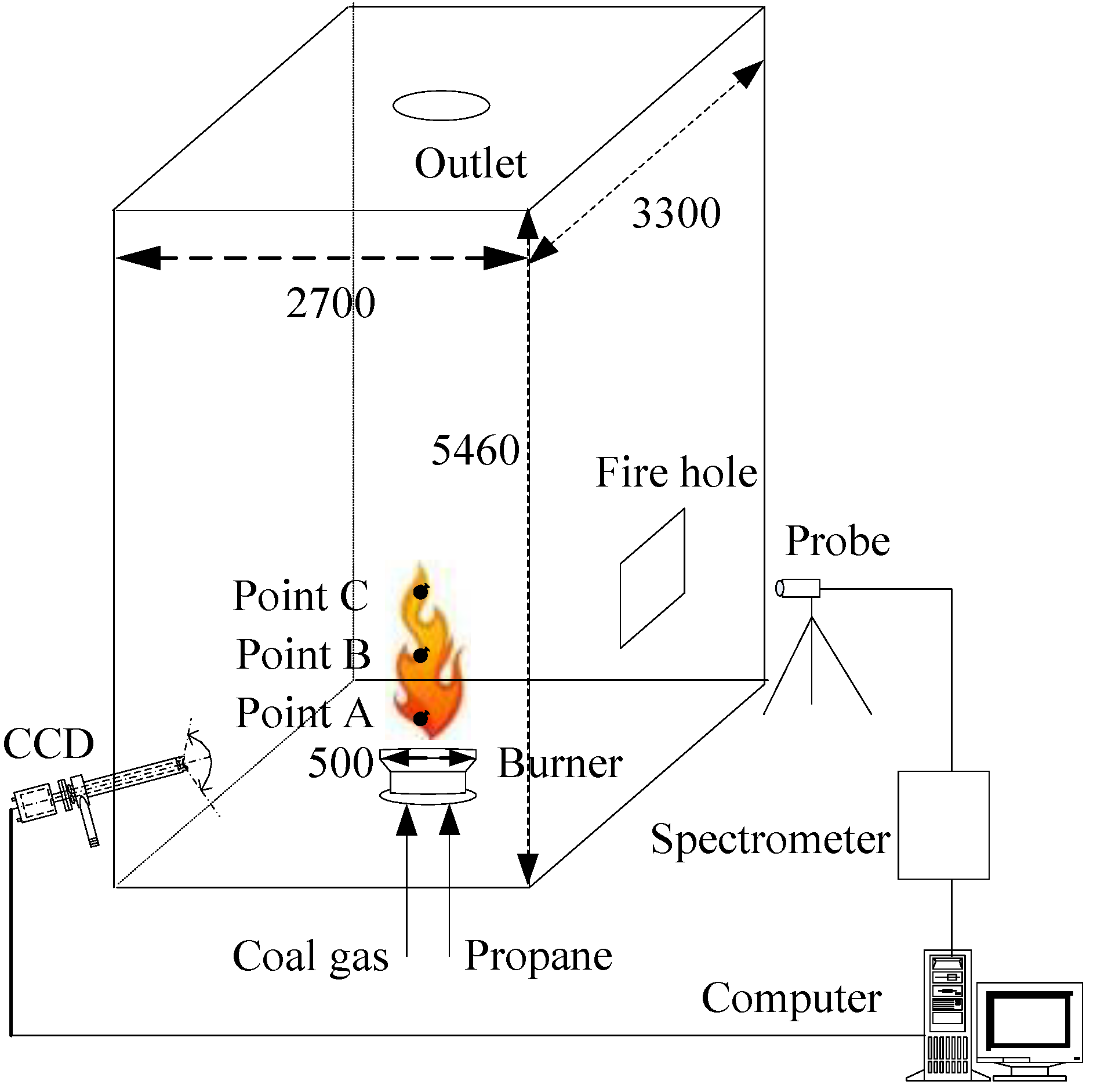
:1. Introduction
2. Emissivity Characteristics and Temperature Measurement
2.1. Methodology
- The characteristic profiles of spectrometer and the filter profiles of CCD camera are calibrated, in order to extract the radiation intensities from the distorted detection signals;
- The approximate polynomial of monochromatic emissivity is detected from the spectrometer signals by a Newton-type iterative method; and
- The ratio pyrometry by color image processing is corrected by the monochromatic emissivity, and then the temperature is solved from an implicit equation without making approximations of the filter profiles of CCD camera.
2.2. Experimental Results and Discussion
3. Radiation Characteristics of Combustion Products
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Javier, B.; Tatiana, G. Diagnostic techniques for the monitoring and control of practical flames. Prog. Energy Combust. Sci. 2010, 36, 375–411. [Google Scholar]
- Liu, F.; Thomson, K.A.; Smallwood, G.J. Soot temperature volume fraction retrieval from spectrally resolved flame emission measurement in laminar axisymmetric coflow diffusion flames: Effect of self-absorption. Combust. Flame 2013, 160, 1693–1705. [Google Scholar] [CrossRef]
- Coppalle, A.; Joyeux, D. Temperature and soot volume fraction in turbulent diffusion flames: Measurements of mean and fluctuating values. Combust. Flame 1994, 96, 275–285. [Google Scholar] [CrossRef]
- Vattulainen, J.; Nummela, V.; Hernberg, R.; Kytölä, J. A system for quantitative imaging diagnostics and its application to pyrometric in-cylinder flame-temperature measurements in large diesel engines. Meas. Sci. Technol. 2000, 11, 103–119. [Google Scholar] [CrossRef]
- Kuhn, P.B.; Ma, B.; Connelly, B.C.; Smooke, M.D.; Long, M.B. Soot and thin-filament pyrometry using a color digital camera. Proc. Combust. Inst. 2011, 33, 743–750. [Google Scholar] [CrossRef]
- Huang, Q.X.; Wang, F.; Liu, D.; Ma, Z.Y.; Yan, J.H.; Chi, Y.; Cen, K.F. Reconstruction of soot temperature and volume fraction profiles of an asymmetric flame using stereoscopic tomography. Combust. Flame 2009, 156, 565–573. [Google Scholar] [CrossRef]
- Lou, C.; Li, W.H.; Zhou, H.C.; Salinas, C.T. Experimental investigation on simultaneous measurement of temperature distributions and radiative properties in an oil-fired tunnel furnace by radiation analysis. Int. J. Heat Mass Transf. 2011, 54, 1–8. [Google Scholar] [CrossRef]
- Keyvan, S.; Rossow, R.; Romero, C.; Li, X.-X. Comparison between Visible and Near-IR Flame Spectra from Natural Gas-FireFurnace for Blackbody Temperature Measurements. Fuel 2004, 83, 1175–1181. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, S.; Zhou, H.; Zhang, B.; Wang, H.; Xu, H. Simultaneously reconstruction of inhomogeneous temperature and radiative properties by radiation image processing. Int. J. Therm. Sci. 2016, 107, 121–130. [Google Scholar]
- Cheng, Q.; Zhang, X.Y.; Wang, Z.C.; Zhou, H.; Shao, S. Simultaneous Measurement of Three-Dimensional Temperature Distributions and Radiative Properties Based on Radiation Image Processing Technology in a Gas-fired Pilot Tubular Furnace. Heat Transf. Eng. 2014, 35, 770–779. [Google Scholar] [CrossRef]
- Christian, G.P.; Alexander, C.W.; David, M.S.; Donaldson, A.B.; Jonathan, L.H. Aluminum Flame Temperature Measurements in Solid Propellant Combustion. Appl. Spectrosc. 2014, 68, 362–366. [Google Scholar]
- Arias, L.; Sbarbaro, D.; Torres, S. Removing baseline flame’s spectrum by using advanced recoveringspectrum techniques. Appl. Opt. 2012, 51, 6111–6116. [Google Scholar] [CrossRef]
- Sun, Y.; Lou, C.; Zhou, H. A simple judgment method of gray property of flames based on spectral analysis and the two-color method for measurements of temperatures and emissivity. Proc. Combust. Inst. 2011, 33, 735–741. [Google Scholar] [CrossRef]
- Fu, T.; Duan, M.; Tang, J.; Shi, C. Measurements of the directional spectral emissivity based on a radiation heating source with alternating spectral distributions. Int. J. Heat Mass Transf. 2015, 90, 1207–1213. [Google Scholar] [CrossRef]
- Snelling, D.R.; Thomson, K.A.; Smallwood, G.J.; G-uacute, L.; Weckman, E.J.; Fraser, R.A. Spectrally Resolved Measurement of Flame Radiation to Determine Soot Temperature and Concentration. AIAA J. 2002, 40, 1789–1795. [Google Scholar] [CrossRef]
- Yan, W.; Chen, D.; Yang, Z.; Yan, E.; Zhao, P. Measurement of Soot Volume Fraction and Temperature for Oxygen-Enriched Ethylene Combustion Based on Flame Image Processing. Energies 2017, 10, 750. [Google Scholar] [CrossRef]
- Yan, W.; Ya, Y.; Du, F.; Shao, H.; Zhao, P. Spectrometer-Based Line-of-Sight Temperature Measurements during Alkali-Pulverized Coal Combustion in a Power Station Boiler. Energies 2017, 10, 1375. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; Zhou, H.; Qi, C. Measurement of distributions of temperature and wavelength-dependent emissivity of a laminar diffusion flame using hyper-spectral imaging technique. Meas. Sci. Technol. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Hossain, M.M.; Lu, G.; Sun, D.; Yan, Y. Three-dimensional reconstruction of flame temperature and emissivity distribution using optical tomographic and two-color pyrometric techniques. Meas. Sci. Technol. 2013, 24, 1–10. [Google Scholar] [CrossRef]
- Chu, H.; Liu, F.; Zhou, H. Calculations of gas radiation heat transfer in a two-dimensional rectangular enclosure using the line-by-line approach and the statistical narrow-band correlated-k model. Int. J. Therm. Sci. 2012, 59, 66–74. [Google Scholar] [CrossRef]
- Hofgren, H.; Sundén, B. Evaluation of Planck-mean coefficients for particle radiative properties in combustion environments. Heat Mass Transf. 2015, 51, 507–519. [Google Scholar] [CrossRef]
- Ludwig, D.B.; Malkmus, W.; Reardon, J.E.; Thomson, J.A.L.; Goulard, R. Handbook of Infrared Radiation from Combustion Gases. NASA SP3080; NASA Marshall Space Flight Center: Huntsville, AL, USA, 1973. [Google Scholar]
- Joseph, D.; Perez, P.; Cuenot, B. Discrete Ordinates and Monte Carlo Methods for Radiative Transfer Simulation applied to CFD combustion modeling. J. Heat Transf. 2018, 131. [Google Scholar] [CrossRef]
- Shimogori, M.; Yoshizako, H.; Shimogori, Y.; Richardson, M. Characterization of Coal Ash Emissivity in High Temperature Atmospheres. JSME Int. J. Ser. B 2006, 49, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Charalampopoulos, T.T. Determination of the wavelength dependence of refractive indices of flame soot. Proc. R. Soc. A 1990, 430, 577–591. [Google Scholar] [CrossRef]
- Zhou, K.; Jiang, J. Thermal Radiation from Vertical Turbulent Jet Flame: Line Source Model. J. Heat Transf. 2016, 138. [Google Scholar] [CrossRef]
- Zhang, H.; Modest, M.F. Evaluation of the Planck-mean absorption coefficients from HITRAN and HITEMP databases. J. Quant. Spectrosc. Radiat. Transf. 2002, 73, 649–653. [Google Scholar] [CrossRef]













| Blackbody Furnace | Spectrometer | CCD Camera | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T (K) | ε | T (K) | Error (%) | ε | Error (%) | T(K) | Error (%) | ε | Error (%) |
| 1143 | 1.0 | 1116.1 | 2.35 | 1.05 | 4.71 | 1144.6 | 0.14 | 0.97 | 2.53 |
| 1173 | 1.0 | 1140.4 | 2.78 | 1.05 | 5.43 | 1162.9 | 0.87 | 1.05 | 5.25 |
| 1203 | 1.0 | 1178.3 | 2.05 | 1.04 | 4.33 | 1209.1 | 0.51 | 0.96 | 3.94 |
| 1233 | 1.0 | 1219.2 | 1.12 | 1.03 | 2.78 | 1246.4 | 1.08 | 0.96 | 4.03 |
| 1263 | 1.0 | 1281.2 | 1.44 | 0.97 | 2.57 | 1279.7 | 1.31 | 0.93 | 6.72 |
| 1293 | 1.0 | 1308.1 | 1.17 | 0.98 | 2.24 | 1307 | 1.07 | 0.95 | 5.06 |
| 1323 | 1.0 | 1335.3 | 0.93 | 0.98 | 2.02 | 1339.1 | 1.22 | 0.96 | 3.67 |
| 1353 | 1.0 | 1362.3 | 0.69 | 0.99 | 1.14 | 1364.3 | 0.84 | 0.97 | 3.18 |
| 1383 | 1.0 | 1390.0 | 0.50 | 0.99 | 1.02 | 1386.7 | 0.26 | 0.98 | 1.93 |
| 1413 | 1.0 | 1425.2 | 0.86 | 0.98 | 1.93 | 1410.2 | 0.21 | 1.02 | 1.72 |
| Case | Propane (m3/h) | Coal Gas (m3/h) | Capacity of the Burner (%) | Oxygen Content (%) | Components of Flue Gas |
|---|---|---|---|---|---|
| 1 | 0 | 113.6 | 50 | 3.92 | 8.9%CO2 + 18.4%H2O + 69.7%N2 |
| 2 | 7.92 | 71.32 | 50 | 3.99 | 8.8%CO2 + 15.6%H2O + 71.5%N2 |
| 3 | 11.88 | 106.98 | 75 | 3.64 | 9.0%CO2 + 15.9%H2O + 71.3%N2 |
| 4 | 22.21 | 51.82 | 75 | 2.69 | 9.8%CO2 + 15.2%H2O + 72.2%N2 |
| Cases | Spectrometer (K) | Point A | Point B | Point C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thermal Couple (K) | CCD (K) | Error (%) | Thermal Couple (K) | CCD (K) | Error (%) | Thermal Couple (K) | CCD (K) | Error (%) | ||
| 1 | 1195.5 | 1295 | 1325.8 | 2.38 | 1216 | 1301.3 | 7.01 | 1126 | 1219.9 | 8.34 |
| 2 | 1343 | 1405 | 1457.3 | 3.72 | 1346 | 1370 | 1.78 | 1309 | 1349.3 | 3.08 |
| 3 | 1461.4 | 1508 | 1528.7 | 1.37 | 1458 | 1498.5 | 2.78 | 1409 | 1488.4 | 5.64 |
| 4 | 1497.7 | 1533 | 1540.5 | 0.49 | 1474 | 1524.3 | 3.41 | 1440 | 1519 | 5.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Zhang, X.; Liu, K.; Zhang, W. Emissivity Characteristics of Hydrocarbon Flame and Temperature Measurement by Color Image Processing. Energies 2019, 12, 2185. https://doi.org/10.3390/en12112185
Lin J, Zhang X, Liu K, Zhang W. Emissivity Characteristics of Hydrocarbon Flame and Temperature Measurement by Color Image Processing. Energies. 2019; 12(11):2185. https://doi.org/10.3390/en12112185
Chicago/Turabian StyleLin, Junyi, Xiangyu Zhang, Kaiyun Liu, and Wenjie Zhang. 2019. "Emissivity Characteristics of Hydrocarbon Flame and Temperature Measurement by Color Image Processing" Energies 12, no. 11: 2185. https://doi.org/10.3390/en12112185
APA StyleLin, J., Zhang, X., Liu, K., & Zhang, W. (2019). Emissivity Characteristics of Hydrocarbon Flame and Temperature Measurement by Color Image Processing. Energies, 12(11), 2185. https://doi.org/10.3390/en12112185





